Abstract
Fluoroquinolones have been in clinical use for over 50 years with significant efficacy. However, increasing resistance and emergence of some marked adverse events have limited their usage. The most recently approved class member, delafloxacin, is the only available anionic (non-zwitterionic) fluoroquinolone. Its unique molecular structure provides improved in vitro activity against most Gram-positive pathogens, including quinolone-resistant strains, which is further enhanced at acid pH. Delafloxacin shows favorable pharmacological properties, with about 60% bioavailability after oral administration, only mild inhibition of cytochrome P450 3A, and no evidence of cardiac- or phototoxicity in healthy volunteers (tested against positive controls). Its twice daily dosing, suitability for intravenous, oral, or switch dosing, the lack of many clinically significant drug-drug interactions, and acceptable adverse event profile in registration clinical trials supports its use in the treatment of acute bacterial skin and skin structure infections, and potentially in other infections, where resistance to other agents, safety, and/or the need for early discharge is of concern.
Keywords: fluoroquinolones, delafloxacin, skin infections
Acute bacterial skin and skin structure infections (ABSSSIs) are associated with significant morbidity and mortality. Several studies have documented increasing patient encounters for treatment of ABSSSIs both in ambulatory and inpatient settings [1–4], but this trend may now be decreasing [5]. A variety of Gram-positive and Gram-negative pathogens have been identified as etiologic agents. However, the predominant causative pathogen across geographic regions is Staphylococcus aureus, followed by other Gram-positive pathogens (eg, coagulase-negative staphylococci, Enterococcus spp., Streptococcus agalactiae [Group B Streptococcus], and Streptococcus pyogenes) and Gram-negative pathogens including Pseudomonas aeruginosa and Escherichia coli, which are more frequently seen in surgical site infections [6, 7]. Morbidity, mortality, and costs associated with hospitalization for treatment of these infections are significant and are appreciably higher in patients with mixed infections compared with those caused by Gram-positive or Gram-negative pathogens alone [8]. Another significant concern is the emergence of pathogens resistant to antimicrobial agents, including methicillin-resistant S. aureus (MRSA) [9], which contributes to higher morbidity and mortality as well as high treatment costs [10, 11], resulting primarily from longer hospital stays [12].
Current guidelines on the treatment of ABSSSIs classify them into nonpurulent (necrotizing infections, cellulitis, and erysipelas) and purulent (furuncles, carbuncles, and abscesses) and further on the basis of severity (mild, moderate, and severe) [13]. A variety of antimicrobial agents are recommended depending upon the type and severity of infection, and if caused by S. aureus, the methicillin susceptibility of the causative strain. As recently reviewed [14], oxacillin (or another β-lactamase resistant penicillin such as dicloxacillin or nafcillin) or cefazolin (in case of allergy to penicillin) are usually recommended for the treatment of infections caused by methicillin-sensitive S. aureus (MSSA), whereas vancomycin, linezolid, daptomycin, or ceftaroline are most often specifically recommended when the infection is caused by MRSA. Older agents such as clindamycin, doxycycline/minocycline, or trimethoprim-sulfamethoxazole are also used to treat infections caused by MSSA or MRSA. However, all of these drugs are associated with limitations that include local high level of resistance (clindamycin or doxycycline), high cost and toxicity (linezolid), decreased susceptibility (vancomycin; often requiring higher dosing that results in renal toxicity), and heightened risk of Clostridium difficile infection (eg, clindamycin) [13, 15]. Although these drugs still form the mainstay of current treatment strategies, recent approvals of agents including dalbavancin, tedizolid, oritavancin, and delafloxacin have provided additional options for the treatment of ABSSSIs, including those caused by MRSA [14, 16].
An additional concern is the ability of S. aureus to survive in the acidic environment of the skin. Their survival is dependent on expression of an enzyme that confers resistance to polyamines, anti-inflammatory compounds capable of promoting wound healing and tissue regeneration, which are present in the acidic environment of the skin and are toxic to S. aureus [17, 18]. Moreover, S. aureus can adopt specific modes of life (eg, in biofilms or intracellularly after phagocytosis by permissive cells) that play a role in the development of persistent/recurrent infections, including in skin and skin-associated structures [19, 20]. Thus, there is a need for therapeutic agents that are not only effective against resistant pathogens but also retain or even increase their activity at the acid pH prevailing at the surface of the skin [21], deep in biofilms [22], or in phagolysosomes [23].
Among the newly approved agents, the anionic fluoroquinolone delafloxacin uniquely shows improved activity at acidic pH (as opposed to most other antibiotics including currently approved fluoroquinolones) and exhibits broad spectrum activity that includes most Gram-positive bacteria involved in ABSSSI and, to some extent, important Gram-negative bacteria. It was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of ABSSSI [16]. We review some of the pertinent data about the key features of delafloxacin to provide a concise overview of its basic properties that may be of interest to clinicians. More clinically oriented reviews are available elsewhere in this Special Issue and in other recent publications [24–27].
CHEMICAL STRUCTURE AND MECHANISM OF ACTION
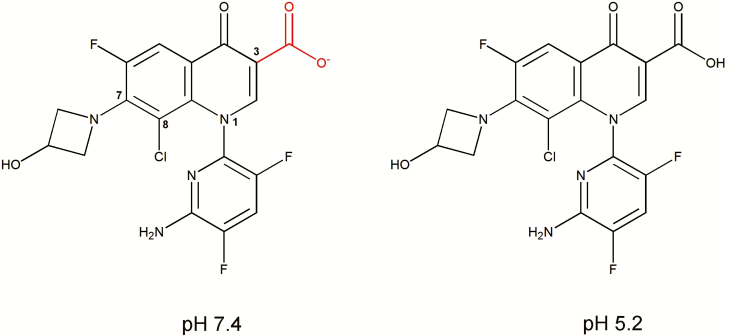
Delafloxacin (CAS registry number 189279-58-1; PubChem CID 487101; formerly known as WQ-3034 and ABT-492) has the molecular formula C18H12ClF3N4O4 and the chemical structure 1-(6-amino-3,5-difluoropyridin-2-yl)-8-chloro-6-fluoro-7-(3-hydroxyazetidin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (Figure 1) [28–30]. The compound differs from other fluoroquinolones in 3 main respects: (1) lack of a basic group at position C7, which makes it a weak acid and therefore predominantly anionic at neutral pH (and not zwitterionic as for most other fluoroquinolones), (2) addition of a chlorine at position C8, which serves as an electron-withdrawing group on the aromatic ring and confers polarity and enhanced activity, and (3) a voluminous heteroaromatic substitution at position N1 that imparts a larger molecular surface to delafloxacin compared to most other fluoroquinolones [28, 31]. These combined structural features directly impact on the activity of delafloxacin (with very low minimal inhibitory concentrations [MICs] against a large array of Gram-positive organisms) and may explain its enhanced potency at acid pH relative to other fluoroquinolones (eg, ciprofloxacin, levofloxacin, moxifloxacin), for which activities decrease (higher MICs) in acidic environments. This enhanced potency at acid pH likely relates to increased accumulation by S. aureus, whereas lower accumulation was seen with moxifloxacin [32]. Delafloxacin may therefore fulfill one of the important requisites for enhanced activity in ABSSSI [33], particularly in infections caused by S. aureus [31, 33] and where high local concentrations are considered essential (see [22, 34]).
