Six Salmonella Typhi or Paratyphi human challenge studies were conducted, and daily stool cultures performed. Vi-containing vaccines reduced bacterial shedding, Ty21a or an experimental vaccine did not. Higher Vi immunoglobulin G titers were associated with reduced shedding.
Keywords: stool shedding, Salmonella Typhi, indirect effects, typhoid conjugate vaccine, Vi-polysaccharide vaccine
Abstract
Background
Shedding of Salmonella Typhi or Paratyphi in the stool or urine leads to contamination of food or water, which is a prerequisite for transmission of enteric fever. Currently, there are limited data on the effect of vaccination or prior exposure on stool shedding.
Methods
Six Salmonella Typhi or Paratyphi human challenge studies were conducted between 2011 and 2017. Participants were either unvaccinated or vaccinated with 1 of 4 vaccines: Vi-polysaccharide (Vi-PS), Vi-tetanus-toxoid conjugate vaccine (Vi-TT), live oral Ty21a vaccine, or an experimental vaccine (M01ZH09). Daily stool cultures were collected for 14 days after challenge.
Results
There were 4934 stool samples collected from 430 volunteers. Participants who received Vi-PS or Vi-TT shed less than unvaccinated participants (odds ratio [OR], 0.34; 95% confidence interval [CI], 0.15–0.77; P = .010 and OR, 0.41; 95% CI, 0.19–0.91, P = .029 for Vi-PS and Vi-TT, respectively). Higher anti-Vi immunoglobulin G titers were associated with less shedding of S. Typhi (P < .0001). A nonsignificant reduction in shedding was associated with Ty21a vaccine (OR, 0.57; 95% CI, 0.27–1.20; P = .140). Individuals previously exposed to S. Typhi shed less than previously unexposed individuals (OR, 0.30; 95% CI, 0.1–0.8; P = .016). Shedding of S. Typhi was more common than S. Paratyphi.
Conclusions
Prior vaccination with Vi vaccines, or natural infection, reduces onward transmission of S. Typhi. Field trials of Vi-TT should be designed to detect indirect protection, reflecting the consequence of reduced stool shedding observed in the human challenge model.
Infection with Salmonella enterica subspecies enterica serovars Typhi or Paratyphi (S. Typhi or S. Paratyphi) is estimated to be responsible for between 11.6 and 26.9 million cases of enteric fever and 75000–216510 deaths annually [1–5]. Transmission of S. Typhi and S. Paratyphi occurs primarily through consumption of contaminated food or water, via short-cycle or long- cycle transmission. Short-cycle transmission is defined as the contamination of food and water in the immediate environment, whereas long-cycle transmission is defined as contamination of the broader environment, such as pollution of water supplies by sewage or inadequate treatment of piped water [6]. The relative contribution of each transmission mode may vary depending on the epidemiological context and may differ between S. Typhi and S. Paratyphi [7]. As these serovars are human restricted, all modes of transmission involve shedding of the organism by infected individuals during incubation, acute disease, or convalescence or by chronic long-term carriers, ultimately resulting in contamination of food or water consumed by susceptible individuals. Disease control is therefore likely to require the integration of initiatives to improve water quality, sanitation, and hygiene, coupled with the deployment of effective vaccines [8].
Vaccines that both protect against clinical disease as well as reduce shedding would likely have enhanced effectiveness by interrupting transmission and providing indirect protection to unvaccinated individuals. Live attenuated oral typhoid vaccine, Ty21a, reduced stool shedding in the early Maryland challenge studies [9] and appears to induce herd immunity in field trials [10]. However, there are conflicting data on the indirect protection conferred by Vi-polysaccharide vaccines (Vi-PS) [11, 12] and limited data on the impact of new Vi-tetanus-toxoid conjugate vaccines (Vi-TT) on stool shedding [13]. Finally, the impact of previous exposure to typhoidal Salmonella on stool shedding after subsequent exposure has not been previously explored.
Experimental challenge studies of closely monitored volunteers can be used to describe microbial dynamics in clinical and subclinical typhoid and paratyphoid infections, including the timing and pattern of stool shedding after challenge in naive or vaccinated individuals [14]. Early experimental human challenge studies in Maryland indicate that shedding is more common in individuals who develop typhoid disease. However, individuals who fail to develop disease following challenge can continue to shed bacteria for several weeks [15]. Differences in stool shedding patterns between S. Typhi and S. Paratyphi A are poorly understood. Improved estimates of stool shedding dynamics in enteric fever are needed, particularly in the context of different immune states, as these form important variables in models of typhoid transmission dynamics and estimates of vaccine impact [16, 17].
