In this multicenter, dose-ranging study of maribavir for the treatment of cytomegalovirus (CMV) infections deemed refractory or resistant to current antivirals, 67% of patients achieved clearance of CMV viremia within 6 weeks, with responses maintained for up to 24 weeks.
Keywords: cytomegalovirus, foscarnet, ganciclovir, maribavir, transplantation
Abstract
Background
Cytomegalovirus (CMV) infections that are refractory or resistant (RR) to available antivirals ([val]ganciclovir, foscarnet, cidofovir) are associated with higher mortality in transplant patients. Maribavir is active against RR CMV strains.
Methods
Hematopoietic-cell or solid-organ transplant recipients ≥12 years old with RR CMV infections and plasma CMV deoxyribonucleic acid (DNA) ≥1000 copies/mL were randomized (1:1:1) to twice-daily dose-blinded maribavir 400, 800, or 1200 mg for up to 24 weeks. The primary efficacy endpoint was the proportion of patients with confirmed undetectable plasma CMV DNA within 6 weeks of treatment. Safety analyses included the frequency and severity of treatment-emergent adverse events (TEAEs).
Results
From July 2012 to December 2014, 120 patients were randomized and treated (40 per dose group): 80/120 (67%) patients achieved undetectable CMV DNA within 6 weeks of treatment (95% confidence interval, 57–75%), with rates of 70%, 63%, and 68%, respectively, for maribavir 400, 800, and 1200 mg twice daily. Recurrent on-treatment CMV infections occurred in 25 patients; 13 developed mutations conferring maribavir resistance. Maribavir was discontinued due to adverse events in 41/120 (34%) patients, and 17/41 discontinued due to CMV infections. During the study, 32 (27%) patients died, 4 due to CMV disease. Dysgeusia was the most common TEAE (78/120; 65%) and led to maribavir discontinuation in 1 patient. Absolute neutrophil counts <1000/µL were noted in 12/106 (11%) evaluable patients, with rates similar across doses.
Conclusions
Maribavir ≥400 mg twice daily was active against RR CMV infections in transplant recipients; no new safety signals were identified.
Clinical Trials Registration
Cytomegalovirus (CMV) infections that are refractory or resistant (RR) to currently available antivirals (ganciclovir, valganciclovir, foscarnet, or cidofovir) are a major cause of morbidity and mortality among allogeneic hematopoietic-cell transplant (HCT) and solid-organ transplant (SOT) recipients [1–5].
Most current anti-CMV agents inhibit the CMV deoxyribonucleic acid (DNA) polymerase, and resistance is caused by mutations in the CMV genes coding for UL97 protein kinase or UL54 DNA polymerase [6]. Ganciclovir resistance has been reported in up to 14% of transplant recipients [7–10]. Resistance to foscarnet and cidofovir has also been well described [11]. Moreover, myelosuppression is frequently associated with (val)ganciclovir use [12, 13] and nephrotoxicity with foscarnet and cidofovir use [14]. Letermovir, recently approved for CMV prophylaxis in HCT recipients [15], inhibits CMV replication by binding to components of the CMV-terminase complex, but the role of letermovir in the treatment of active CMV infection, RR or not, has not been well studied [16].
Maribavir [17] is active in vitro against CMV strains resistant to ganciclovir, foscarnet, or cidofovir [18–20]. Maribavir has anti-CMV effects on CMV DNA synthesis, viral gene expression, encapsidation, and viral capsid egress through the inhibition of UL97-mediated phosphorylation of nuclear lamin A/C [21–23]. Phase 1 data demonstrated a favorable safety profile up to 2400 mg/day [24, 25]. A phase 2 dose-escalation study of maribavir (up to 400 mg twice daily [BID]) for CMV prophylaxis in HCT was encouraging [26]. However, 100 mg BID maribavir prophylaxis failed to prevent CMV disease in HCT or liver-transplant recipients in phase 3 trials [27–29]. A case series of maribavir for RR CMV disease suggested that maribavir 400–800 mg BID could be used for treatment of active CMV infections [30].
We assessed the efficacy, safety, and tolerability of different doses of maribavir among transplant recipients experiencing RR CMV infections.
METHODS
Patients and Study Design
We recruited HCT and SOT recipients ≥12 years old, who had documented CMV infections RR to ≥1 available US Food and Drug Administration-approved antiviral treatments and a central-laboratory plasma CMV DNA level ≥1000 copies/mL, from 27 centers in the United States. Detailed eligibility criteria are in the Supplementary Appendix.
Refractory CMV infection was defined as a documented failure to achieve >1 log10 decrease in CMV DNA level after ≥2 weeks of ganciclovir, valganciclovir, or foscarnet treatment. Resistant CMV infection was defined as a refractory CMV infection with documentation of at least 1 genetic mutation associated with resistance to ganciclovir or foscarnet by local testing results.
Randomization and Blinding
Patients were stratified by transplant type (HCT, SOT) and randomized using permuted blocks of 3 via an interactive voice- and Web-response system at a ratio of 1:1:1 to receive maribavir 400, 800, or 1200 mg BID for up to 24 weeks (Supplementary Figure S1). Patients, investigators, and study personnel were blinded to dose. Patients received a box with 3 bottles at each study visit. Each bottle contained either maribavir 200 mg tablets or matching placebo tablets. Patients took 2 tablets from each bottle twice daily. Depending on the dose allocated (400, 800, or 1200 mg BID), patients took 400 mg of maribavir from 1, 2, or 3 bottles, respectively.
