Abstract
The US burden of acute skin infections is substantial. While Staphylococcus aureus and Streptococcus spp. are the most common causes, gram-negative bacteria and mixed infections can occur in some settings. These mixed infections are more likely to result in inappropriate empiric antibiotic therapy. Important challenges remain in diagnosing and treating acute skin infections.
Keywords: burden, acute bacterial skin and skin structure infections, epidemiology, etiology, United States
Skin and soft tissue infections (SSTIs) are characterized by microbial invasion of the skin layers and underlying soft tissues and range in severity from mild to life threatening [1]. Among the most common infections encountered in both ambulatory and hospital settings [2–4], SSTIs in the United States have increased dramatically in incidence in recent decades [3, 4]. The burden of SSTIs and their complications are considerable, resulting in hospitalization, surgery, bacteremia, and, on occasion, death [2]. Although Staphylococcus aureus and beta-hemolytic streptococci represent the traditional culprits in SSTI [5], more recently Gram-negative organisms, as well as mixed pathogens (both Gram-negative and Gram-positive bacteria), have become significant causes of acute skin infections [5–8]. These include healthcare-associated (HCA) complicated SSTIs (cSSTIs) [8], such as diabetic foot infections.
Several schemes exist for SSTI classification, each of which typically relies on numerous variables, such as location where infection occurred, causative pathogen, progression rates, depth of extension, and clinical presentation or severity [1]. In 2013, the US Food and Drug Administration (FDA) proposed a new categorization schema: “acute bacterial skin and skin structure infections” (ABSSSI). In a formal guidance for the development of drugs to treat such infections [9], the FDA defined ABSSSI as a bacterial cellulitis/erysipelas; wound infection; or major cutaneous abscess, with a lesion size area of at least 75 cm2 (measured by area of redness, edema, or induration) [9]. For many clinicians, however, the 2014 Infectious Diseases Society of America (IDSA) classification of SSSIs [10] is more useful and practical [1]. The IDSA classifies SSTIs as either nonpurulent (including cellulitis, erysipelas, and necrotizing infection) or purulent (including furuncle, carbuncle, and abscess) [10], with abscess and cellulitis being most common. Because of the variety of taxonomies to describe acute skin infections in the literature and to avoid confusion, we uniformly use “SSTI” throughout the article, regardless of how these infections are designated in individual studies. Here, we review the epidemiology, etiology, and outcomes of SSTIs in the United States.
SSTI EPIDEMIOLOGY
Burden
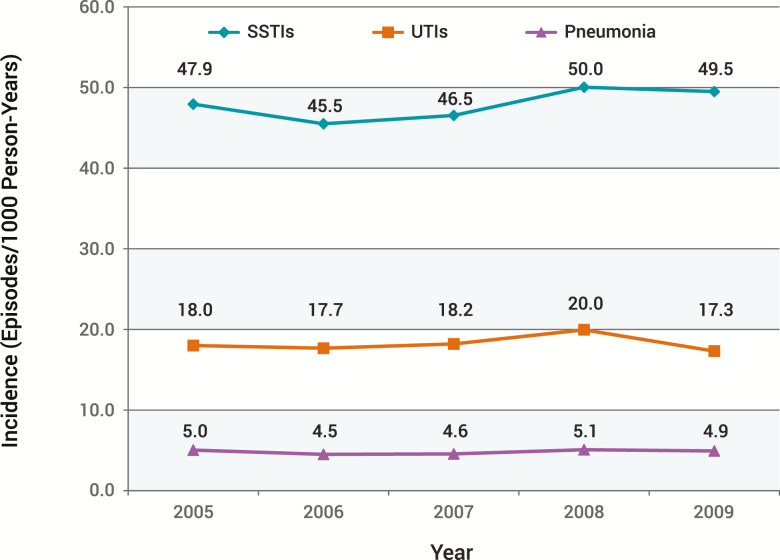
SSTI is a common infection in all healthcare settings in the United States. For example, Hersh and colleagues utilized the National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey from 1997 to 2005 to assess SSTI visits to physician offices, hospital outpatient departments, and hospital emergency departments (EDs) [4]. They found that total visits increased by 65% from 8.6 million in 1997 to 14.2 million in 2005, while the overall rate of ambulatory visits increased by 50% from 32.1 visits/1000 population in 1997 to 48.1 (P = .003 for trend) in 2005 [4]. Between 2005 and 2010, in a large US-based, multicenter, retrospective cohort of ambulatory and inpatient encounters among nearly 50 million commercially insured individuals aged 0–64 years, Miller and colleagues reported the frequency of SSTIs to be 2.3 million cases, which was far higher than that of either urinary tract infections or pneumonia (Figure 1) [2]. In this study, however, a rise in the incidence density of SSTIs over time was not observed (2005: 47.9 infections per 1000 patient-years vs 2010: 48.5 infections per 1000 patient-years), echoing prior findings [5]. In contrast, Kaye and colleagues recently noted that not only did the absolute volume of SSTI hospitalizations rise in the United States from 641 863 in 2005 to 752 770 in 2011, but so did SSTI diagnoses as a proportion of all admissions, increasing from 1.6% to 2.0% [11]. Similarly, Lee and colleagues assessed US trends in SSTIs between 2000 and 2012 and found a 40% increase (2.4 million to 3.3 million) in the overall incidence of SSTIs during this period [12]. Thus, while it is clear that SSTI is a high-volume condition in both in- and outpatient settings, only some studies demonstrate a rising incidence in recent history, with one reporting a decline in some EDs [13].