Figure 1.
Chemical structure of delafloxacin with atom numbering for the key positions discussed in the text. Due to the lack of basic group in the C7 substituent, the only ionizable group is the carboxylate function attached to position C3 (calculated pKa = 5.43). The figure shows the calculated predominant forms at pH 7.4 (left; anionic [to 98.5%]) and at pH 5.2 (right; neutral [62.7%]). Calculations were made with MarvinSketch version 18.9.0 (academic license) available from http://www.chemaxon.com.
The structural characteristics of delafloxacin also enable it to target both DNA gyrase and DNA topoisomerase IV from Gram-positive (S. aureus) and Gram-negative (E. coli) pathogens with equal affinity [35]. The dual targeting of gyrase and topoisomerase IV decreases likelihood of resistance, which requires the accumulation of multiple mutations affecting both enzymes [36]. This feature may contribute to the activity of delafloxacin against MRSA isolates, including those harboring mutations in the quinolone resistance determining region (QRDR) and to the low levels of resistance to delafloxacin among these MRSA isolates [29].
ANTIBACTERIAL ACTIVITY
As explained above, delafloxacin has structural attributes that confer activity at low pH. This has been demonstrated in a series of studies comparing its activity with other fluoroquinolones in media at different pH levels. Against S. aureus ATCC25923, delafloxacin MIC was 5 log2 dilutions lower at pH 5.5 (0.00003 mg/L) than at pH 7.4 (0.001 mg/L), whereas moxifloxacin MIC was 2 log2 dilutions higher at pH 5.5 (0.125 mg/L) than at pH 7.4 (0.03 mg/L). Similar observations were made in a series of clinical isolates [32]. Another study examined the in vitro activity of delafloxacin and ciprofloxacin at pH values ranging from 6 to 8 [37]. Variable MICs reported for delafloxacin against MRSA strain W44 at pH values of 6, 7, and 8 were 0.006 mg/L, 0.05 mg/L, and 0.2 mg/L, respectively. Also, time-kill experiments against MRSA W44 that evaluated delafloxacin across the pH range at concentrations of 0.025 mg/L and 0.1 mg/L (ie, half and 2 times the MIC at pH 7) showed bactericidal activity at both pH 6 and 7 but not at pH 8. Accumulation of delafloxacin within MRSA W44 was also pH dependent, being highest at pH 6 and lowest at pH 8.
An early study that evaluated the in vitro activity of delafloxacin against a panel of fluoroquinolones including trovafloxacin, levofloxacin, and ciprofloxacin demonstrated its activity against multiple quinolone-susceptible pathogens [35]. Activity against 7 quinolone-susceptible Enterobacteriaceae was comparable with that of other fluoroquinolones. Delafloxacin was more active than the other agents against fastidious Gram-negative pathogens including Haemophilus influenzae, Moraxella catarrhalis, Neisseria gonorrhoeae, and Legionella spp. and other Gram-negative pathogens such as P. aeruginosa and Helicobacter pylori. Delafloxacin was more potent than trovafloxacin and levofloxacin against multidrug-resistant Streptococcus pneumoniae (including isolates resistant to penicillin and macrolides) and H. influenzae (including β-lactam-resistant isolates). A subsequent study that included an expanded panel of comparators including moxifloxacin, gatifloxacin, and gemifloxacin in addition to trovafloxacin, levofloxacin, and ciprofloxacin reported that delafloxacin was more active against quinolone-susceptible and -resistant Gram-positive pathogens but was equipotent against quinolone-susceptible, nonfermentative Gram-negative pathogens [38]. This study also reported that delafloxacin was bactericidal against quinolone-resistant strains of E. coli within 6 h, S. aureus within 10 h, and S. pneumoniae by 24 h.
The in vitro activities of delafloxacin and a comprehensive panel of comparators (levofloxacin, ceftaroline, ciprofloxacin, clindamycin, daptomycin, erythromycin, linezolid, oxacillin, tetracycline, tigecycline, trimethoprim-sulfamethoxazole, and vancomycin) against 6485 isolates collected from multiple sites in Europe and the United States in 2014 have been evaluated (Table 1) [39]. This study applied 2016 interpretation criteria of the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) for comparator agents. Although there are neither CLSI nor EUCAST interpretive criteria (breakpoints) for delafloxacin, those set by the FDA for the United States in 2017 (see prescribing information and medication guide [16]) have been listed below Table 1 for comparison. (Since 2006, the FDA has reasserted its rights to define breakpoints for antibiotics, which affects all new drugs registered in the United States since then. EUCAST breakpoints are set up during the registration process of new antibiotics with the European Medicine Agency [EMA], but this has not yet been finalized for delafloxacin at the time of the final writing and revision of this review [December 8, 2018].) Delafloxacin had the lowest MICs among agents tested against MSSA, MRSA, S. pneumoniae, and viridans group and beta-hemolytic Streptococci and MICs comparable to those of ciprofloxacin and levofloxacin against some Enterobacteriaceae. Its low MICs against pathogens associated with ABSSSI as well as respiratory and urinary tract infections (UTIs) were confirmed. These findings have been corroborated by a more recent susceptibility analysis of 36 683 Gram-positive and Gram-negative isolates (of which 10 153 were identified as associated with skin and skin structure infections [SSSI]) collected between 2014 and 2016 from sites in the United States and Europe [40]. Application of the CLSI and EUCAST 2017 breakpoints for comparator agents and FDA breakpoints for delafloxacin from the 2017 package insert enabled confirmation of the broad-spectrum in vitro activity of delafloxacin against this contemporary panel of isolates. Delafloxacin demonstrates lower MICs than levofloxacin and moxifloxacin against S. aureus (MIC50/MIC90 0.008/0.5 mg/L, 0.25/>4 mg/L, and ≤0.06/4 mg/L for delafloxacin, levofloxacin, and moxifloxacin, respectively), coagulase-negative staphylococci (CoNS) (MIC50/MIC90 0.015/0.5 mg/L, 0.25/>4 mg/L, and 0.12/4 mg/L, respectively), S. pneumoniae (MIC50/MIC90 0.015/0.03 mg/L, 1/1 mg/L, and ≤0.12/0.25 mg/L, respectively), and S. pyogenes and S. agalactiae (MIC50/MIC90 0.015/0.03 mg/L and 0.5/1 mg/L for delafloxacin and levofloxacin, respectively, against both pathogens). Other studies have shown lower MICs for delafloxacin than comparators against clinical isolates of Neisseria gonorrhoeae and Chlamydophila pneumoniae [41, 42] and against isolates of S. aureus resistant to methicillin [29] and other fluoroquinolones (eg, levofloxacin and ciprofloxacin) [43]. The latter study examined the in vitro activity of delafloxacin against S. aureus isolates from participants in Phase 3 studies harboring mutations in the QRDR, including isolates with the most frequently encountered mutations in clinical trials—the S84L mutation at the gyrA locus and the S80Y mutation at the parC locus—and documented high rates of microbiological response against such isolates. Notably, the MIC values for isolates with single mutations were considerably larger than for susceptible isolates but did not exceed 0.5 mg/L (a value considered as “intermediate” by the FDA; see definition in [16]). A sole isolate harboring both mutations showed an MIC of 4 mg/L (thus reported as resistant) but was presumed eradicated by delafloxacin treatment.