Study Assessments
Patients were evaluated weekly through treatment week 6, every 2 weeks through week 12, and every 4 weeks thereafter, through week 24. Patients who discontinued maribavir had study evaluations at 1, 4, 8, and 12 weeks post-treatment. Blood samples were collected at each visit for central laboratory CMV testing using the ARTUS CMV TM PCR kit (Qiagen, Valencia, CA; quantification range, 200–100000 copies/mL). Additional study visits and laboratory assessments are outlined in Supplementary Table S1.
Patients were required to achieve minimum virologic responses for treatment to continue: by week 3, they needed to achieve any decrease in CMV DNA between baseline and week 2’s test; and by week 6, they needed to achieve a decrease ≥2 log10 between baseline and week 5’s test or have undetectable CMV DNA on week 5’s test. Patients continued treatment beyond week 6 at the investigator’s discretion, through a maximum of 24 weeks, to achieve or maintain undetectable CMV DNA levels. Treatment could be discontinued due to recovery (ie, CMV response was adequate), as judged by site investigators.
Investigator-reported local laboratory results of mutations associated with ganciclovir or foscarnet resistance were used for the eligibility designation of “resistant.” Baseline plasma samples for all patients were assessed at a central laboratory for known maribavir resistance mutations in the UL97 and UL27 regions of the CMV genome, and for known ganciclovir, foscarnet, or cidofovir mutations in the UL97 and UL54 genes [31]. During the study, plasma samples from selected patients (including those who had a CMV recurrence during the study) were also submitted for CMV genotyping for resistance mutations.
Safety assessments included monitoring of adverse events (AEs) and serious AEs (SAEs).
Study Endpoints
The primary efficacy endpoint was the proportion of patients with confirmed undetectable (<200 copies/mL) central-laboratory plasma CMV DNA in 2 consecutive post-baseline and on-treatment samples that were separated by at least 5 days and were within the first 6 weeks of treatment. CMV recurrence was defined as plasma CMV DNA ≥200 copies/mL in ≥2 consecutive samples separated by ≥5 days in a patient who had previously achieved plasma CMV DNA <200 copies/mL.
Secondary efficacy analyses included the time to the first undetectable plasma CMV DNA at any time, proportion of patients with CMV recurrence, and time to first CMV recurrence. Efficacy was also evaluated for pre-specified subgroups based on baseline transplant and CMV infection characteristics. For patients with a symptomatic CMV infection or organ disease at baseline, clinical manifestations were classified by the investigator at each study visit as worse, unchanged, improved, or absent. The primary safety analysis was the incidence of treatment-emergent AEs (TEAEs).
Statistical Analysis
Enrollment of 120 patients into 3 dose groups (1:1:1) was planned based on the feasibility of assessing maribavir safety in a medically complex transplant population. All analyses were performed in the intent-to-treat (ITT) population of all randomized patients who received at least 1 dose of maribavir; the primary endpoint is also reported for the per-protocol (PP) population (defined in the Supplementary Appendix). Due to the small number of patients per group, no formal statistical comparisons between groups were planned. The expected proportion of patients with undetectable viremia was 70% by week 7 [32]. Summary statistical analyses were performed to evaluate the overall treatment effect and dose-group effects. Kaplan-Meier plots were used for time-to-event analyses. SAS Version 9.2 (SAS Institute, Cary, NC) was used for all calculations.
Study Oversight
The study was designed and initially sponsored by ViroPharma. On 29 April 2014, sponsorship was transferred to Shire Development, LLC. All investigators and laboratories provided study data. Trial statisticians performed the study analyses and vouch for the integrity and validity of the analyses; the authors affirm the study was conducted as specified in the protocol.
The study was conducted in accordance with International Conference on Harmonisation of Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. The trial was approved by relevant ethics committees at each site. An independent, unblinded Data Monitoring Committee reviewed available safety and safety-related efficacy data at pre-defined time points during the study. All patients provided written informed consent prior to any study-specific procedures.
RESULTS
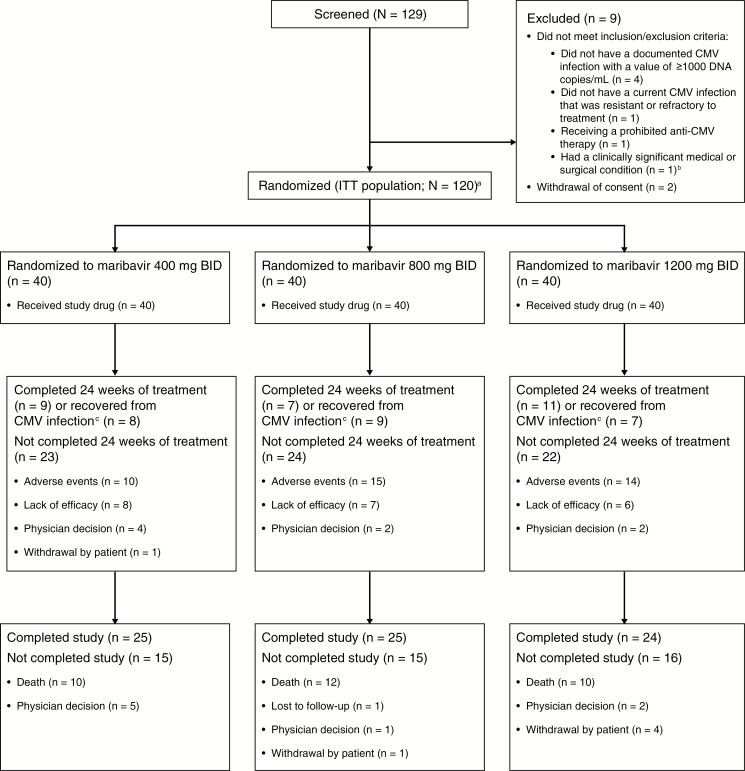
Between 17 July 2012 and 5 December 2014, 129 patients consented and were screened, and 120 (47 HCT and 73 SOT recipients) were randomized (Figure 1). Baseline characteristics were similar between groups (Table 1, Supplementary Table S2). Despite not meeting 1 or more eligibility criteria, 14 patients were randomized into the study: 6 patients had screening CMV DNA <1000 copies/mL; 2 patients weighed <40 kg; 2 patients had current CMV infections not RR to available antivirals; 6 patients were receiving concomitant, prohibited anti-CMV therapies; and 1 patient required advanced life support at the time of enrollment. These patients were excluded from the PP population.