Figure 1.
Incidence of skin and soft tissue infections, urinary tract infections, and pneumonia: 2005–2009 [2]. Reprinted with Permission from Miller et al [2]. Abbreviations: SSTI, skin and soft tissue infection; UTI, urinary tract infection.
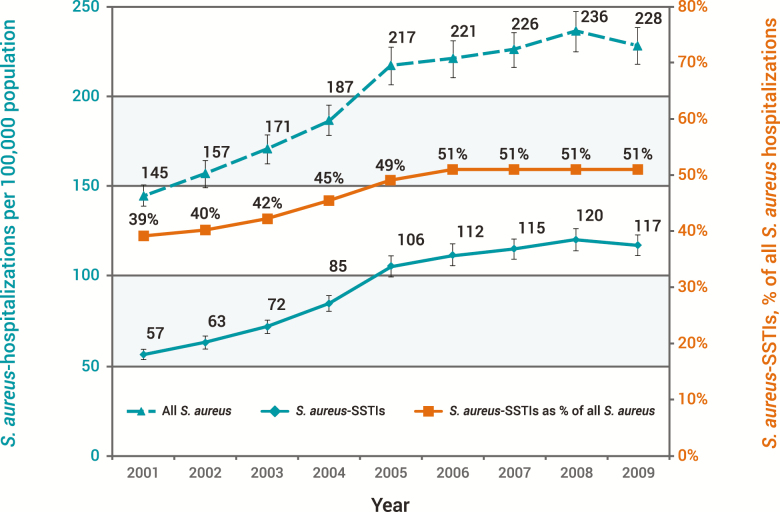
An increase in S. aureus-associated SSTIs has been a major contributor to the US burden of skin infections. According to Suaya and colleagues, who analyzed the Kaiser Permanente database, the incidence of S. aureus SSTIs doubled between 2001 and 2009 from 57 to 117 cases per 100 000 population (P < .01). Additionally, the volume of hospitalizations due to S. aureus-associated SSTIs increased 123% from 160 811 to 358 212 (Figure 2) [14]. A concurrent rise in SSTIs due to methicillin-resistant S. aureus (MRSA) has also been observed in some studies. In a US population-based study, Casey and coworkers assessed the incidence of HCA-MRSA, community-associated MRSA (CA-MRSA), and SSTIs in rural and urban areas of Pennsylvania within the Geisinger System [15]. From 2005 to 2009, the annual incidence of CA-MRSA increased by 34%, HCA-MRSA by 7%, and SSTIs by 4%. Complementing the rising incidence, Ray and colleagues also reported increases in the percentage of SSTIs due to MRSA between 1998 and 2009 in a large integrated US health plan, where the proportion of S. aureus isolates that were MRSA increased steadily from 13% in 1998 to 48% in 2009 [16]. Notwithstanding the result of these studies, others have reported a recent decline in MRSA SSTIs in some US populations [17, 18].
Figure 2.
Incidence of all Staphylococcus aureus hospitalizations and those due to skin and soft tissue infections in the United States: 2001–2009 [14]. Reprinted with Permission from Suaya et al [14]. Abbreviation: SSTI, skin and soft tissue infection.
Etiology
Because the microbiology of SSTIs remains elusive, arguably more so than in other common conditions such as bloodstream infections, clinicians must make many treatment decisions based on an educated guess with regards to which pathogens are causing infection. In cellulitis, in particular, it is challenging to obtain a microbiologic diagnosis. When a culture is obtained, the probability of isolating an organism is low. For example, needle aspiration of inflamed skin is positive in only 5%–40% of cases, while punch biopsy cultures yield results in 20%–30%, primarily due to Streptococcus spp. and S. aureus [10]. Since yield from blood cultures is low (≤5%) in patients with cellulitis and erysipelas, cultures are not routinely recommended [10]. Therefore, SSTIs with known pathogens are not necessarily representative of all SSTIs [5]. This fact certainly makes the etiology of the vast majority of SSTIs uncertain.
As with uncomplicated cellulitis, the majority of SSTIs where purulent material can be obtained are caused by Gram-positive pathogens, mainly S. aureus and streptococci [1]. Gram-positive pathogens are typically identified in more than 80% of culture-positive skin infections (such as abscess or surgical-site infections) [5–7, 19], and S. aureus is the most common cause of culture-confirmed SSTIs in the United States [5].