Table 1.
Comparative In Vitro Activities of Delafloxacin and Comparators Against Relevant Gram-positive and Gram-negative Clinical Isolates From the United States and Europe
| % of Isolates Susceptible by Following Criteria: | MIC (mg/L) | ||||
|---|---|---|---|---|---|
| Organism Group (No. of Isolates Tested)/Antibiotic |
CLSI | EUCAST | 50% | 90% | Range |
| Gram-positive pathogens | |||||
| Staphylococcus aureus (1350) | |||||
| Delafloxacina | ≤0.004 | 0.25 | ≤0.004 to 4 | ||
| Levofloxacin | 64.4 | 64.4 | 0.25 | >4 | ≤0.12 to 4 |
| Ciprofloxacin | 0.0 | 0.0 | 64 | >128 | 64 to >128 |
| Ceftaroline | 98.0 | 98.0 | 0.25 | 1 | 0.03 to 2 |
| Clindamycin | 87.0 | 86.8 | ≤0.25 | >2 | ≤0.25 to >2 |
| Daptomycin | 99.8 | 99.8 | 0.25 | 0.5 | ≤0.06 to 2 |
| Linezolid | 100.0 | 100.0 | 1 | 1 | 0.25 to 2 |
| Oxacillin | 57.6 | 57.6 | 0.5 | >2 | ≤0.25 to >2 |
| Trimethoprim-sulfamethoxazole | 98.5 | 98.5 | ≤0.5 | ≤0.5 | ≤0.5 to >4 |
| Vancomycin | 100.0 | 100.0 | 1 | 1 | 0.25 to 2 |
| MSSA (777) | |||||
| Delafloxacina | ≤0.004 | 0.008 | ≤0.004 to 4 | ||
| Levofloxacin | 89.8 | 89.8 | 0.25 | 2 | ≤0.12 to >4 |
| Ciprofloxacin | 0.0 | 0.0 | >128 | >128 | >128 to >128 |
| Ceftaroline | 100.0 | 100.0 | 0.25 | 0.25 | 0.03 to 1 |
| Clindamycin | 94.0 | 93.7 | ≤0.25 | ≤0.25 | ≤0.25 to >2 |
| Daptomycin | 100.0 | 100.0 | 0.25 | 0.5 | ≤0.06 to 1 |
| Linezolid | 100.0 | 100.0 | 1 | 1 | 0.25 to 2 |
| Oxacillin | 100.0 | 100.0 | 0.5 | 0.5 | ≤0.25 to 2 |
| Trimethoprim-sulfamethoxazole | 99.0 | 99.0 | ≤0.5 | ≤0.5 | ≤0.5 to >4 |
| Vancomycin | 100.0 | 100.0 | 1 | 1 | 0.25 to 2 |
| MRSA (573) | |||||
| Delafloxacina | 0.06 | 0.5 | ≤0.004 to 4 | ||
| Levofloxacin | 30.0 | 30.0 | 4 | >4 | ≤0.12 to >4 |
| Ciprofloxacin | 0.0 | 0.0 | >128 | >128 | 64 to >128 |
| Ceftaroline | 95.3 | 95.3 | 1 | 1 | 0.25 to 2 |
| Clindamycin | 77.5 | 77.5 | ≤0.25 | >2 | ≤0.25 to >2 |
| Daptomycin | 99.5 | 99.5 | 0.25 | 0.5 | 0.12 to 2 |
| Linezolid | 100.0 | 100.0 | 1 | 1 | 0.25 to 2 |
| Oxacillin | 0.0 | 0.0 | >2 | >2 | >2 to >2 |
| Trimethoprim-sulfamethoxazole | 97.9 | 97.9 | ≤0.5 | ≤0.5 | ≤0.5 to >4 |
| Vancomycin | 100.0 | 100.0 | 1 | 1 | 0.5 to 2 |
| Enterococcus faecalis (450) | |||||
| Delafloxacinb | 0.06 | 1 | ≤0.004 to 2 | ||
| Levofloxacin | 70.7 | 70.7 | 1 | >4 | 0.25 to >4 |
| Ceftaroline | 2 | 8 | 0.25 to >32 | ||
| Clindamycin | >2 | >2 | ≤0.25 to >2 | ||
| Daptomycin | 100.0 | 1 | 2 | 0.12 to 4 | |
| Linezolid | 99.8 | 100.0 | 1 | 1 | ≤0.12 to 4 |
| Trimethoprim-sulfamethoxazole | ≤0.5 | ≤0.5 | ≤0.5 to >4 | ||
| Vancomycin | 97.8 | 97.8 | 1 | 2 | 0.5 to >16 |
| Streptococcus pyogenes (433) | |||||
| Delafloxacinc | 0.008 | 0.015 | ≤0.004 to 0.03 | ||
| Levofloxacin | 99.8 | 96.5 | 0.5 | 1 | 0.25 to >4 |
| Moxifloxacin | 100.0 | ≤0.12 | 0.25 | ≤0.12 to 0.5 | |
| Ceftaroline | 100.0 | 100.0 | ≤0.015 | ≤0.015 | ≤0.015 to 0.03 |
| Clindamycin | 91.5 | 91.9 | ≤0.25 | ≤0.25 | ≤0.25 to >2 |
| Vancomycin | 100.0 | 100.0 | 0.25 | 0.5 | ≤0.12 to 0.5 |
| Streptococcus agalactiae (225) | |||||
| Delafloxacind | 0.008 | 0.015 | ≤0.004 to 0.5 | ||
| Levofloxacin | 97.8 | 96.9 | 0.5 | 1 | 0.25 to >4 |
| Moxifloxacin | 97.8 | ≤0.12 | 0.25 | ≤0.12 to >4 | |
| Ceftaroline | 100.0 | 100.0 | ≤0.015 | 0.03 | ≤0.015 to 0.03 |
| Clindamycin | 70.7 | 72.4 | ≤0.25 | >2 | ≤0.25 to >2 |
| Vancomycin | 100.0 | 100.0 | 0.5 | 0.5 | 0.25 to 1 |
| Gram-negative pathogens | |||||
| Enterobacteriaceae (2250) | |||||
| Delafloxacine | 0.06 | 4 | ≤0.004 to ≥4 | ||
| Ceftazidime | 86.3 | 82.8 | 0.25 | 16 | 0.03 to >32 |
| Ceftriaxone | 80.3 | 80.3 | 0.12 | >8 | ≤0.06 to >8 |
| Ciprofloxacin | 81.6 | 79.3 | ≤0.03 | >4 | ≤0.03 to >4 |
| Piperacillin-tazobactam | 89.3 | 85.7 | 2 | 32 | ≤0.5 to >64 |
| Escherichia coli (500) | |||||
| Delafloxacine | 0.03 | 4 | ≤0.004 to >4 | ||
| Ceftazidime | 89.2 | 83.4 | 0.12 | 8 | 0.03 to >32 |
| Ceftriaxone | 84.0 | 84.0 | ≤0.06 | >8 | ≤0.06 to >8 |
| Ciprofloxacin | 69.4 | 68.8 | ≤0.03 | >4 | ≤0.03 to >4 |
| Piperacillin-tazobactam | 94.2 | 90.0 | 2 | 8 | ≤0.05 to >64 |
| Klebsiella pneumoniae (389) | |||||
| Delafloxacine | 0.06 | >4 | 0.015 to >4 | ||
| Ceftazidime | 76.9 | 74.8 | 0.12 | >32 | 0.03 to >32 |
| Ceftriaxone | 75.3 | 75.3 | ≤0.06 | >8 | ≤0.06 to >8 |
| Ciprofloxacin | 77.4 | 75.6 | ≤0.03 | >4 | ≤0.03 to >4 |
| Piperacillin-tazobactam | 81.2 | 75.8 | 4 | >64 | ≤0.5 to >64 |
| Pseudomonas aeruginosa (200) | |||||