Figure 1.
Patient disposition. Abbreviations: BID, twice daily; CMV, cytomegalovirus; DNA, deoxyribonucleic acid; ITT, intent-to-treat. aA total of 14 patients were randomized in the study, despite not meeting 1 or more protocol eligibility criteria. See text for details. bA clinically significant medical or surgical condition that could have interfered with the administration of the study drug or the interpretation of study results, or compromised the safety or well-being of the patient. cAccording to the study design, treatment could be stopped if the patient achieved confirmed undetectable levels of CMV DNA, indicating successful treatment. Patients achieving undetectable CMV DNA levels could also be maintained on study treatment at the discretion of the investigator.
Table 1.
Patient Demographics and Baseline Characteristics
| Characteristic | Maribavir 400 mg BID (n = 40) |
Maribavir 800 mg BID (n = 40) |
Maribavir 1200 mg BID (n = 40) |
All Doses (N = 120) |
|---|---|---|---|---|
| Median age, years (range) |
54.5 (18–74) |
61.0 (19–74) |
50.5 (20–70) |
55.0 (18–74) |
| Female sex, n (%) | 19 (47.5) | 16 (40.0) | 16 (40.0) | 51 (42.5) |
| Race, n (%) | ||||
| Asian | 2 (5.0) | 1 (2.5) | 1 (2.5) | 4 (3.3) |
| Black or African American | 6 (15.0) | 8 (20.0) | 4 (10.0) | 18 (15.0) |
| Native Hawaiian or Pacific Islander | 0 | 0 | 1 (2.5) | 1 (0.8) |
| White | 32 (80.0) | 31 (77.5) | 32 (80.0) | 95 (79.2) |
| Other | 0 | 0 | 2 (5.0)a | 2 (1.7) |
| Median body mass index, kg/m2 (range) |
26.3 (18.4–40.6) | 22.8 (16.1–45.8)b |
24.0 (15.6–49.9) |
24.3 (15.6–49.9)b |
| Most recent transplant, n (%) | ||||
| Hematopoietic-cell transplantc | 16 (40.0) | 16 (40.0) | 15 (37.5) | 47 (39.2) |
| Solid-organ transplantc,d | 24 (60.0) | 24 (60.0) | 25 (62.5) | 73 (60.8) |
| Kidney | 9 (22.5) | 11 (27.5) | 10 (25.0) | 30 (25.0) |
| Lung | 6 (15.0) | 7 (17.5) | 7 (17.5) | 20 (16.7) |
| Pancreas | 5 (12.5) | 1 (2.5) | 5 (12.5) | 11 (9.2) |
| Liver | 5 (12.5) | 2 (5.0) | 3 (7.5) | 10 (8.3) |
| Heart | 2 (5.0) | 2 (5.0) | 2 (5.0) | 6 (5.0) |
| Intestine | 3 (7.5) | 1 (2.5) | 0 | 4 (3.3) |
| Other | 2 (5.0) | 0 | 1 (2.5) | 3 (2.5) |
| Asymptomatic CMV, n (%) | 24 (60.0) | 26 (65.0) | 27 (67.5) | 77 (64.2) |
| Symptomatic CMV, n (%) | 10 (25.0) | 7 (17.5) | 10 (25.0) | 27 (22.5) |
| CMV organ disease, n (%) | 6 (15.0) | 7 (17.5) | 3 (7.5) | 16 (13.3) |
| Median viral load at baseline, log10 copies/mL (range)e | 3.7 (2.0–6.0) | 3.8 (2.0–5.7) | 3.7 (2.0–7.0) | 3.7 (2.0–7.0) |
| High viral load (≥104 copies/mL) | 4.5 (4.0–6.0) | 4.8 (4.0–5.7) | 4.7 (4.0–7.0) | 4.7 (4.0–7.0) |
| Low viral load (<104 copies/mL) | 3.0 (2.0–4.0) | 3.3 (2.0–3.9) | 3.3 (2.0–4.0) | 3.3 (2.0–4.0) |
| Known CMV genetic mutations associated with resistance to ganciclovir or foscarnet, n (%)f | 22 (55.0) | 25 (62.5) | 24 (60.0) | 71 (59.2) |
| Had net immunosuppression reduced prior to starting study treatment, n (%) | 13 (32.5) | 16 (40.0) | 16 (40.0) | 45 (37.5) |
Abbreviations: BID, twice daily; CMV, cytomegalovirus.
aMulti-racial (n = 1) and both Black/African American and White/Caucasian (n = 1).
bData missing from 1 male patient.
cDenominator is the number of patients in the intent-to-treat safety group.
dPatients may have received multi-organ transplants and therefore appear in more than 1 category of solid-organ transplant.
eVisit values of <200 copies/mL are included as 100 copies/mL for analysis.
fAntiviral resistance done at study sites for clinical reasons. This testing was not a requirement of the study.
Patients received maribavir for a median of 75 (range, 5–177) days. Detailed pharmacokinetic data are in Supplementary Table S3.