We performed an analysis of stool shedding dynamics in healthy volunteers enrolled into closely monitored S. Typhi and S. Paratyphi human challenge studies. Our aims in this study were to model stool shedding after experimental challenge and to compare those challenged with S. Typhi vs S. Paratyphi, participants who did or did not develop enteric fever after challenge, those who received typhoid vaccines vs those who were unvaccinated, rechallenged individuals previously exposed to S. Typhi or S. Paratyphi vs previously unexposed individuals, differences according to demographic variables, and the relationship between antibody levels and stool shedding.
METHODS
Typhoid and Paratyphoid Human Challenge Studies
Data were available from 6 enteric fever human challenge studies conducted in Oxford between 2011 and 2017. A list of included studies is provided in Table 1.
Table 1.
List of Included Studies
| Study | Challenge Agent | Study Type | Vaccine | Description | References | |
|---|---|---|---|---|---|---|
| 1 | OVG2009/10 (T1) |
Salmonella Typhi Quailes strain | Observational | … | Dose-finding study Low dose: 1–5 × 103 CFU (n = 20) High dose: 1–5 × 104 CFU (n = 20) |
[18] |
| 2 | OVG2011/02 NCT01405521 (T2) |
S. Typhi Quailes strain | Vaccine RCT | M01ZH09 (n = 31)a Ty21a (n = 30)a Placebo (n = 30) |
Ty21a (3 dose) or M01ZH09 (single dose) vaccines compared with control Challenge dose 1–5 × 104 CFU 28 days after vaccination |
[19] |
| 3 | OVG2013/07 NCT02100397 (P1) |
S. Paratyphi A NVGH308 strain | Observational | … | Dose-finding study High dose: 1–5 × 103 CFU (n = 20) Low dose: 0.5–1 × 103 CFU (n = 20) |
[14] |
| 4 | OVG2014/08 NCT02324751 (VAST) |
S. Typhi Quailes strain | Vaccine RCT | Vi-PS (n = 35)a Vi-TT conjugate (n = 37)a Placebo (n = 31) |
Single dose Vi-PS (Typhim Vi®, Sanofi Pasteur) or Vi-TT (TypbarTCV®, Bharat Biotech) vaccines compared with control MenACWY Challenge dose 1–5 × 104 CFU 28 days after vaccination |
[13] |
| 5 | OVG2014/01 NCT02192008 (PATCH) |
S. Paratyphi A NVGH308 strain S. Typhi Quailes strain |
RCT | … | Naive challenge (S. Typhi and S. Paratyphi) vs rechallenge (homotypic and heterotypic) S. Typhi challenge dose 1–5 × 104 CFU S. Paratyphi challenge dose 1–5 × 103 CFU |
[20] |
| 6 | OVG2016/03 NCT03067961 (TYGER) |
S. Typhi Quailes strain and S. Typhi SB6000 (TT deficient) |
RCT | … | Wild-type S. Typhi Quailes strain (n = 20) SB6000 TT negative strain (n = 20) Challenge dose 1–5 × 104 CFU |
[21] |
Abbreviations: CFU, colony-forming unit; PS, polysaccharide; RCT, randomized controlled trial: TT, tetanus-toxoid.
aVaccinated and completed challenge.
All challenge studies followed comparable protocols, detailed elsewhere (Supplementary Materials) [13, 18, 19, 20, 21, 22]. Briefly, healthy adults drank 120 mL of sodium bicarbonate solution prior to challenge. After challenge, daily blood and stool cultures were collected for 14 days. Participants were diagnosed with enteric fever if they had fever of 38oC for ≥12 hours and/or S. (Para)Typhi bacteremia detected ≥72 hours from challenge. Antibiotics were initiated at the time of diagnosis or at day 14 for those not diagnosed. All participants were effectively treated, and no chronic carriers were identified.
Typhoid challenge was performed using the Qualies strain (genotype 3.1.0) [15, 23]. Paratyphoid challenge was performed using the S. Paratyphi A NVGH308 strain [14]
Stool Culture
Stool cultures were performed according to local procedures based on national guidance at Oxford University Hospital National Health Service (NHS) Foundation Trust (Supplementary Materials) [24].