| Delafloxacinf | 0.25 | >4 | 0.015 to >4 | ||
| Ceftazidime | 78.5 | 78.5 | 2 | >32 | 0.25 to >32 |
| Ceftriaxone | >8 | >8 | 1 to >8 | ||
| Ciprofloxacin | 75.0 | 70.0 | 0.25 | >4 | ≤0.03 to >4 |
| Piperacillin-tazobactam | 78.0 | 78.0 | 8 | >64 | ≤0.5 to >64 |
Adapted from Pfaller et al [39]. Only data for pathogens for the treatment of which delafloxacin is approved (only in the United States so far [16]) and data on antimicrobial agents recommended for the treatment of skin and soft tissue infections in the 2014 Infectious Diseases Society of America guidelines [13] are presented.
Abbreviations: CLSI, Clinical and Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility Testing; MIC, minimal inhibitory concentration; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus.
US Food and Drug Administration–designated breakpoints (for use in the United States [16]) against the following pathogens are listed below.
a Staphylococcus aureus (MRSA and MSSA isolates): Susceptible, ≤0.25 mg/L; intermediate, 0.5 mg/L; resistant, ≥1 mg/L.
b Enterococcus faecalis: Susceptible, ≤0.12 mg/L; intermediate, 0.25 mg/L; resistant, ≥0.5 mg/L.
c Streptococcus pyogenes: Susceptible, ≤0.06 mg/L; intermediate, –; resistant, –. Isolates yielding results other than “susceptible” should be submitted to a reference laboratory for testing.
d Streptococcus agalactiae: Susceptible, ≤0.06 mg/L; intermediate, 0.12 mg/L; resistant, ≥0.25 mg/L.
eEnterobacteriaceae (including Escherichia coli and Klebsiella pneumoniae): Susceptible, ≤0.25 mg/L; intermediate, 0.5 mg/L; resistant, ≥1 mg/L.
f Pseudomonas aeruginosa: Susceptible, ≤0.5 mg/L; intermediate, 1 mg/L; resistant, ≥2 mg/L.
Delafloxacin showed two- to five-fold lower broth MICs than ciprofloxacin against Enterobacteriaceae (E. coli and K. pneumoniae) isolated from the urine of patients with suspected urinary tract infections (UTIs) [44]. In addition, delafloxacin proved more active than moxifloxacin against S. aureus intracellularly [32] as well as in biofilms, both in vitro and in vivo [22, 45]. In this case, its activity was enhanced by agents capable of disrupting the biofilm, such as the antifungal agent caspofungin, which inhibits the synthesis of polysaccharide constituents of the biofilm matrix [46]. Taken together, these studies highlight the utility of delafloxacin in the treatment of a variety of infections caused by most Gram-positive pathogens. The situation is more difficult for E. faecalis and for Gram-negative pathogens (Enterobacteriaceae [current name: Enterobacterales] or P. aeruginosa), for which the MIC90 may exceed the FDA breakpoints (see [39]), requiring documentation of the susceptibility and making empiric treatments more risky.
PHARMACOKINETICS
Evaluation of the pharmacokinetics (PK) and disposition of delafloxacin following a single intravenous dose administered to healthy male volunteers showed the mean Cmax, area under the curve (AUC0-∞), Tmax, and T½ to be 8.98 mg/L, 21.31 mg.h/L, 1 h, and 2.35 h, respectively [47]. Excretion was predominantly (66%) via the kidney, with a lower proportion (29%) of the dose excreted in the feces. The predominant circulating components were determined to be delafloxacin and its direct glucuronide conjugate. Delafloxacin exhibits linear PK that reach steady-state following 3 days of daily oral dosing, with minimal accumulation [48]. Delafloxacin oral bioavailability is 58.8%, which is lower than for levofloxacin or moxifloxacin, but total systemic exposure (AUC0-t and AUC0-∞) following a single intravenous (300 mg) and a single oral dose (450 mg) of delafloxacin was equivalent (Table 2) [49]. Thus, a transition between dosing routes with daily dose adjustment is possible and has been approved in the United States [16]. The mean absolute bioavailability of delafloxacin was not affected by food. The steady state volume of distribution of delafloxacin is 30–48 L, which approximates total body water. The plasma protein binding of delafloxacin is approximately 84% (involving primarily albumin). Plasma protein binding of delafloxacin is not significantly affected by renal impairment. In a mass balance study, the mean half-life for delafloxacin was 3.7 h (standard deviation [SD] 0.7 h) after a single dose intravenous administration. The mean half-life values for delafloxacin ranged from 4.2 to 8.5 h following multiple oral administrations.
Table 2.