Efficacy
For the ITT population, 67% (80/120; 95% confidence interval [CI], 57–75%) of patients had CMV DNA <200 copies/mL by week 6; the proportion was similar across doses (Table 2). There were 86 (72%) patients who achieved confirmed undetectable plasma CMV DNA. The median time to confirmed undetectable plasma CMV DNA was 24 (95% CI, 15–31), 28 (95% CI, 15–38), and 22 (95% CI, 19–30) days in the 400, 800, and 1200 mg BID groups, respectively (Figure 2A). At baseline, 9 patients had CMV DNA <200 copies/mL: 7 of the 9 had blood/plasma CMV infections with a value of ≥1000 DNA copies/mL at screening, but their viral loads had decreased to <200 copies/mL at randomization; the other 2 patients were enrolled without meeting this criterion. Of these 9 patients, 7 achieved confirmed undetectable plasma CMV DNA by week 6 in the primary analysis (3 of whom had symptomatic CMV infections or CMV disease at baseline), and 2 did not achieve confirmed undetectable plasma CMV DNA before being discontinued from the study drug (1 patient had CMV disease at baseline). These 9 patients were excluded from the PP analysis.
Table 2.
Efficacy Outcomes for Maribavir (ITT Population)
| Outcome | Maribavir 400 mg BID (n = 40) |
Maribavir 800 mg BID (n = 40) |
Maribavir 1200 mg BID (n = 40) |
All Doses (N = 120) |
|---|---|---|---|---|
| Primary efficacy endpoint: patients with confirmed undetectable plasma CMV DNA within 6 weeks, n (%)a | ||||
| Yesb | 28 (70.0) | 25 (62.5) | 27 (67.5) | 80 (66.7) |
| No | 12 (30.0) | 15 (37.5) | 11 (27.5) | 38 (31.7) |
| Patients with missing data (no post-baseline CMV measurement within 6 weeks), n (%) |
0 | 0 | 2 (5.0) | 2 (1.7) |
| Treatment effect estimate by group | ||||
| Estimated ratea | 0.70 | 0.63 | 0.68 | 0.67 |
| 95% CI | 0.53–0.83 | 0.46–0.77 | 0.51–0.81 | 0.57–0.75 |
| Secondary efficacy endpoint: CMV recurrence in patients achieving undetectable CMV DNA | ||||
| Patients achieving confirmed undetectable CMV DNA during the study, as defined in the recurrence analysis, n | 29 | 27 | 30 | 86 |
| Patients with CMV recurrence at any time during the study, n (%)c | ||||
| Yesd | 7 (24.1) | 11 (40.7) | 12 (40.0) | 30 (34.9) |
| Noe,f | 22 (75.9) | 14 (51.9) | 17 (56.7) | 53 (61.6) |
| Treatment effect estimate by group | ||||
| Estimated ratec | 0.24 | 0.41 | 0.40 | 0.35 |
| 95% CI | 0.10–0.44 | 0.22–0.61 | 0.23–0.59 | 0.25–0.46 |
| Patients with CMV recurrence on treatment, n (%)c | 6 (20.7) | 9 (33.3) | 10 (33.3) | 25 (29.1) |
| Patients with CMV recurrence off treatment, n (%)c,g | 1 (3.4) | 2 (7.4) | 2 (6.7) | 5 (5.8) |
Abbreviations: AE, adverse event; BID, twice daily; CI, confidence interval; CMV, cytomegalovirus; DNA, deoxyribonucleic acid; ITT, intent-to-treat.
aNumerator is the number of “yes” patients; denominator is the number of ITT patients.
bPatients who died after achieving CMV DNA <200 copies/mL within 6 weeks were included as responders in the primary analysis; 5 patients who achieved CMV DNA <200 copies/mL within 6 weeks died within 42 days of the first dose of the study drug (fatal AEs: pneumonia [bacterial], n = 1; sepsis with multi-organ failure, n = 1; multi-organ failure, n = 2; post-transplant lymphoproliferative disorder, n = 1). None of these deaths was related to the study drug and none of the fatal AEs was likely to be related to CMV.
cNumerator is all recurrences; denominator is the number of patients achieving confirmed undetectable CMV DNA during the study, as specifically defined in the recurrence analysis.
dAny recurrence during the study, including early-withdrawal patients who had a recurrence before withdrawal from the study.
eDid not have recurrence during the study and had data after achieving confirmed undetectable CMV DNA, including early-withdrawal patients who did not have recurrence before withdrawal from the study.
fCalculated using the exact (Clopper-Pearson) confidence limits for the binomial proportion.
gFollow-up assessments through 12 weeks post-treatment.
Figure 2.
Kaplan-Meier plots for (A) time to confirmed undetectable plasma CMV DNA at any time during the study and (B) time from confirmed undetectable plasma CMV DNA to CMV recurrence. Abbreviations: CMV, cytomegalovirus; DNA, deoxyribonucleic acid.
For the PP population, 71% (65/91; 95% CI, 61–80%) of patients had CMV DNA <200 copies/mL by week 6; the proportion was numerically higher in the 1200 mg BID group (78% [21/27]; 95% CI, 58–91%) compared with the 400 mg BID (71% [22/31]; 95% CI, 52–86%) and 800 mg BID (67% [22/33]; 95% CI, 48–82%) groups.
Of the 86 patients who achieved confirmed undetectable plasma CMV DNA during the study, 30 (35%; 95% CI, 25–46%) subsequently experienced recurrent CMV viremia (Table 2); 25 were on maribavir treatment at the time of recurrence. Of these patients, 13/25 (52%; 400 mg, n = 4; 800 mg, n = 6; 1200 mg, n = 3) developed de novo UL97 mutations, known to confer resistance to maribavir (T409M in 10, H411Y in 3). Patients with recurrent CMV viremia remained on or resumed maribavir treatment for a median of 5 (range, 0–19) weeks after recurrence. The probability of recurrence at 6 and 12 weeks after becoming undetectable was 23% and 30%, respectively (Figure 2B).