Antibody Measures
Anti-Vi immunoglobulin (Ig) G titers were measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit (VaccZyme, The Binding Site, Birmingham, UK) according to the manufacturer’s guidelines [13]. IgG and IgA isotype responses to S. Typhi lipopolysaccharide (LPS) (Sigma L2387), S. Typhi Hd (University of Maryland 01-CVD0150622-01), S. Paratyphi A O:2 (GSK Vaccines for Global Health) [25], and S. Paratyphi Ha (University of Maryland CVD 1902D lot CVD141113-01) antigens were measured with an in-house ELISA in serum samples collected immediately prior to challenge [14, 18, 19].
Statistical Analyses
Stool culture data were combined in mixed effects logistic regression models, which included participant-specific random intercepts to account for the multiple samples per person. Models were adjusted for the vaccine received, study, and, where applicable, challenge dose (high or low). “Day” was included in the model as a categorical factor to allow the odds of stool shedding to vary by day.
An overall interaction term (day-by-vaccine) was tested to determine if vaccination altered the pattern of shedding over time. An interaction term for day-by-diagnosis status was used to compare the pattern of shedding in those who were diagnosed with enteric fever and those who remained undiagnosed at day 14.
The linear predictor from the model was exported for each participant for each day and converted into a probability by taking the inverse logit. These probabilities are presented in figures with a loess smooth to illustrate the findings from the logistic regression models. Odds ratios (ORs) presented from logistic regression models represent the ratio of the odds of shedding in comparative groups on average across all 14 days.
All models were fitted in SAS version 9.4, and code is displayed in the Supplementary Materials.
RESULTS
In total, 4934 stool samples from 430 participants were analyzed; 3698 samples were from S. Typhi challenge participants and 1236 samples were from S. Paratyphi challenge participants (Table 2). In total, 14.5% of stool samples from S. Typhi-challenged participants were positive compared with 7.5% of samples from those challenged with S. Paratyphi A.
Table 2.
Stool Microbiology by Challenge Agent and Study
| Study | Number of Participants | Number of Participants With ≥1 Positive Stool Sample | Number of Days Shedding (Median [Interquartile Range]) |
Number of Samples from Salmonella Typhi-challenged Participants | Number of Samples from Salmonella Paratyphi A-challenged Participants | Total | |||
|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | ||||||
| 1 | OVG2009/10 (T1) |
40 | 22 (55%) | 1 [0–2] | 520 | 54 (9%) | ... | ... | 574 |
| 2 | OVG2011/02 (T2) |
92 | 72 (78%) | 2 [1–4] | 908 | 217 (19%) | ... | ... | 1125 |
| 3 | OVG2013/07 (P1) |
40 | 17 (43%) | 0 [0–1] | ... | ... | 510 | 36 (7%) | 546 |
| 4 | OVG2014/08 (VAST) |
103 | 64 (62%) | 1 [0–2] | 842 | 126 (13%) | ... | ... | 968 |
| 5 | OVG2014/01 (PATCH) |