Mean (Standard Deviation) Pharmacokinetic Parameters and Statistical Analysis of Pharmacokinetic Parameters Following Administration of a Single 1-Hour Intravenous Infusion or a Single Oral Dose of Delafloxacin in Healthy Volunteers
| Parameter | Oral Delafloxacin (450 mg) (n = 55) |
Intravenous Delafloxacin (300 mg) (n = 55) |
|---|---|---|
| Tmax, ha | 0.817 (0.50–4.00) | 1.00 (0.75–1.13) |
| Cmax, mg/L | 6.12 (1.96) | 10.7 (2.29) |
| AUC0-t, mg.h/L | 23.3 (7.00) | 26.9 (5.78) |
| AUC0-∞, mg.h/Lb | 24.2 (6.45) | 26.7 (6.03) |
| Fc | 58.8 (10.5)d | |
| Statistical Analysis | ||
| Parameter | Geometric Least Squares Mean (90% CI) | Ratio of Geometric Least Squares Mean (Oral/IV), % (90% CI) |
| Cmax, mg/L | ||
| Oral (N = 55) | 5.80 (5.44–6.17) | 55.16 (51.50–59.08) |
| IV (N = 55) | 10.51 (9.87–11.19) | |
| AUC0-∞, mg.h/L | ||
| Oral (N = 42) | 22.97 (21.61–24.41) | 87.68 (83.56– 92.00) |
| IV (N = 49) | 26.20 (24.71–27.78) | |
| AUC0-t, mg.h/L | ||
| Oral (N = 55) | 22.24 (20.99–23.57) | 84.45 (80.90– 88.15) |
| IV (N = 55) | 26.34 (24.85–27.91) |
Adapted from Hoover et al [49].
Abbreviations: AUC, area under the curve; CI, confidence interval; IV, intravenous.
aMedian (range).
bn = 42 for the oral dose and n = 49 for the IV infusion.
cF was calculated for each participant as (AUC0-∞ after oral) (IV dose)/(AUC0-∞ after IV) (oral dose).
dn = 37.
Following a single intravenous (300 mg) administration to subjects with mild (estimated Glomerular Filtration Rate [eGFR] = 51–80 mL/min/1.73 m2), moderate (eGFR = 31–50 mL/min/1.73 m2), severe (eGFR = 15–29 mL/min/1.73 m2) renal impairment, and end-stage renal disease with hemodialysis receiving intravenous delafloxacin within 1 h before and 1 h after hemodialysis, mean total delafloxacin exposure (AUCt) was 1.3, 1.6, 1.8, 2.1, and 2.6-fold higher, respectively, than that for matched normal control subjects [49, 50]. Mild, moderate, or severe hepatic impairment does not adversely affect either exposure or clearance of delafloxacin, indicating that dose adjustments are not required in this population [51]. Also, delafloxacin does not significantly affect the PK of midazolam, a cytochrome P450 [CYP] 3A substrate [52]. A small change in the Cmax of 1-hydroxymidazolam was documented in this study but was not considered clinically relevant. Neither sex nor age had any significant effect on the pharmacology of delafloxacin.
PHARMACODYNAMICS
Monte Carlo simulation analyses using clinical PK and non-clinical PK/pharmacodynamic (PD) data were used to determine target attainment (TA) probabilities, which were used to support dose selection decisions [53]. Probabilities were determined for delafloxacin doses of 200–450 mg given intravenously every 12 hours, revealing high percent probabilities of TA for MIC values ≤0.5 mg/L with intravenous and oral doses of 300 mg and 450 mg respectively, which were chosen for the Phase 3 studies [53].
Several studies have evaluated the comparative PD of delafloxacin versus other fluoroquinolones, mainly levofloxacin and ciprofloxacin, against multiple clinically relevant pathogens including S. aureus, E. coli, S. pneumoniae, and K. pneumoniae in both in vitro and in vivo model systems [54–58]. Exposure of ciprofloxacin-susceptible and ciprofloxacin-resistant clinical isolates of S. aureus to clinically achievable ratios of AUC to MIC of delafloxacin and levofloxacin in a model simulating the PK of single and multiple doses of the 2 fluoroquinolones showed that delafloxacin was capable of producing greater anti-staphylococcal effects than levofloxacin at clinically achievable AUC/MICs [54]. Moreover, delafloxacin was more effective in the prevention of the selection of resistant mutants in S. aureus, as shown by appreciable differences in the clinically achievable AUC24/MIC ratios (for the same organism, delafloxacin was capable of reaching an AUC24h/MIC ratio of 870 h, which significantly exceeded the protective value of 240 h, whereas levofloxacin achieved a value of only 70 h, which was considerably lower than its protective value of 200 h) [54]. Examination of the killing kinetics of E. coli and P. aeruginosa exposed to single and multiple doses of delafloxacin and ciprofloxacin at clinically achievable AUC/MIC ratios showed that the killing effect of delafloxacin on E. coli at its clinically achievable AUC/MIC ratio (1740 h) was significantly higher than that seen with ciprofloxacin at its clinically achievable AUC/MIC ratio (2200 h) [55]. In the case of P. aeruginosa, two 12 h doses of delafloxacin (AUC/MIC 2 × 140 h) were more efficient at killing than ciprofloxacin (AUC/MIC 120 h). This study showed that clinically achievable AUC/MICs of delafloxacin and ciprofloxacin were comparable with regard to efficacy against E. coli (quaque die [QD] vs bis in die [BID] dosing) and against P. aeruginosa (at BID dosing but not QD dosing of delafloxacin). A subsequent animal study predicted significantly greater efficacy of clinically achievable AUC/MIC ratios of delafloxacin versus levofloxacin against ciprofloxacin-resistant S. pneumoniae and similar efficacy against ciprofloxacin-susceptible isolates [56]. Evaluation of the PK/PD targets of delafloxacin for S. aureus, S. pneumoniae, and K. pneumoniae in a murine lung infection model showed its activity against these pathogens, including isolates exhibiting resistance to other classes of antimicrobial agents [57] (in this study, the authors measured the free AUC24h/MIC ratio and observed that at least 1 log10 kill was achieved for S. aureus when exposing the animals to values similar to those observed in humans during conventional therapy). A more recent study evaluated the PD of delafloxacin against a panel of pathogens causing community-acquired pneumonia including S. pneumoniae, MSSA, MRSA, and K. pneumoniae in a neutropenic murine lung infection model and documented in vitro and in vivo activity (as measured by the change in log10 colony forming unit (CFU) at 24 h compared to 0 h controls) as well as its high degree of penetration into the lung compartment, as evidenced by significantly higher concentrations in epithelial lining fluid compared with free drug in plasma [58].