Subgroup analyses are shown in Supplementary Table S4. Similar proportions of HCT and SOT recipients had virologic responses within 6 weeks (70% vs 64%); the CMV recurrence rate was lower among HCT recipients (26% vs 40%).
Among patients with a symptomatic CMV infection or disease at baseline, CMV-associated manifestations had improved by week 6 in 24/34 (71%) evaluable patients and resolved in 6/34 (18%) of these patients. CMV-associated symptoms/disease were not analyzable in 9 patients at week 6 (total deaths, n = 4: SOT deaths, n = 3 [CMV disease, n = 1; symptomatic CMV infection, n = 2] and HCT death, n = 1 [CMV disease]; withdrawn by investigator, n = 2; withdrawn from the study drug due to AEs, n = 3 [withdrawn from study, n = 1; continued in study, n = 2]).
Safety
Dysgeusia was the most common TEAE overall (65%), with an incidence numerically higher in the 1200 mg BID group (Table 3); the majority of dysgeusia TEAEs were described as “metallic” or “bitter” tastes. Nausea and vomiting were reported in 34% and 29%, respectively, with similar incidences across doses. A TEAE of CMV infection was reported in 23% of patients overall, and the incidence or progression of CMV disease was reported in 7 patients (CMV pneumonia, n = 3; CMV gastroenteritis, n = 2; CMV chorioretinitis, n = 1; CMV encephalitis, n = 1).
Table 3.
Summary of AEs (ITT Population)
| Maribavir 400 mg BID (n = 40) |
Maribavir 800 mg BID (n = 40) |
Maribavir 1200 mg BID (n = 40) |
All Doses (N = 120) |
|
|---|---|---|---|---|
| AEs | ||||
| Patients with ≥1 TEAE | 40 (100.0) | 40 (100.0) | 40 (100.0) | 120 (100.0) |
| Dysgeusia | 24 (60.0) | 25 (62.5) | 29 (72.5) | 78 (65.0) |
| Nausea | 15 (37.5) | 12 (30.0) | 14 (35.0) | 41 (34.2) |
| Vomiting | 11 (27.5) | 13 (32.5) | 11 (27.5) | 35 (29.2) |
| CMV infectiona | 6 (15.0) | 12 (30.0) | 10 (25.0) | 28 (23.3) |
| Diarrhea | 5 (12.5) | 13 (32.5) | 10 (25.0) | 28 (23.3) |
| Fatigue | 8 (20.0) | 10 (25.0) | 7 (17.5) | 25 (20.8) |
| Anemia | 7 (17.5) | 7 (17.5) | 10 (25.0) | 24 (20.0) |
| Peripheral edema | 11 (27.5) | 6 (15.0) | 6 (15.0) | 23 (19.2) |
| Headache | 9 (22.5) | 4 (10.0) | 6 (15.0) | 19 (15.8) |
| Renal impairment | 3 (7.5) | 7 (17.5) | 9 (22.5) | 19 (15.8)b |
| Depression | 2 (5.0) | 8 (20.0) | 1 (2.5) | 11 (9.2) |
| Discontinuation owing to an AE | 11 (27.5) | 17 (42.5) | 13 (32.5) | 41 (34.2) |
| ≥1 treatment-emergent SAE | 28 (70.0) | 27 (67.5) | 26 (65.0) | 81 (67.5) |
| Deaths | 10 (25.0) | 12 (30.0) | 10 (25.0) | 32 (26.7) |
| Laboratory evaluation: incidence of treatment-emergent neutropenia | ||||
| Patients with baseline and ≥1 post-baseline ANCc | 37 (92.5) | 35 (87.5) | 34 (85.0) | 106 (88.3) |
| Patients with ANC <1000/µLd | ||||
| At baseline | 4 (10.8) | 7 (20.0) | 6 (17.6) | 17 (16.0) |
| ≥1 occurrence at any time during the study | 4 (10.8) | 4 (11.4) | 4 (11.8) | 12 (11.3) |
| Patients with ANC <500/µLd | ||||
| At baseline | 3 (8.1) | 1 (2.9) | 1 (2.9) | 5 (4.7) |
| ≥1 occurrence at any time during the study | 0 | 1 (2.9) | 1 (2.9) | 2 (1.9) |
All data are n (%). Individual TEAEs are shown for those occurring in ≥20% of patients in any treatment group.
Abbreviations: AE, adverse event; ANC, absolute neutrophil count; BID, twice daily; CMV, cytomegalovirus; ITT, intent-to-treat; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
aAny CMV event was recorded by the investigator as either an AE or SAE, with CMV organ disease reported as an SAE. Therefore, this represents new CMV infections as reported by the investigator, which may have differed from the protocol definition of CMV infection.
b19 patients had renal impairment TEAEs: hematopoietic-cell transplant, n = 5; solid-organ transplant, n = 14 (kidney, n = 5; lung, n = 4; liver, heart, intestine, pancreas, and liver/pancreas, all n = 1).
cDenominator is the number of patients in each treatment group.
dDenominator is the number of patients with baseline and ≥1 post-baseline ANC.
Maribavir was discontinued because of AEs in 41/120 (34%) patients. The most common AEs leading to maribavir discontinuation were CMV infections (17/41, 42%) or disease (4/41, 10%). Gastrointestinal AEs (nausea, vomiting, diarrhea) resulted in treatment discontinuation in 3 patients; dysgeusia led to discontinuation of maribavir in 1 patient.