At the same time, because of the emergence and spread of resistance among both Gram-positive (in particular, MRSA) and Gram-negative organisms, treatment of SSTIs has become more challenging over the last 2 decades. In a large, retrospective, observational study, Ray and colleagues assessed the microbiology of SSTIs within a US health plan [5]. Between 2009 and 2011 among 376 262 individual plan members, 471 550 episodes of SSTI occurred, more than half of which were coded as cellulitis or abscess. Only 23% of infections were cultured, 81% of which were S. aureus, and 46% of these were MRSA. Importantly, previous history of MRSA infection, advanced age, chronic open wounds, underlying chronic disease, and frequent contact with a healthcare facility were risk factors for MRSA SSTIs [20] (Table 1). While MRSA accounted for 37% of overall culturable infections in this study [5], among purulent SSTIs presenting to EDs, the rate of MRSA infection is 59% [21].
Table 1.
Risk Factors for Types of Bacterial Skin and Soft Tissue Infections
| Methicillin-resistant Staphylococcus aureus [Russo 2016] | Gram-negative, Anaerobe, and Polymicrobial [Russo 2016] | Mixed [Zilberberg 2012] |
|---|---|---|
| Previous colonization Contact with patients colonized Antibiotic therapy in previous 12 months Hospitalization in previous 12 months History of previous infection Recent travel to Latin America, Africa, or Southeast Asia Long-term care facility residence Previous ICU admission |
Surgical site infection in the following locations: Axillary cavity Gastrointestinal tract Perineum Female genital tract |
Previous ICU admission Nursing home residence |
| Comorbidities: Cardiovascular disease Diabetes Peripheral vascular disease Chronic wounds Central venous catheter Chronic renal disease Dialysis IV drug abuse |
Comorbidities: Diabetes Cirrhosis IV drug abuse Subcutaneous drug abuse |
… |
Gram-negative pathogens, though less frequent, are no less challenging to treat [19]. They appear more commonly in HCA-cSSTIs than in CA-cSSTIs [8] and are often associated with surgical-site infections of the abdominal wall or infections in the anal and perineal region [22]. In the same US population–based study, Ray and colleagues reported a 14% prevalence of Gram-negative pathogens [5]. More recent studies that enrolled hospitalized patients with SSTIs found that more than 30% of patients whose cultures recovered an organism had either Gram-negative or mixed pathogens isolated. Both of these groups were at heightened risk for receiving inappropriate initial antibiotic therapy [6, 7].
The prevalence of mixed Gram-positive and Gram-negative infections ranges from 10% to 24% [6–8] and, as is the case with purely Gram-negative infections, they are also more frequent among patients with HCA-SSTI [8]. Importantly, several studies indicate that these mixed infections are associated with increased risk of inappropriate empiric antibiotic therapy [8, 19, 23]. In a single-center, retrospective, cohort study of 717 patients hospitalized with a cSSTI from 2006 to 2007, Zilberberg and colleagues found that nearly 40% of individuals with mixed SSTIs received inappropriate empiric therapy [8]. Multiple studies have underscored the importance of appropriate empiric treatment vis-à-vis clinical and economic consequences, and they are reviewed later in this article [19, 24–28]. Risk factors for Gram-negative and polymicrobial SSTIs, which are summarized in Table 1, may help to identify these patients and help providers choose appropriate empiric therapy [20, 23].
Risk Factors for Initial and Recurrent SSTI
Although relatively common among healthy populations, SSTIs are more frequent in persons who share certain clinical and demographic features. Ray and colleagues reported that children aged <5 years and adults aged ≥65 years had the highest rates of SSTIs compared to all other age groups [5]. The same study reported that Asians (risk ratio [RR], 0.51; 95% confidence interval [CI], 0.50 to 0.52), African-Americans (RR, 0.94; 95% CI, 0.92 to 0.96), and Hispanics (RR, 0.81; 95% CI, 0.80 to 0.83) had a lower risk for SSTIs than whites [5]. However, when the pathogen was known to be S. aureus, African-Americans (odds ratio [OR], 1.79; 95% CI, 1.67 to 1.92) and Hispanics (OR, 1.24; 95% CI, 1.18 to 1.31) had a higher risk of MRSA infections compared with whites, while Asians had a further reduction in risk beyond that of white patients (OR, 0.73; 95% CI, 0.68 to 0.78) [5].
Certain comorbid conditions, such as diabetes, obesity, critical illness, immune compromise, liver and kidney disease, and vascular insufficiency, affect not only the development of SSTIs but also their outcomes [29]. For example, individuals with diabetes have nearly double the rate of SSTIs compared to those without diabetes (RR, 1.93; 95% CI, 1.90 to 1.96) [5]. Obesity is not only a risk factor for surgical-site infections but also for recurrent skin infections due to MRSA and clinical failure in patients hospitalized with cellulitis or cutaneous abscess [30–32].