SAFETY AND PHARMACOLOGY
Fluoroquinolones have a long history of adverse effects with several of them being considered as class-related such as tendinitis, tendon rupture, peripheral neuropathy, central nervous system effects, and exacerbation of myasthenia gravis. As a result, all fluoroquinolones approved in the United States (including delafloxacin [16]) carry a general boxed warning about these effects (significant decreases in blood sugar and certain mental health side effects have been added recently or will be soon [59]). Both the US FDA and the European Medicines Agency (EMA) are also concerned with rare but severe and permanent or long-lasting serious side effects (see [59, 60], which led to the FDA statement that risks of fluoroquinolones may outweigh benefits for patients with mild infections such as acute sinusitis, acute bronchitis, and uncomplicated UTIs [61]. Likewise, the EMA may reduce the indications of fluoroquinolones to “severe infections when other antibiotics cannot be used” [62]. Most of these class-related adverse effects and/or permanent effects were uncommon in the safety data bases of registration and post-marketing studies undertaken by or under the control of Industry (see, for example, the safety profile of moxifloxacin as compiled from such studies involving about 15 000 patients [63]). The observation period in these studies is limited and they usually exclude patients with known risk factors. In this context, although the FDA label mentions that peripheral neuropathy and central nervous system effects have been observed with delafloxacin (also hypersensitivity, and Clostridium difficile-associated diarrhea), these were not specifically observed or reported more frequently in the delafloxacin arm than in the comparator arm in the clinical trials published to date [50, 64, 65, 66]. The current developer of delafloxacin undertook a series of studies aimed at examining specific fluoroquinolone-related side effects. In this context, cardiac safety was examined in clinical models that showed that neither a therapeutic (300 mg administered by IV) nor a supratherapeutic (900 mg IV) dose of delafloxacin was associated with clinically meaningful disturbances in cardiac repolarization (as measured by the corrected QT [QTc] interval) under conditions in which moxifloxacin, used as comparator, gave an unambiguous signal demonstrwating that the study was adequately sensitive to assess QTc prolongation [67]. Also, no relationship was reported between plasma concentrations and the placebo-corrected change from pre-dose baseline in the QTc (ΔΔQTcF) [49]. Because of concern about photosensitivity, commonly associated with a halogen substituent in position C8 (see [68] for review), a study of the photosensitizing potential of delafloxacin to ultraviolet (UVA and UVB) and visible radiation was conducted in 52 healthy volunteers. Neither delafloxacin given for 7 days at 200 mg/day and 400 mg/day (0.22 and 0.44 times the approved recommended daily oral dosage, respectively, nor placebo demonstrated clinically significant phototoxic potential at any wavelengths tested (295 to 430 nm), including solar simulation. The active comparator (lomefloxacin, which possesses a fluorine substituent in C8) demonstrated a moderate degree of phototoxicity at UVA 335 nm and 365 nm and solar simulation wavelengths [69]. Finally, significant drug-drug interactions are unlikely [16, 49], which is a consideration when choosing non-fluoroquinolone alternatives such as macrolides.
KEY MESSAGES AND CONCLUSION
Delafloxacin is the only anionic member of the fluoroquinolone class approved (in the United States only at the date of writing) for clinical use by intravenous and/or oral routes. This unique biochemical characteristic results in several features, most notably an increased antibacterial activity (lower MICs) in acidic conditions that might occur in many infected sites such as abscesses, biofilms, and/or intracellularly in phagolysosomes. Like other fluoroquinolones, delafloxacin shows highly bactericidal activity. Based on breakpoints currently defined for the United States by the FDA, delafloxacin shows useful activity against most Gram-positive pathogens including strains that are resistant to other currently approved fluoroquinolones. Its activity against Gram-negative species, if confirmed by appropriate susceptibility testing, would support its utility in ABSSSIs caused by these organisms. Additional indications, such as respiratory tract infections for which the in vitro spectrum of activity of delafloxacin seems promising, need to be confirmed in comprehensive clinical trials. Although convincing safety features can only be demonstrated through large-scale clinical use, delafloxacin’s safety record in the clinical registration trials was favorable. Moreover, specific studies examining cardiac- and phototoxicities were negative. The pharmacology of delafloxacin supports twice daily dosing and easy transition (with dose adaptation) from intravenous to oral routes, whereas the lack of clinically significant drug-drug interactions provides some assurance of safe use in the out-patient setting. In summary, as a result of its chemical, microbiological, and pharmacological properties, and its adverse event profile to date, delafloxacin may complement our current antibacterial armamentarium for effective treatment of skin/skin structure infections in the face of increasing antimicrobial resistance to other agents.
Author contributions. All authors were involved in the drafting, review, and approval of the manuscript for submission.
Acknowledgments. The authors thank Glenn Tillotson of GST Micro LLC for his expert feedback during the development of this manuscript. Assistance with the preparation of this manuscript was provided by Rich Friel and Alexandra Rayser of the Health Care Alliance Group, Voorhees, New Jersey.
Financial support. This study was funded by Melinta Therapeutics, New Haven, Connecticut.
Supplement sponsorship. This supplement is sponsored by Melinta Therapeutics, Inc.
Potential conflicts of interest. F. V. B. is Research Director from the Belgian Fonds de la Recherche Scientifique (FRS-FNRS). F. V. B. and P. M. T. received research grants for laboratory work about delafloxacin from Melinta Therapeutics, and P. M. T. was a speaker for Menarini S.r.L. about delafloxacin. S. H. Z. reports no conflicts of interest relative to this manuscript. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 2008; 168:1585–91. [DOI] [PubMed] [Google Scholar]
- 2. Qualls ML, Mooney MM, Camargo CA Jr., Zucconi T, Hooper DC, Pallin DJ. Emergency department visit rates for abscess versus other skin infections during the emergence of community-associated methicillin-resistant Staphylococcus aureus, 1997–2007. Clin Infect Dis 2012; 55:103–5. [DOI] [PubMed] [Google Scholar]
- 3. Edelsberg J, Taneja C, Zervos M, et al. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 2009; 15:1516–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaye KS, Patel DA, Stephens JM, Khachatryan A, Patel A, Johnson K. Rising United States hospital admissions for acute bacterial skin and skin structure infections: recent trends and economic impact. PLoS One 2015; 10:e0143276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morgan E, Hohmann S, Ridgeway J, Daum R, David MZ. Decreasing incidence of skin and soft tissue infections at 86 U.S. emergency departments, 2009–2014. Clin Infect Dis 2018; doi:10.1093/cid/ciy509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Summanen PH, Talan DA, Strong C, et al. Bacteriology of skin and soft-tissue infections: comparison of infections in intravenous drug users and individuals with no history of intravenous drug use. Clin Infect Dis 1995; 20:S279–82. [DOI] [PubMed] [Google Scholar]
- 7. Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagnostic Microbiol Infect Dis 2007; 57:7–13. [DOI] [PubMed] [Google Scholar]
- 8. Itani KM, Merchant S, Lin SJ, Akhras K, Alandete JC, Hatoum HT. Outcomes and management costs in patients hospitalized for skin and skin-structure infections. Am J Infect Control 2011; 39:42–9. [DOI] [PubMed] [Google Scholar]
- 9. Lee GC, Dallas SD, Wang Y, et al. Emerging multidrug resistance in community-associated Staphylococcus aureus involved in skin and soft tissue infections and nasal colonization. J Antimicrob Chemother 2017; 72:2461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA Jr. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med 2008; 51:291–8. [DOI] [PubMed] [Google Scholar]
- 11. Suaya JA, Mera RM, Cassidy A, et al. Incidence and cost of hospitalizations associated with Staphylococcus aureus skin and soft tissue infections in the United States from 2001 through 2009. BMC Infect Dis 2014; 14:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nathwani D. Impact of methicillin-resistant Staphylococcus aureus infections on key health economic outcomes: does reducing the length of hospital stay matter? J Antimicrob Chemother 2003; 51: ii37–44. [DOI] [PubMed] [Google Scholar]
- 13. Stevens DL, Bisno AL, Chambers HF, et al. ; Infectious Diseases Society of America Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–52. [DOI] [PubMed] [Google Scholar]
- 14. Russo A, Concia E, Cristini F, et al. Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin Microbiol Infect 2016; 22:S27–36. [DOI] [PubMed] [Google Scholar]
- 15. Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother 2013; 68:1951–61. [DOI] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration. BAXDELA (delafloxacin) Prescribing Information and Medication Guide Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208610s000,208611s000lbl.pdf. Accessed December 8, 2018.