During the study, 27% (32/120) of patients died (Table 3) after a median follow-up of 22 (range, 1–41) weeks; mortality was similar across treatments. There were 4 deaths attributed to CMV (CMV pneumonia, n = 2; CMV infection, n = 1; CMV encephalitis, n = 1). The site investigator considered 1 death, in the 800 mg BID group, to be possibly related to maribavir (multi-organ failure).
Renal impairment was reported in 19/120 (16%) patients (400 mg BID, n = 3; 800 mg BID, n = 7; 1200 mg, BID n = 9) and met SAE criteria in 7/120 (6%) patients. All except 1 of these 19 patients had a history of chronic renal disease or elevated creatinine; worsening renal impairment was considered related to the study drug in 3 patients, 1 of which was an SAE. In 1 patient, renal impairment was reported as related to concomitant administration of foscarnet (for herpes simplex virus meningoencephalitis). In all, 3 events (1 per group) were fatal; none of these were considered related to maribavir. The frequency of renal TEAEs were similar in kidney transplant patients compared to other SOT or HCT recipients.
Treatment-emergent increased immunosuppressant drug levels were reported in 12/120 (10%) patients (11 involving tacrolimus, 1 involving sirolimus); 11 were considered to be related to maribavir. Due to an AE of increased tacrolimus levels, 1 patient discontinued treatment.
Neutropenia (absolute neutrophil count <1000/µL) was present in 17/106 (16%) patients at baseline. A total of 12/106 (11%) patients had ≥1 occurrence of neutropenia during the study, with rates similar across doses (Table 3).
Changes from baseline in vital signs, electrocardiograms, and clinical laboratory data were generally small, and no clinically meaningful differences were shown across the maribavir dose groups.
DISCUSSION
The treatment of transplant recipients who had refractory or resistant CMV infections with maribavir 400–1200 mg BID led to the resolution of CMV viremia in two-thirds of patients within 6 weeks of treatment. No new safety signals were identified [26–28], and the results support the safety and tolerability of maribavir administration for up to 24 weeks.
These results are encouraging given the severity of illness and comorbidities in these patients. The VICTOR trial (NCT00431353), which compared valganciclovir and ganciclovir in SOT recipients with CMV disease without baseline ganciclovir resistance, reported undetectable plasma CMV DNA (<600 copies/mL) in 67–70% of patients at week 7 [32]. While undetectable plasma CMV DNA (<200 copies/mL) was achieved by a similar proportion (67%) by week 6 with maribavir, this study had a more diverse transplant population, with more severe CMV disease cases and limited treatment options.
No new safety concerns were identified. Given the patients enrolled, it was expected that all patients would experience TEAEs. Although no previous prospective data in RR CMV are available, the mortality of 27% after a median of 22 weeks is within the range reported in retrospective studies (17–31%) [1, 2, 4]. CMV was the primary cause of death in 4/32 patients. The most frequent TEAE related to maribavir administration was dysgeusia (65%), which was tolerable in most patients.
We found no evidence of dose-related increased myelosuppression in this study, in line with previous studies of maribavir [26–28]. The neutropenia rate (11%) was lower than those reported in studies of SOT recipients receiving pre-emptive valganciclovir/ganciclovir (22%) [12] and HCT recipients receiving ganciclovir prophylaxis (41%) [13]. In a recent randomized trial for pre-emptive treatment of CMV reactivation in SOT and HCT patients, treatment-emergent neutropenia (absolute neutrophil count <1000/µL) occurred in 18% of valganciclovir-treated patients versus 5% of maribavir-treated patients [33].
In this study, renal impairment was reported as a TEAE in 16% of patients (and as an SAE in 6%). An assessment of renal statuses was confounded by frequent histories of chronic kidney disease and other comorbidities, and concomitant treatment with known nephrotoxic medications. In foscarnet-treated RR patients, renal dysfunction occurred in 20/39 (51%) patients by the end of treatment [1].
Coadministration of maribavir and tacrolimus may increase calcineurin inhibitor plasma concentrations [34]. In this study, treatment-emergent increased immunosuppressant drug levels (tacrolimus or sirolimus) were reported in 10% (12/120) of patients. Overall, 38% of patients had immunosuppressive drug dosing reduced prior to starting treatment (Table 1), at least partly in response to CMV infections. Careful monitoring of immunosuppressant levels in patients who receive maribavir remains important.
We found no appreciable differences between the 3 maribavir doses with regard to CMV viremia clearance or recurrence rates, suggesting that all doses were similarly effective at viral clearance. While the reasons for the lack of an apparent maribavir dose-response within the dosing ranges studied remain unclear, the results are consistent with an earlier dose-ranging study [26]. It is possible that this lack of dose-response may be due to the relatively small dosing cohorts’ sample size. Factors other than dosage may play a role in response. Numerically higher response rates were observed among certain subgroups, including those with low versus high baseline plasma CMV DNA levels and those with no use of antilymphocyte antibody versus use. These findings support the impact of host and viral characteristics on virologic responses—and possibly CMV recurrences—and may require further study.
Study limitations included the lack of a non-maribavir comparator arm, study population heterogeneity (transplant types, comorbidities), and the relatively small size of each cohort. As there is no therapy currently approved for RR CMV, and a suitable comparator for both SOT and HCT recipients has yet to be determined, non-maribavir therapy was not evaluated. Furthermore, the complexity of administering multiple comparators may have compromised study blinding, and the drawbacks of an open-label design would have substantially limited the utility of the data obtained.
Although cohorts were small and diverse, all centers were subject to audits and adhered to the study protocol. Accordingly, virologic and clinical responses were observed across transplant types and, together with the phase 2 trial of pre-emptive maribavir or valganciclovir for CMV in HCT and SOT recipients [33], support further development of maribavir treatment for RR CMV infections. A phase 3 trial program is underway (ClinicalTrials.gov: NCT02931539; NCT02927067) to confirm the findings.