MRSA colonization is also an important risk factor for SSTIs [33]. The majority of patients who develop MRSA infections are colonized beforehand, most often in the anterior nares. While approximately 20% of the general population has persistent S. aureus colonization, 66% are intermittent carriers [33]. Among modifiable risk factors, smoking has also been found to increase the risk of surgical-site infections among patients who have undergone elective plastic surgery procedures [34], as well as women who undergo cesarean delivery [35].
In one recent study, recurrent infections were shown to be a major contributor to the overall SSTI burden [36]. In a study of 272 patients treated in the ED for cutaneous abscess, consistent with observations from other settings, SSTI recurred within 3 months in 28% of patients [37, 38]. Factors found to increase the risk of SSTI recurrence included having contact with an individual infected with MRSA and use of wound packing [36].
Outcomes
Few studies have reported on the overall mortality rates related to SSTIs in the United States. In a study of adult patients hospitalized in the United States with a primary diagnosis of SSTI, Kaye and colleagues found that mortality was relatively low and decreased from 0.56% in 2005 to 0.46% in 2011 [11]. The investigators speculated that the decrease in SSTI-related mortality over time may have been due to earlier administration of antibiotics with activity against MRSA [11].
A number of studies have examined economic consequences of SSTIs [12, 39]. The most recent data from Lee and colleagues indicate that the total cost of SSTIs in the United States was $13.8 billion in 2012 [12]. As with many other disease states, hospitalizations were major drivers of these costs, with an average expenditure of $22 706 per person [12]. The significantly higher costs associated with inpatient treatment [39], along with patient preferences, bring into focus the importance of trying to avoid hospitalization to the extent possible [40]. The fact that administration of intravenous (IV) antibiotics is the sole reason for 42% of SSTI hospital admissions [32] suggests that restructuring healthcare delivery away from the inpatient setting, as well as use of long-acting antibiotics (eg, oritavancin, dalbavancin), may help to alleviate a substantial proportion of costs.
Length of stay (LOS) for SSTI has been shown in multiple recent studies to vary according to the type of infection and pathogen. In the multicenter study by Kaye et al, for example, it was the patients with postoperative wound infections (22% of SSTI admissions) who had the longest hospital stays (adjusted, 5.81 days) and highest total costs (adjusted, $9388) [11]. Patient comorbidities, such as diabetes, renal insufficiency, and immune compromise, also have an impact on treatment costs for SSTIs, since comorbidities often result in prolonged hospitalization [6, 39].
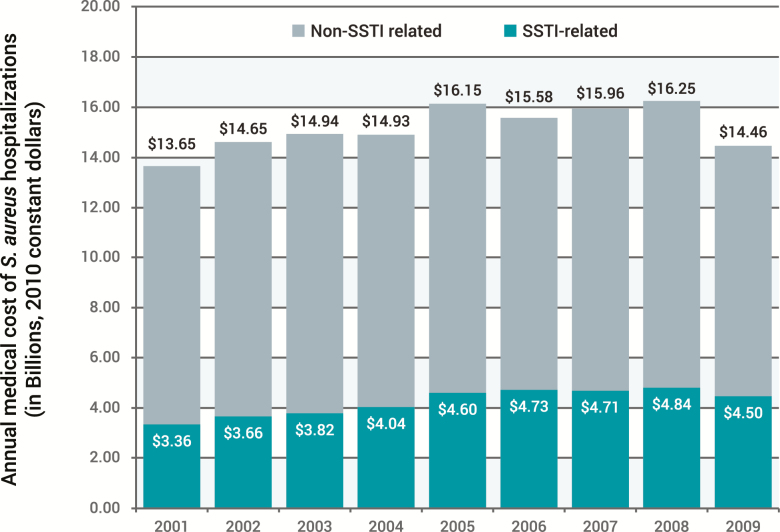
In addition to the differential impact on LOS and costs related to infection type, the culprit pathogen may also contribute to these outcomes in several ways. For example, in an analysis of all SSTI admissions in the United States, Suaya and colleagues reported the hospitalization cost of S. aureus SSTIs to be more than $11 000 per patient, with the national costs rising by 26% between 2001 and 2009 [14]. While overall S. aureus-related hospitalization costs increased as well, those associated with SSTI increased to a greater degree (Figure 3) [14]. Although Gram-positive pathogens are the most common causes of SSTIs, Itani et al found that infections that involve mixed and resistant pathogens are associated with worse outcomes among patients hospitalized for SSTIs [41]. Among 5156 SSTI cases in 42 US hospitals with identifiable pathogens, 59.7% were Gram positive, 21.5% Gram negative, and 18.8% mixed. Patients with mixed pathogens compared to patients with SSTIs due to Gram-negative or Gram-positive pathogens had significantly longer unadjusted LOS (17.2 days vs 10.1 and 9.5 days; P < .0001), a higher mortality rate (10.2% vs 6.5% and 4.8%; P < .0001), and higher mean patient costs ($80 093 vs $41 634 and $40 046; P < .001) [38]. Longer unadjusted LOS (17.2 vs 10.2 days; P < .0001) and higher total costs ($73 779 vs 44 103; P = .0004) were also observed in SSTI cases due to Pseudomonas aeruginosa compared with non–P. aeruginosa cases. Moreover, in this study, SSTI cases that involved MRSA (isolated from 21.6% of all cases) were associated with significantly longer unadjusted LOS (12.6 vs 10.7 days; P = .0019) and greater mortality (8.7% vs 5.5%; P = .001) than non-MRSA cases, although total costs were similar between the groups [41].