115 | 56 (45%) | 0 [0–2] | 562 | 84 (13%) | 633 | 57 (8%) | 1336 |
| 6 | OVG2016/03 (TYGER) |
40 | 24 (60%) | 1 [0–2] | 329 | 56 (15%) | ... | … | 385 |
| Total | 430 | 255 (59%) | 3161 | 537 (14.5%) | 1143 | 93 (7.5%) | 4934 | ||
Differences in S. Typhi Stool Shedding According to Diagnosis Status
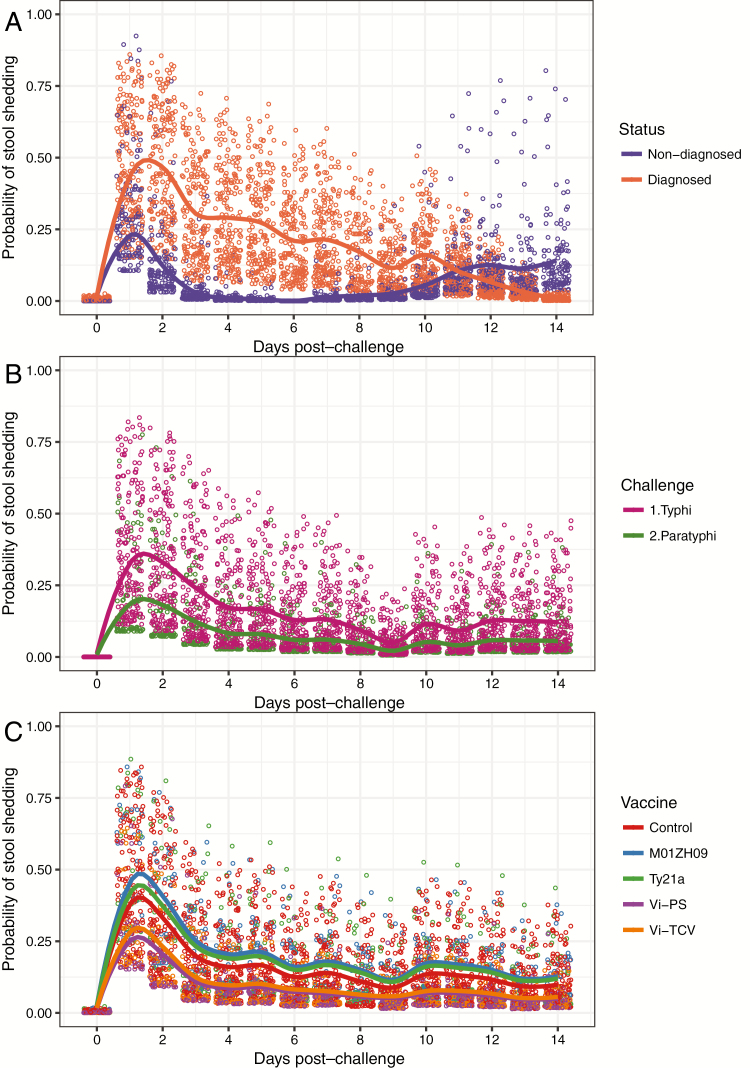
There were 331 participants challenged with S. Typhi, of whom 186 (56.2%) met the diagnostic criteria for typhoid fever [18]. The highest incidence of shedding was observed on day 1 and day 2 after challenge. The pattern of shedding over time was significantly different between those diagnosed and those who did not develop enteric fever (P < .0001 day-by-diagnosis interaction). The odds of shedding 1 day after challenge were 2.5 times greater in those who were later diagnosed than in those who remained undiagnosed (OR, 2.49; 95% confidence interval [CI], 1.32–4.69; P = .0049). On day 2 odds were 9 times higher in those later diagnosed (OR, 8.93; 95% CI, 3.86–20.61; P < .0001) and on day 3 were 23 times higher (OR, 22.59; 95% CI, 6.83–74.7; P < .0001). In the second week after challenge, many diagnosed participants had commenced antibiotics, and shedding ceased. In the undiagnosed participants, increased shedding was observed from day 10 onward, until those participants also received treatment on day 14 (Figure 1A). A similar pattern was observed in historic challenge studies (Supplementary Figure S1).
Figure 1.
Probability of bacterial shedding in stool by day in controlled human infection enteric fever studies. (A) N = 331 participants challenged with Salmonella Typhi according to diagnosis status. Nondiagnosed N = 145, diagnosed N = 186. (B) Unvaccinated participants exposed to oral challenge with S. Typhi (N = 197) or S. Paratyphi (N = 109) bacteria. (C) Vaccinated and unvaccinated participants challenged with 1–5 × 104 colony-forming units S. Typhi wild-type bacteria according to vaccine received. Control vaccine or no vaccine (N = 158); M01ZH09, experimental typhoid vaccine (N = 32); Ty21a, live attenuated oral typhoid vaccine (N = 30); Vi-PS, Vi-polysaccharide typhoid vaccine (Typhim Vi®, Sanofi Pasteur; N = 35); Vi-TT, Vi-tetanus toxoid conjugate vaccine (TypbarTCV®, Bharat Biotech; N = 37)
Differences in Stool Shedding After S. Typhi or S. Paratyphi Challenge in Unvaccinated Participants
There were 197 unvaccinated participants exposed to S. Typhi and 109 unvaccinated participants exposed to S. Paratyphi. The odds of shedding in participants exposed to S. Typhi were twice as high as in those exposed to S. Paratyphi (OR, 1.97; 95% CI, 1.00–3.88; P = .049). A sensitivity analysis excluding low-dose challenge gave a very similar estimate (OR, 1.97; P = .044). The dose received was nonsignificant in the model (Figure 1B).
Effect of Vaccination on Stool Shedding After S. Typhi Challenge
Data from 5 S. Typhi studies were analyzed. Participants challenged with a low dose or a genetically modified S. Typhi were excluded [21].