In conclusion, maribavir at doses of ≥400 mg BID was effective for the treatment of RR CMV among HCT and SOT recipients, representing a promising therapy for patients with limited treatment options and a significant risk of allograft rejection, opportunistic infections, and mortality [1–5]. In line with previous studies, there was no evidence of dose-related myelosuppression; these data support the safety of maribavir administration for up to 24 weeks.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. All authors (a) made substantial contributions to either the conception and design or the acquisition, analysis, or interpretation of the data; (b) drafted the article or critically revised it for important intellectual content; (c) gave final approval of the version to be published; and (d) agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Acknowledgments. The authors thank Sharon Donato, the clinical trial manager for ViroPharma/Shire, and Kevin Rockich, the clinical pharmacology lead for Shire, for their role in coordinating the trial; Clyde Crumpacker (Division of Infectious Disease, Beth Israel Deaconess Medical Center) for his informative discussion of resistance mutations; Yelena Shvenke from Shire for her review and retrieval of data; and Jeff Wang from Shire for statistical input. Under the direction of the authors, Joanna Wright, DPhil, and Debby Moss, PhD, of Caudex Health (Oxford, UK), and Susan Brackenridge, DPhil, on behalf of Caudex Health, provided writing assistance for this publication, funded by Shire International GmbH. Editorial assistance in formatting, proofreading, copy editing, and fact checking the manuscript and coordination and collation of comments was also provided by Caudex Health, funded by Shire International GmbH. Tien Bo from Shire also reviewed and edited the manuscript for scientific accuracy.
Financial support. The study was supported by ViroPharma/Shire Development, LLC. Shire International GmbH provided funding to Caudex Health (Oxford, UK) for support in writing, editing, and managing this manuscript. Although employees of Shire were involved in the design, collection, analysis, interpretation, and fact checking of information, the content of this manuscript, the interpretation of the data, and the decision to submit the manuscript for publication were made independently by the authors.
Potential conflicts of interest. G. A. P. has acted as a consultant to and investigator for Astellas, Chimerix, Merck, and Shire, and her organization has received research funding for the conduct of the current study from Shire. F. P. S. has received grants to her organization and support for travel to meetings for other purposes from Shire, and her organization has received grants/has grants pending from Ansun Biopharma, Qiagen, and Whiscon. A. A. L. has been an investigator for Astellas, Chimerix, Merck, and Shire. M. R. P. has been an investigator for Shire, ViroPharma, and Chimerix. R. K. A. has received an institutional grant for a multicenter trial from Shire, and her organization has received grants/has grants pending for multicenter trials from Aicuris, Astellas, Chimerix, Merck, and Oxford Immunotec. M. U. was an employee of and held stock/stock options in Shire at the time of the study. A. W. and J. W. are employees of and hold stock/stock options in Shire. M. B. has served as a consultant to Microbiotix, Shire, Astellas, Chimerix, Helocyte, Oxford Immunotec, Artemis Therapeutics, and Merck, and has been an investigator for Astellas, Chimerix, Shire, and Merck. F. M. M. has received grants and personal fees from Shire during the conduct of the study; grants and personal fees from Astellas, Chimerix, and Merck; and personal fees from Fate Therapeutics, Roche Molecular Diagnostics, GlaxoSmithKline, and LFB S.A. outside the submitted work. S. V. was an employee of and held stock/stock options in Shire/ViroPharma at the time of the study. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: IDWeek 2016, New Orleans, LA, 26–30 October 2016, Abstract no. 78; BMT Tandem Meetings, Orlando, FL, 22–27 February 2017, Abstract no. 45; and the American Transplant Congress, Chicago, IL, 29 April–3 May 2017, Abstract no. 562.
References
- 1. Avery RK, Arav-Boger R, Marr KA, et al. Outcomes in transplant recipients treated with foscarnet for ganciclovir-resistant or refractory cytomegalovirus infection. Transplantation 2016; 100:e74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Minces LR, Nguyen MH, Mitsani D, et al. Ganciclovir-resistant cytomegalovirus infections among lung transplant recipients are associated with poor outcomes despite treatment with foscarnet-containing regimens. Antimicrob Agents Chemother 2014; 58:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Limaye AP, Corey L, Koelle DM, Davis CL, Boeckh M. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet 2000; 356:645–9. [DOI] [PubMed] [Google Scholar]
- 4. Liu J, Kong J, Chang YJ, et al. Patients with refractory cytomegalovirus (CMV) infection following allogeneic haematopoietic stem cell transplantation are at high risk for CMV disease and non-relapse mortality. Clin Microbiol Infect 2015; 21:1121.e9–15. [DOI] [PubMed] [Google Scholar]
- 5. Fisher CE, Knudsen JL, Lease ED, et al. Risk factors and outcomes of ganciclovir resistant cytomegalovirus infection in solid organ transplant recipients. Clin Infect Dis 2017; 65:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Razonable RR, Humar A; AST Infectious Diseases Community of Practice Cytomegalovirus in solid organ transplantation. Am J Transplant 2013; 13(Suppl 4):93–106. [DOI] [PubMed] [Google Scholar]
- 7. Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Emergence of drug-resistant cytomegalovirus in the era of valganciclovir prophylaxis: therapeutic implications and outcomes. Clin Transplant 2008; 22:162–70. [DOI] [PubMed] [Google Scholar]