Figure 3.
Annual medical costs of Staphylococcus aureus skin and soft tissue infection hospitalizations in the United States and proportion of total costs for all S. aureus hospitalizations: 2001–2009 [14]. Reprinted with Permission from Suaya et al [14]. Abbreviation: SSTI, skin and soft tissue infection.
Initial Antibiotic Treatment and Outcomes in Patients With SSTIs
Because initial management of serious infections relies on the clinician’s judgment regarding the likelihood of different potential causative pathogens, in the setting of shifting resistance patterns, choosing effective coverage becomes challenging. Some early indirect evidence that supports the associations between initial treatment failure and higher mortality, longer hospital stays, and higher hospital charges was reported from large administrative data analyses [24–26]. More recently, clinically focused studies confirmed that poor outcomes are frequently associated with the use of initial antibiotic regimens that fail to cover the pathogen that eventually grows in culture. Thus, it is imperative that clinicians understand their local antibiograms, identify patient risk factors for resistance, and provide prompt empiric treatment that includes appropriate coverage for suspected pathogens [7, 19, 25, 27].
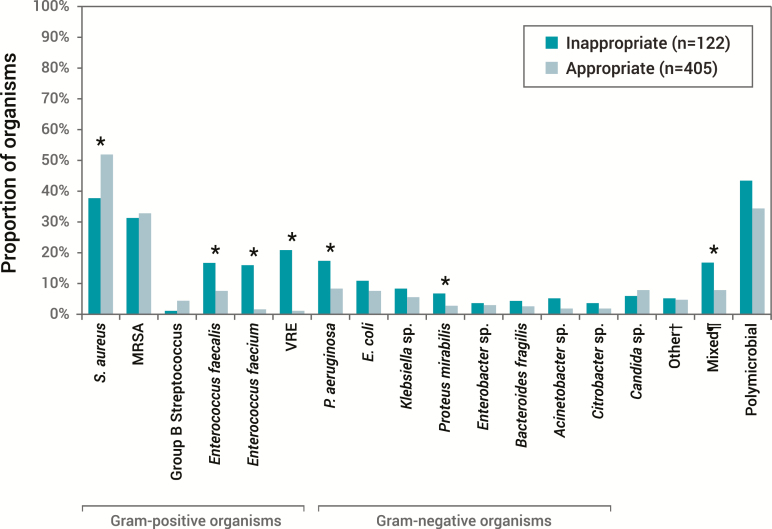
Patients with HCA-SSTI are at risk for acquiring different pathogens than those with CA-SSTI. Compared to patients with CA-SSTI, patients admitted with HCA infection are at increased risk for infections due to antimicrobial-resistant pathogens, and this high risk of resistance makes them more likely to receive inappropriate empiric therapy and have poor outcomes (Table 2) [19, 25, 27, 28]. In a single-center, retrospective analysis that included 527 patients with HCA SSTI, nearly 25% received inappropriate empiric treatment, with an almost 2-day prolongation of LOS [27]. Furthermore, those patients with a mixed infection (approximately 10% of the total population) were at higher risk for receiving inappropriate empiric coverage than those without mixed pathogens [27] (Figure 4). A more recent multicenter, prospective, observational study supports these findings [7] with regards to patients with polymicrobial infections being more likely to receive inappropriate initial antibiotic therapy (OR, 4.52; 95% CI, 2.62–7.78). Monomicrobial Gram-negative infections were associated with increased risk for inappropriate empiric treatment compared to monomicrobial Gram-positive infections (OR, 3.43; 95% CI, 1.79–6.60). Zervos and colleagues found a similar association between increased risk of inappropriate empiric therapy in patients with MRSA, Gram-negative infections, or mixed pathogens compared to patients with methicillin-susceptible S. aureus or streptococci (P < .05) [19]. Thus, in some scenarios, in order to administer appropriate empiric treatment, clinicians need to consider the probability of pathogens other than susceptible Gram-positive organisms as the cause of SSTI.
Table 2.