Participants who received Vi-PS or Vi-TT vaccine had lower rates of shedding than unvaccinated participants (OR, 0.34; 95% CI, 0.15–0.77; P = .010 and OR, 0.41; 95% CI, 0.19–0.91; P = .029 for Vi-PS and Vi-TT, respectively). In Ty21a vaccine recipients, there was a nonsignificant reduction in shedding compared with unvaccinated controls (OR, 0.57; 95% CI, 0.27–1.20; P = .14; Figure 1C). There were no differences between unvaccinated participants and those who received the M01ZH09 vaccine or between any other groups after adjusting for study-specific variation. The vaccine-by-day interaction term was nonsignificant, showing that the pattern of shedding over time was similar for all vaccines even though the amount of shedding differed.
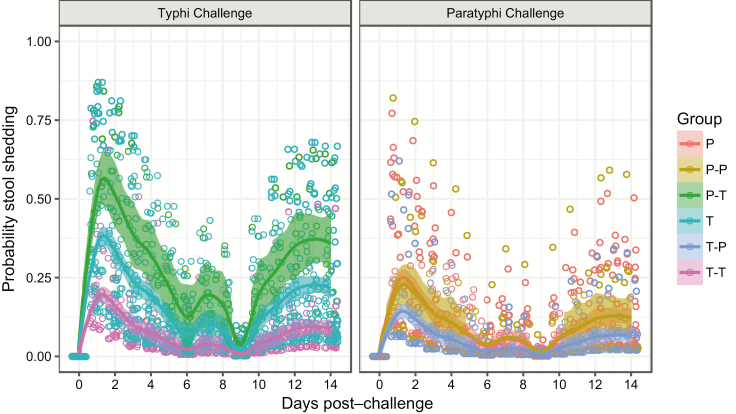
Effect of Previous Exposures
Participants previously exposed to S. Typhi had less stool shedding than previously unexposed individuals (T-T vs T, OR, 0.33; 95% CI, 0.1–0.8; P = .016; Figure 2). For those participants who received S. Typhi challenge, previous exposure to S. Paratyphi significantly increased stool shedding compared with previous S. Typhi exposure (P-T vs T-T: OR, 7.5; 95% CI, 2.0–28.4; P = .003) and nonsignificantly increased the rate of bacterial shedding compared with previously unexposed individuals (P-T vs T: OR, 2.5; 95% CI, 0.8–8.0; P = .125). For those receiving S. Paratyphi challenge, there were no differences between groups, and a lower rate of shedding in general was observed in comparison with those exposed to S. Typhi challenge.
Figure 2.
Bacterial shedding in stool after S. Typhi or S. Paratyphi challenge, according to previous exposure. P = S. Paratyphi naive (n = 39); P-P = S. Paratyphi rechallenge after previous S. Paratyphi exposure (n = 13); P-T = S. Typhi challenge after previous S. Paratyphi exposure (n = 10); T = S. Typhi challenge in S. Typhi naive participants (n = 71); T-P = S. Paratyphi challenge after previous S. Typhi exposure (n = 27); T-T = S. Typhi rechallenge after previous S. Typhi exposure (n = 27). See Supplementary Table S1 for model outputs.
Variation in Shedding According to Age and Sex
In 197 unvaccinated participants (34% female), the median number of samples provided was 11 (interquartile range [IQR], 8–14) for women and 12 (IQR, 9–15) for men. Men had proportionately more positive stool samples than women (OR, 1.78; 95% CI, 1.10–2.87; P = .0187). Age was not significantly related to shedding (P = .795).
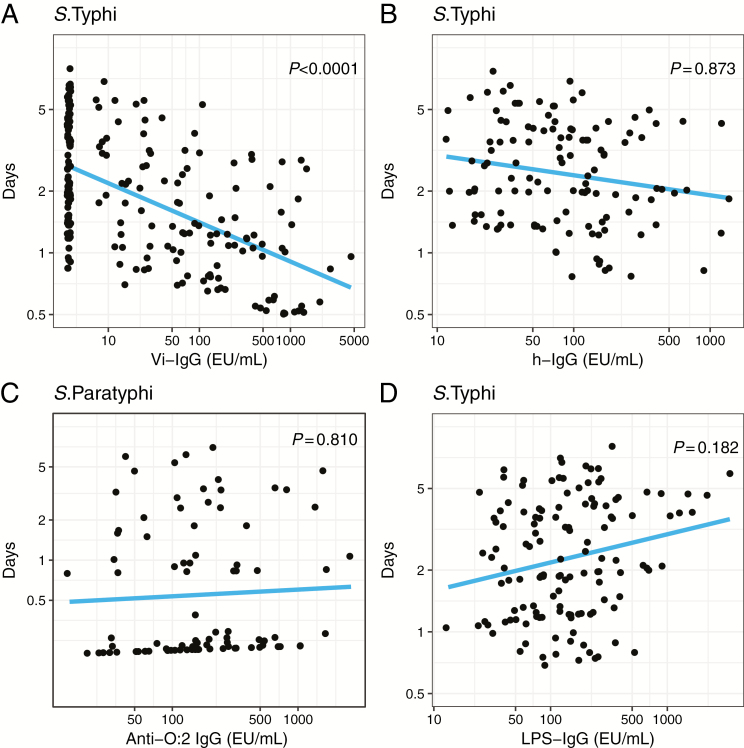
Antibody Levels Prior to Challenge
Higher anti-Vi IgG antibody titers prior to challenge were associated with less bacterial shedding after challenge with S. Typhi (P < .0001). There was no relationship between anti-LPS IgG or anti-Hd IgG and shedding after challenge with either S. Typhi or S. Paratyphi (Figure 3). IgA and IgM responses to S. Typhi LPS and Hd antigens were nonsignificant (Supplementary Figure S2).