- 17. Alonzo F 3rd, Torres VJ. A lesson in survival: S. aureus versus the skin. Cell Host Microbe 2013; 13:3–5. [DOI] [PubMed] [Google Scholar]
- 18. Thurlow LR, Joshi GS, Clark JR, et al. Functional modularity of the arginine catabolic mobile element contributes to the success of USA300 methicillin-resistant Staphylococcus aureus. Cell Host Microbe 2013; 13:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garzoni C, Kelley WL. Staphylococcus aureus: new evidence for intracellular persistence. Trends Microbiol 2009; 17:59–65. [DOI] [PubMed] [Google Scholar]
- 20. James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen 2008; 16:37–44. [DOI] [PubMed] [Google Scholar]
- 21. Ohman H, Vahlquist A. In vivo studies concerning a pH gradient in human stratum corneum and upper epidermis. Acta Derm Venereol 1994; 74:375–9. [DOI] [PubMed] [Google Scholar]
- 22. Siala W, Mingeot-Leclercq MP, Tulkens PM, Hallin M, Denis O, Van Bambeke F. Comparison of the antibiotic activities of daptomycin, vancomycin, and the investigational fluoroquinolone delafloxacin against biofilms from Staphylococcus aureus clinical isolates. Antimicrob Agents Chemother 2014; 58:6385–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A 1978; 75:3327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bassetti M, Pecori D, Cojutti P, Righi E, Pea F. Clinical and pharmacokinetic drug evaluation of delafloxacin for the treatment of acute bacterial skin and skin structure infections. Expert Opin Drug Metab Toxicol 2017; 13:1193–200. [DOI] [PubMed] [Google Scholar]
- 25. Righi E, Carnelutti A, Vena A, Bassetti M. Emerging treatment options for acute bacterial skin and skin structure infections: focus on intravenous delafloxacin. Infect Drug Resist 2018; 11:479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jorgensen SCJ, Mercuro NJ, Davis SL, Rybak MJ. Delafloxacin: place in therapy and review of microbiologic, clinical and pharmacologic properties. Infect Dis Ther 2018; 7:197–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cho JC, Crotty MP, White BP, Worley MV. What is old is new again: delafloxacin, a modern fluoroquinolone. Pharmacotherapy 2018; 38:108–21. [DOI] [PubMed] [Google Scholar]
- 28. Duffy EM, DeVito JA, Remy JM, Burak ES. Delafloxacin chemical properties lead to increased potency against gram-positive pathogens, including quinolone-resistant pathogens (II) (Poster E-183). 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) Boston, Massachusetts: American Society for Microbiology, 2010. [Google Scholar]
- 29. Remy JM, Tow-Keogh CA, McConnell TS, Dalton JM, Devito JA. Activity of delafloxacin against methicillin-resistant Staphylococcus aureus: resistance selection and characterization. J Antimicrob Chemother 2012; 67:2814–20. [DOI] [PubMed] [Google Scholar]
- 30. Mogle BT, Steele JM, Thomas SJ, Bohan KH, Kufel WD. Clinical review of delafloxacin: a novel anionic fluoroquinolone. J Antimicrob Chemother 2018; 73:1439–51. [DOI] [PubMed] [Google Scholar]
- 31. Kocsis B, Domokos J, Szabo D. Chemical structure and pharmacokinetics of novel quinolone agents represented by avarofloxacin, delafloxacin, finafloxacin, zabofloxacin and nemonoxacin. Ann Clin Microbiol Antimicrob 2016; 15:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lemaire S, Tulkens PM, Van Bambeke F. Contrasting effects of acidic pH on the extracellular and intracellular activities of the anti-Gram-positive fluoroquinolones moxifloxacin and delafloxacin against Staphylococcus aureus. Antimicrob Agents Chemother 2011; 55:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van Bambeke F. Delafloxacin, a non-zwitterionic fluoroquinolone in Phase III of clinical development: evaluation of its pharmacology, pharmacokinetics, pharmacodynamics and clinical efficacy. Future Microbiol 2015; 10:1111–23. [DOI] [PubMed] [Google Scholar]
- 34. Foulston L, Elsholz AK, DeFrancesco AS, Losick R. The extracellular matrix of Staphylococcus aureus biofilms comprises cytoplasmic proteins that associate with the cell surface in response to decreasing pH. MBio 2014; 5:e01667–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nilius AM, Shen LL, Hensey-Rudloff D, et al. In vitro antibacterial potency and spectrum of ABT-492, a new fluoroquinolone. Antimicrob Agents Chemother 2003; 47:3260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Candel FJ, Peñuelas M. Delafloxacin: design, development and potential place in therapy. Drug Des Devel Ther 2017; 11:881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ohshita Y, Yazaki A. In vitro studies with WQ-3034, a newly synthesized acidic fluoroquinolone (abstract F164). In: 37th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) Toronto, Canada, 1997. [Google Scholar]
- 38. Almer LS, Hoffrage JB, Keller EL, Flamm RK, Shortridge VD. In vitro and bactericidal activities of ABT-492, a novel fluoroquinolone, against Gram-positive and Gram-negative organisms. Antimicrob Agents Chemother 2004; 48:2771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pfaller MA, Sader HS, Rhomberg PR, Flamm RK. In vitro activity of delafloxacin against contemporary bacterial pathogens from the United States and Europe, 2014. Antimicrobial Agents and Chemotherapy 2017; 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Flamm RK, Shortridge D, Huband MD, McCurdy S, Pfaller MA. Activity of delafloxacin when tested against bacterial surveillance isolates collected in the US and Europe during 2014–2016 as part of a global surveillance program (poster 1222). ID Week 2017. San Diego, California, 2017. [Google Scholar]
- 41. Hammerschlag MR, Roblin PM. The in vitro activity of a new fluoroquinolone, ABT-492, against recent clinical isolates of Chlamydia pneumoniae. J Antimicrob Chemother 2004; 54:281–2. [DOI] [PubMed] [Google Scholar]
- 42. Soge OO, Salipante SJ, No D, Duffy E, Roberts MC. In vitro activity of delafloxacin against clinical Neisseria gonorrhoeae isolates and selection of gonococcal delafloxacin resistance. Antimicrob Agents Chemother 2016; 60:3106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCurdy S, Lawrence L, Quintas M, et al. In vitro activity of delafloxacin and microbiological response against fluoroquinolone-susceptible and nonsusceptible Staphylococcus aureus isolates from two phase 3 studies of acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 2017; 61:e00772–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. So W, Crandon JL, Nicolau DP. Effects of urine matrix and pH on the potency of delafloxacin and ciprofloxacin against urogenic Escherichia coli and Klebsiella pneumoniae. J Urol 2015; 194:563–70. [DOI] [PubMed] [Google Scholar]