Effect of Inappropriate or Inadequate Antibiotic Therapy on Resource Utilization and Outcomes in Patients With Skin and Soft Tissue Infections [19, 25, 27, 28]
| Study | Population | % Patients Receiving Inappropriate/Inadequate Therapy | Additional Burden and Outcomes |
|---|---|---|---|
| Zilberberg 2010 | Hospitalized HCA cSSTI | 23a (N = 717) | • 1.8 additional hospital days • 4.6 additional hospital days in subgroup with cSSTI and bacteremia |
| Eagye 2009 | Hospitalized cSSI | 30b (N = 130) | • 4 additional hospital days • 3 additional days of therapy • $7667 additional inpatient costs |
| Zervos 2012 | Hospitalized cSSTI | 18.5 (N = 1096) | • 12-times higher mortality and readmission rate within 30 days in subgroup of patients with ulcers |
| Lipsky 2014b | Hospitalized cSSTI | 23.1 (N = 494) | • 1 additional hospital day • 2.43 additional hospital days in MRSA+HCA cohort • 9% more patients with at least 1 composite economic outcomec • 22% more patients with at least 1 composite economic outcome in the MRSA+HCA cohort |
Abbreviations: cSSI, complicated surgical site infection; cSSTI, complicated skin and soft tissue infections; HCA, healthcare associated; MRSA, methicillin resistant S. aureus.
aInappropriate empiric antibiotic therapy defined as patient did not receive treatment within 24 hours of the time the culture was obtained with an agent exhibiting in vitro activity against the isolated pathogen.
bInadequate antibiotic therapy was deemed to have been given if the patient did not receive an antibiotic to which the causative organism(s) was susceptible within 24 hours of identification of infection.
cComposite economic outcome = hospital admission, emergency department visit, or unscheduled visits to a healthcare provider due to study infection after hospital discharge.
Figure 4.
Distribution of pathogens among hospitalized patients (N = 717) with culture-positive complicated skin and soft tissue infections treated with appropriate or inappropriate empiric therapy [27]. Reprinted with Permission from Zilberberg et al [27]. Abbreviations: E. coli, Escherichia coli; MRSA, methicillin-resistant Staphylococcus aureus; P. aeruginosa, Pseudomonas aeruginosa; S. aureus, Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus. *P < .05 for inappropriate vs appropriate groups. †Other: Morganella morganii, Serratia marcescens, Stenotrophomonas maltophilia, Streptococcus pyogenes, and Streptococcus pneumoniae. ¶Includes both a Gram-positive and a Gram-negative organism.
Gibbons et al implemented a stewardship intervention that consisted of an SSTI evidence-based treatment algorithm and education to providers, including calls and medical record notes from stewardship personnel regarding antimicrobial therapy targeted to physicians [42]. Compared to the control period, the percentage of antibiotic use concordant with an evidence-based SSTI treatment algorithm rose significantly during the intervention period (33% vs 19%; P = .04), the median number of days of IV antibiotic therapy to oral conversion diminished (3 vs 5; P < 0.0001), and the number of documented SSTI treatment complications was reduced (1% vs 8%; P = .04) [42].
CURRENT CHALLENGES IN THE DIAGNOSIS AND MANAGEMENT OF SSTIS
While rapid diagnostics have improved the outcomes for patients with other severe infections, limited data and strategies are available for SSTIs. Hence, more studies are urgently needed to establish the role of various novel diagnostic technologies in these infections [43]. Even when such diagnostics are available, due to the high rates of culture-negative SSTIs, therapy will remain challenging in patients at increased risk for Gram-negative and mixed infections.
The optimal time to switch from IV to oral antibiotic therapy as well as the optimal duration of therapy remain uncertain [43]. Switching from IV to oral antibiotics when clinical improvement is apparent and antibiotic deescalation are important components of antimicrobial stewardship [43]. In addition, there is likely a role for short-course therapy in some SSTI populations, although there is a dearth of clinical evidence on this topic.
SUMMARY
The burden and cost of acute skin infections in the United States, including ambulatory visits and hospitalizations, are substantial. While S. aureus (including MRSA) and Streptococcus spp. remain common SSTI causes, Gram-negative and mixed infections, though often underappreciated by clinicians when choosing empiric coverage, are present in up to 30% of culture-positive SSTIs. To optimize outcomes, it is important for clinicians to recognize factors that may increase the risk for MRSA as well as for a Gram-negative or a mixed infection. Future research should address the optimal duration of SSTI therapy, including the timing of IV to oral switch, and further characterize risk factors for polymicrobial infections. Such data would augment antibiotic stewardship efforts to provide effective empiric antimicrobial therapy to patients with SSTIs while also avoiding unnecessarily broad-spectrum treatment.
Acknowledgments. The authors appreciate the expert feedback provided by Glenn Tillotson of GST Micro LLC. Assistance with the preparation of this manuscript was provided by Rich Friel and Alexandra Rayser of the HealthCare Alliance Group, Voorhees, New Jersey.
Financial support. Editorial support was funded by Melinta Therapeutics and was provided by HealthCare Alliance Group.
Supplement sponsorship. This supplement is sponsored by Melinta Therapeutics, Inc.