- 8. Limaye AP, Raghu G, Koelle DM, Ferrenberg J, Huang ML, Boeckh M. High incidence of ganciclovir-resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy. J Infect Dis 2002; 185:20–7. [DOI] [PubMed] [Google Scholar]
- 9. Hantz S, Garnier-Geoffroy F, Mazeron MC, et al. ; French CMV Resistance Survey Study Group. Drug-resistant cytomegalovirus in transplant recipients: a French cohort study. J Antimicrob Chemother 2010; 65:2628–40. [DOI] [PubMed] [Google Scholar]
- 10. Myhre HA, Haug Dorenberg D, Kristiansen KI, et al. Incidence and outcomes of ganciclovir-resistant cytomegalovirus infections in 1244 kidney transplant recipients. Transplantation 2011; 92:217–23. [DOI] [PubMed] [Google Scholar]
- 11. Strasfeld L, Chou S. Antiviral drug resistance: mechanisms and clinical implications. Infect Dis Clin North Am 2010; 24:413–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martín-Gandul C, Pérez-Romero P, González-Roncero FM, et al. ; Spanish Network for Research in Infectious Diseases REIPI. Clinical impact of neutropenia related with the preemptive therapy of CMV infection in solid organ transplant recipients. J Infect 2014; 69:500–6. [DOI] [PubMed] [Google Scholar]
- 13. Salzberger B, Bowden RA, Hackman RC, Davis C, Boeckh M. Neutropenia in allogeneic marrow transplant recipients receiving ganciclovir for prevention of cytomegalovirus disease: risk factors and outcome. Blood 1997; 90:2502–8. [PubMed] [Google Scholar]
- 14. Jacobsen T, Sifontis N. Drug interactions and toxicities associated with the antiviral management of cytomegalovirus infection. Am J Health Syst Pharm 2010; 67:1417–25. [DOI] [PubMed] [Google Scholar]
- 15. Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med 2017; 377:2433–44. [DOI] [PubMed] [Google Scholar]
- 16. Stoelben S, Arns W, Renders L, et al. Preemptive treatment of Cytomegalovirus infection in kidney transplant recipients with letermovir: results of a Phase 2a study. Transpl Int 2014; 27:77–86. [DOI] [PubMed] [Google Scholar]
- 17. Koszalka GW, Johnson NW, Good SS, et al. Preclinical and toxicology studies of 1263W94, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob Agents Chemother 2002; 46:2373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Biron KK, Harvey RJ, Chamberlain SC, et al. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob Agents Chemother 2002; 46:2365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drew WL, Miner RC, Marousek GI, Chou S. Maribavir sensitivity of cytomegalovirus isolates resistant to ganciclovir, cidofovir or foscarnet. J Clin Virol 2006; 37:124–7. [DOI] [PubMed] [Google Scholar]
- 20. Williams SL, Hartline CB, Kushner NL, et al. In vitro activities of benzimidazole D- and L-ribonucleosides against herpesviruses. Antimicrob Agents Chemother 2003; 47:2186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamirally S, Kamil JP, Ndassa-Colday YM, et al. Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathog 2009; 5:e1000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krosky PM, Baek MC, Coen DM. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J Virol 2003; 77:905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prichard MN. Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir. Rev Med Virol 2009; 19:215–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lalezari JP, Aberg JA, Wang LH, et al. Phase I dose escalation trial evaluating the pharmacokinetics, anti-human cytomegalovirus (HCMV) activity, and safety of 1263W94 in human immunodeficiency virus-infected men with asymptomatic HCMV shedding. Antimicrob Agents Chemother 2002; 46:2969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang LH, Peck RW, Yin Y, Allanson J, Wiggs R, Wire MB. Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti-human cytomegalovirus agent, in healthy and human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother 2003; 47:1334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Winston DJ, Young JA, Pullarkat V, et al. Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem cell transplant recipients: a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood 2008; 111:5403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marty FM, Ljungman P, Papanicolaou GA, et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis 2011; 11:284–92. Erratum: 2011; 11:343. [DOI] [PubMed] [Google Scholar]
- 28. Winston DJ, Saliba F, Blumberg E, et al. ; 1263-301 Clinical Study Group. Efficacy and safety of maribavir dosed at 100 mg orally twice daily for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, double-blind, multicenter controlled trial. Am J Transplant 2012; 12:3021–30. [DOI] [PubMed] [Google Scholar]
- 29. Marty FM, Boeckh M. Maribavir and human cytomegalovirus-what happened in the clinical trials and why might the drug have failed?Curr Opin Virol 2011; 1:555–62. [DOI] [PubMed] [Google Scholar]
- 30. Avery RK, Marty FM, Strasfeld L, et al. Oral maribavir for treatment of refractory or resistant cytomegalovirus infections in transplant recipients. Transpl Infect Dis 2010; 12:489–96. [DOI] [PubMed] [Google Scholar]
- 31. Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev 2010; 23:689–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Asberg A, Humar A, Rollag H, et al. ; VICTOR Study Group. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 2007; 7:2106–13. [DOI] [PubMed] [Google Scholar]
- 33. Maertens J, Cordonnier C, Jaksch P, et al. Maribavir versus valganciclovir for preemptive treatment of cytomegalovirus (CMV) viremia: a randomized, dose-ranging, phase 2 study among hematopoietic stem cell transplant (SCT) and solid organ transplant (SOT) recipients. Open Forum Infectious Diseases 2016; 3(Suppl 1):2287. [Google Scholar]
- 34. Pescovitz MD, Bloom R, Pirsch J, Johnson J, Gelone S, Villano SA. A randomized, double-blind, pharmacokinetic study of oral maribavir with tacrolimus in stable renal transplant recipients. Am J Transplant 2009; 9:2324–30. Available at: https://academic.oup.com/ofid/article/3/suppl_1/2287/2636606 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.