- 45. Bauer J, Siala W, Tulkens PM, Van Bambeke F. A combined pharmacodynamic quantitative and qualitative model reveals the potent activity of daptomycin and delafloxacin against Staphylococcus aureus biofilms. Antimicrob Agents Chemother 2013; 57:2726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Siala W, Kucharíková S, Braem A, et al. The antifungal caspofungin increases fluoroquinolone activity against Staphylococcus aureus biofilms by inhibiting N-acetylglucosamine transferase. Nat Commun 2016; 7:13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McEwen A, Lawrence L, Hoover R, et al. Disposition, metabolism and mass balance of delafloxacin in healthy human volunteers following intravenous administration. Xenobiotica 2015; 45:1054–62. [DOI] [PubMed] [Google Scholar]
- 48. Hoover R, Hunt T, Benedict M, et al. Single and multiple ascending-dose studies of oral delafloxacin: effects of food, sex, and age. Clin Ther 2016; 38:39–52. [DOI] [PubMed] [Google Scholar]
- 49. Hoover R, Hunt T, Benedict M, et al. Safety, tolerability, and pharmacokinetic properties of intravenous delafloxacin after single and multiple doses in healthy volunteers. Clin Ther 2016; 38:53–65. [DOI] [PubMed] [Google Scholar]
- 50. Hoover RK, Alcorn H Jr, Lawrence L, Paulson SK, Quintas M, Cammarata SK. Delafloxacin pharmacokinetics in subjects with varying degrees of renal function. J Clin Pharmacol 2018; 58:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hoover R, Marbury TC, Preston RA, et al. Clinical pharmacology of delafloxacin in patients with hepatic impairment. J Clin Pharmacol 2017; 57:328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Paulson SK, Wood-Horrall RN, Hoover R, Quintas M, Lawrence LE, Cammarata SK. The pharmacokinetics of the CYP3A substrate midazolam after steady-state dosing of delafloxacin. Clin Ther 2017; 39:1182–90. [DOI] [PubMed] [Google Scholar]
- 53. Rubino CM, Bhavnani SM, Burak ES, Ambrose PG. Pharmacokinetic-pharmacodynamic target attainment analyses supporting delafloxacin phase 3 dose regimen decisions (poster A1-681). 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) Boston, MA, 2010. [Google Scholar]
- 54. Firsov AA, Vostrov SN, Lubenko IY, Arzamastsev AP, Portnoy YA, Zinner SH. ABT492 and levofloxacin: comparison of their pharmacodynamics and their abilities to prevent the selection of resistant Staphylococcus aureus in an in vitro dynamic model. J Antimicrob Chemother 2004; 54:178–86. [DOI] [PubMed] [Google Scholar]
- 55. Zinner SH, Vostrov SN, Alferova IV, Lubenko IY, Portnoy YA, Firsov AA. Comparative pharmacodynamics of the new fluoroquinolone ABT492 and ciprofloxacin with Escherichia coli and Pseudomonas aeruginosa in an in vitro dynamic model. Int J Antimicrob Agents 2004; 24:173–7. [DOI] [PubMed] [Google Scholar]
- 56. Firsov AA, Alferova IV, Smirnova MV, Lubenko IY, Portnoy YA, Zinner SH. Comparative pharmacodynamics of the new fluoroquinolone ABT492 and levofloxacin with Streptococcus pneumoniae in an in vitro dynamic model. Int J Antimicrob Agents 2005; 25:409–13. [DOI] [PubMed] [Google Scholar]
- 57. Lepak AJ, Andes DR. In vivo pharmacodynamic target assessment of delafloxacin against Staphylococcus aureus, Streptococcus pneumoniae, and Klebsiella pneumoniae in a murine lung infection model. Antimicrob Agents Chemother 2016; 60:4764–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Thabit AK, Crandon JL, Nicolau DP. Pharmacodynamic and pharmacokinetic profiling of delafloxacin in a murine lung model against community-acquired respiratory tract pathogens. Int J Antimicrob Agents 2016; 48:535–41. [DOI] [PubMed] [Google Scholar]
- 59. US Food and Drug Administration. FDA reinforces safety information about serious low blood sugar levels and mental health side effects with fluoroquinolone antibiotics; requires label changes Available at: https://www.fda.gov/drugs/drugsafety/ucm611032.htm. Accessed December 8, 2018.
- 60. European Medicines Agency (EMA). Public hearing on quinolones and fluoroquinolones: summary of safety concerns and list of questions Available at: http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500247105. Accessed December 8, 2018.
- 61. US Food and Drug Administration. FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. Available at: https://www.fda.gov/drugs/drugsafety/ucm500143.htm. Accessed December 8, 2018.
- 62. European Medicines Agency (EMA). Quinolone- and fluoroquinolone-containing medicinal products: disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics. Available at https://www.ema.europa.eu/en/medicines/human/referrals/quinolone-fluoroquinolone-containing-medicinal-products. Accessed December 8, 2018.
- 63. Tulkens PM, Arvis P, Kruesmann F. Moxifloxacin safety: an analysis of 14 years of clinical data. Drugs R D 2012; 12:71–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. O’Riordan W, Mehra P, Manos P, Kingsley J, Lawrence L, Cammarata S. A randomized phase 2 study comparing two doses of delafloxacin with tigecycline in adults with complicated skin and skin-structure infections. Int J Infect Dis 2015; 30:67–73. [DOI] [PubMed] [Google Scholar]
- 65. Pullman J, Gardovskis J, Farley B, et al. ; PROCEED Study Group Efficacy and safety of delafloxacin compared with vancomycin plus aztreonam for acute bacterial skin and skin structure infections: a Phase 3, double-blind, randomized study. J Antimicrob Chemother 2017; 72:3471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kingsley J, Mehra P, Lawrence LE, et al. A randomized, double-blind, Phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother 2016; 71:821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Litwin JS, Benedict MS, Thorn MD, Lawrence LE, Cammarata SK, Sun E. A thorough QT study to evaluate the effects of therapeutic and supratherapeutic doses of delafloxacin on cardiac repolarization. Antimicrob Agents Chemother 2015; 59:3469–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Van Bambeke F, Michot JM, Van Eldere J, Tulkens PM. Quinolones in 2005: an update. Clin Microbiol Infect 2005; 11:256–80. (Erratum in Clin Microbiol Infect 2005; 11:513.) [DOI] [PubMed] [Google Scholar]
- 69. Dawe RS, Ferguson J, Ibbotson S, et al. Lack of phototoxicity potential with delafloxacin in healthy male and female subjects: comparison to lomefloxacin. Photochem Photobiol Sci 2018; 17:773–80. [DOI] [PubMed] [Google Scholar]