Potential conflicts of interest. K. S. K. reports grants and personal fees from Merck and personal fees from Melinta, Nabriva, Paratek, and Shinogi outside the submitted work. M. D. Z. reports grants from Tetraphase, Merck, and Astellas; consulting fees from Shionogi; and grants and consulting fees from Pfizer outside the submitted work; and consulting fees from Melinta during the conduct of the study. A. F. S. reports grants from Astellas, Pfizer, Spero, and Tetraphase and has served as a speaker for or consultant to Astellas, Entatsis, Merck, Nabriva, Paratek, Pfizer, and Tetraphase. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Esposito S, Noviello S, Leone S. Epidemiology and microbiology of skin and soft tissue infections. Curr Opin Infect Dis 2016; 29:109–15. [DOI] [PubMed] [Google Scholar]
- 2. Miller LG, Eisenberg DF, Liu H, et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005–2010. BMC Infect Dis 2015; 15:362–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Edelsberg J, Taneja C, Zervos M, et al. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 2009; 15:1516–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 2008; 168:1585–91. [DOI] [PubMed] [Google Scholar]
- 5. Ray GT, Suaya JA, Baxter R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a U.S. population: a retrospective population-based study. BMC Infect Dis 2013; 13:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGinnis E, Cammarata S, Barnes S, et al. Characteristics of patients hospitalized for acute bacterial skin and skin structure infections from 2009–2013. In: ID Week; 2016; New Orleans, LA. [Google Scholar]
- 7. Lipsky BA, Napolitano LM, Moran GJ, Vo L, Nicholson S, Kim M. Inappropriate initial antibiotic treatment for complicated skin and soft tissue infections in hospitalized patients: incidence and associated factors. Diagn Microbiol Infect Dis 2014; 79:273–9. [DOI] [PubMed] [Google Scholar]
- 8. Zilberberg MD, Shorr AF, Micek ST, et al. Epidemiology and outcomes of hospitalizations with complicated skin and skin-structure infections: implications of healthcare-associated infection risk factors. Infect Control Hosp Epidemiol 2009; 30:1203–10. [DOI] [PubMed] [Google Scholar]
- 9. US Food and Drug Administration. Acute bacterial skin and skin structure infections: developing drugs for treatment. Guidance for industry. 2013. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071185.pdf [Accessed Jan 31 2019] [Google Scholar]
- 10. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:147–59. [DOI] [PubMed] [Google Scholar]
- 11. Kaye KS, Patel DA, Stephens JM, Khachatryan A, Patel A, Johnson K. Rising United States hospital admissions for acute bacterial skin and skin structure infections: recent trends and economic impact. PLoS One 2015; 10:e0143276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee GC, Boyd NK, Lawson KA, Frei CR. Incidence and cost of skin and soft tissue infections in the United States. Value Health 2015; 18:A245. [Google Scholar]
- 13. Morgan E, Hohmann S, Ridgway J, Daum R, David MZ. Decreasing incidence of skin and soft tissue infections at 86 U.S. emergency departments, 2009–2014. Clin Infect Dis 2018. doi: 10.1093/cid/ciy509. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suaya JA, Mera RM, Cassidy A, et al. Incidence and cost of hospitalizations associated with Staphylococcus aureus skin and soft tissue infections in the United States from 2001 through 2009. BMC Infect Dis 2014; 14:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Casey J, Cosgrove S, Stewart W, et al. A population-based study of the epidemiology and clinical features of methicillin-resistant Staphylococcus aureus infection in Pennsylvania, 2001–2010. Epidemiol Infect 2013; 141:1166–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ray GT, Suaya JA, Baxter R. Trends and characteristics of culture-confirmed Staphylococcus aureus infections in a large U.S. integrated health care organization. J Clin Microbiol 2012; 50:1950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sutter DE, Milburn E, Chukwuma U, Dzialowy N, Maranich AM, Hospenthal DR. Changing susceptibility of Staphylococcus aureus in a US pediatric population. Pediatrics 2016; 137. doi: 10.1542/peds.2015–3099. Epub 2016 Mar 1. [DOI] [PubMed] [Google Scholar]
- 18. Acree ME, Morgan E, David MZ. S. aureus infections in Chicago, 2006–2014: increase in CA MSSA and decrease in MRSA incidence. Infect Control Hosp Epidemiol 2017; 38:1226–34. [DOI] [PubMed] [Google Scholar]
- 19. Zervos MJ, Freeman K, Vo L, et al. Epidemiology and outcomes of complicated skin and soft tissue infections in hospitalized patients. J Clin Microbiol 2012; 50:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Russo A, Concia E, Cristini F, et al. Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin Microbiol Infect 2016; 22(Suppl 2):S27–36. [DOI] [PubMed] [Google Scholar]
- 21. Talan DA, Krishnadasan A, Gorwitz RJ, et al. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin Infect Dis 2011; 53:144–9. [DOI] [PubMed] [Google Scholar]
- 22. Dryden MS. Complicated skin and soft tissue infection. J Antimicrob Chemother 2010; 65(Suppl 3):35–44. [DOI] [PubMed] [Google Scholar]
- 23. Zilberberg M, Micek ST, Kollef MH, Shelbaya A, Shorr AF. Risk factors for mixed complicated skin and skin structure infections to help tailor appropriate empiric therapy. Surg Infect (Larchmt) 2012; 13:377–82. [DOI] [PubMed] [Google Scholar]
- 24. Berger A, Oster G, Edelsberg J, Huang X, Weber DJ. Initial treatment failure in patients with complicated skin and skin structure infections. Surg Infect (Larchmt) 2013; 14:304–12. [DOI] [PubMed] [Google Scholar]
- 25. Eagye KJ, Kim A, Laohavaleeson S, Kuti JL, Nicolau DP. Surgical site infections: does inadequate antibiotic therapy affect patient outcomes? Surg Infect (Larchmt) 2009; 10:323–31. [DOI] [PubMed] [Google Scholar]
- 26. Edelsberg J, Berger A, Weber DJ, Mallick R, Kuznik A, Oster G. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol 2008; 29:160–9. [DOI] [PubMed] [Google Scholar]
- 27. Zilberberg MD, Shorr AF, Micek ST, et al. Hospitalizations with healthcare-associated complicated skin and skin structure infections: impact of inappropriate empiric therapy on outcomes. J Hosp Med 2010; 5:535–40. [DOI] [PubMed] [Google Scholar]
- 28. Lipsky BA, Napolitano LM, Moran GJ, et al. Economic outcomes of inappropriate initial antibiotic treatment for complicated skin and soft tissue infections: a multicenter prospective observational study. Diagn Microbiol Infect Dis 2014; 79:266–72. [DOI] [PubMed] [Google Scholar]
- 29. Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol 2008; 19:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sreeramoju P, Porbandarwalla NS, Arango J, et al. Recurrent skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus requiring operative debridement. Am J Surg 2011; 201:216–20. [DOI] [PubMed] [Google Scholar]
- 31. Halilovic J, Heintz BH, Brown J. Risk factors for clinical failure in patients hospitalized with cellulitis and cutaneous abscess. J Infect 2012; 65:128–34. [DOI] [PubMed] [Google Scholar]
- 32. Wiseman JT, Fernandes-Taylor S, Barnes ML, et al. Predictors of surgical site infection after hospital discharge in patients undergoing major vascular surgery. J Vasc Surg 2015; 62:1023–1031.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abad CL, Pulia MS, Safdar N. Does the nose know? An update on MRSA decolonization strategies. Curr Infect Dis Rep 2013; 15:455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Theocharidis V, Katsaros I, Sgouromallis E, et al. Current evidence on the role of smoking in plastic surgery elective procedures: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2018; 71:624–36. [DOI] [PubMed] [Google Scholar]
- 35. Kawakita T, Landy HJ. Surgical site infections after cesarean delivery: epidemiology, prevention and treatment. Matern Health Neonatol Perinatol 2017; 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. May LS, Zocchi M, Zatorski C, et al. Treatment failure outcomes for emergency department patients with skin and soft tissue infections. West J Emerg Med 2015; 16:642–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miller LG, Quan C, Shay A, et al. A prospective investigation of outcomes after hospital discharge for endemic, community-acquired methicillin-resistant and -susceptible Staphylococcus aureus skin infection. Clin Infect Dis 2007; 44:483–92. [DOI] [PubMed] [Google Scholar]
- 38. Graber CJ, Jacobson MA, Perdreau-Remington F, Chambers HF, Diep BA. Recurrence of skin and soft tissue infection caused by methicillin-resistant Staphylococcus aureus in a HIV primary care clinic. J Acquir Immune Defic Syndr 2008; 49:231–3. [DOI] [PubMed] [Google Scholar]
- 39. Linder KE, Nicolau DP, Nailor MD. Epidemiology, treatment, and economics of patients presenting to the emergency department for skin and soft tissue infections. Hosp Pract (1995) 2017; 45:9–15. [DOI] [PubMed] [Google Scholar]
- 40. Ektare V, Khachatryan A, Xue M, Dunne M, Johnson K, Stephens J. Assessing the economic value of avoiding hospital admissions by shifting the management of gram+ acute bacterial skin and skin-structure infections to an outpatient care setting. J Med Econ 2015; 18:1092–101. [DOI] [PubMed] [Google Scholar]
- 41. Itani KM, Merchant S, Lin SJ, Akhras K, Alandete JC, Hatoum HT. Outcomes and management costs in patients hospitalized for skin and skin-structure infections. Am J Infect Control 2011; 39:42–9. [DOI] [PubMed] [Google Scholar]
- 42. Gibbons JA, Smith HL, Kumar SC, et al. Antimicrobial stewardship in the treatment of skin and soft tissue infections. Am J Infect Control 2017; 45:1203–7. [DOI] [PubMed] [Google Scholar]
- 43. Esposito S, Bassetti M, Bonnet E, et al. ; International Society of Chemotherapy Hot topics in the diagnosis and management of skin and soft-tissue infections. Int J Antimicrob Agents 2016; 48:19–26. [DOI] [PubMed] [Google Scholar]