Figure 3.
Relationship between days of bacterial shedding in stool after Salmonella Typhi or S. Paratyphi challenge and antibody levels prior to challenge. (A) Anti-Vi immunoglobulin (Ig) G prior to challenge with S. Typhi. (B) Anti-Hd IgG prior to challenge with S. Typhi. (C) Anti-O:2 IgG prior to challenge with S. Paratyphi. (D) Anti-S. Typhi lipopolysaccharide IgG prior to challenge with S. Typhi. Days: y-axis represents the predicted total number of days of stool shedding (out of 14). The total number of days was determined from the logistic regression model by summing across all 14 days the predicted probability for each day for each person. Abbreviation: Ig, immunoglobulin.
DISCUSSION
Shedding of S. Typhi and S. Paratyphi bacilli is a prerequisite for onward transmission in these human-restricted infections. This is the first comprehensive analysis of bacterial shedding from almost 5000 stool samples taken after deliberate challenge of healthy volunteers with S. Typhi or S. Paratyphi. We demonstrate that stool shedding is more common in individuals who meet the case definition of enteric fever (fever 38oC for ≥12 hours and/or S. (Para)Typhi bacteremia), but shedding can also occur in the absence of bacteremia or clinical symptoms of disease. Vaccination with Vi-PS or Vi-TT significantly reduced stool shedding of S. Typhi following controlled human infection, suggesting that these vaccines are likely to reduce onward transmission of disease. The decreased shedding following vaccination with the live-attenuated Ty21a vaccine was not significant, possibly due to the small sample sizes available for these comparisons and moderate protective efficacy of Ty21a in the challenge model. In earlier challenge studies with Ty21a involving larger numbers of participants who received a freshly harvested formulation of vaccine, shedding of S. Typhi was significantly reduced in recipients of the live oral vaccine [9].
The effects of Vi-PS or Vi-TT vaccination on indirect protection and stool shedding are poorly understood. A cluster randomized control trial in Kolkata, India, demonstrated that Vi-PS vaccination can result in indirect protection against typhoid fever in unvaccinated individuals resident in population clusters randomized to Vi-PS vaccine [11]. However, indirect protection of Vi-PS was not observed in another cluster randomized trial conducted in Karachi, Pakistan. One difference is that the trial in Kolkata vaccinated adults as well as children [12].
The reduction in stool shedding observed in individuals vaccinated with a Vi-TT conjugate vaccine is an important finding. To date, there are no completed cluster randomized trials of typhoid conjugate vaccines. However, 1 trial is ongoing in Bangladesh, and individually randomized trials are ongoing in Nepal and Malawi [26]. A previous Vi-conjugate vaccine with Pseudomonas aeruginosa exotoxin A as a carrier protein was shown to have a high vaccine efficacy (VE) in Vietnam (VE, 91.1%; 95% CI, 78.6%–96.5%) [27]. Vi-TT vaccine is highly immunogenic in children [28], with demonstrated efficacy of 54.6%–87.1% in a controlled human infection model (depending on the diagnostic criteria used) [13] and vaccine efficacy of 85% estimated from serological data [29]. In October 2017 the World Health Organization recommended the introduction of typhoid conjugate vaccines for children aged >6 months in typhoid-endemic countries [8]. It remains to be determined to what degree the reduction in shedding associated with Vi-TT vaccination will translate to indirect protection of unvaccinated persons in field studies. If the reduction in shedding translates to indirect protection of nonvaccinees in field settings, the overall effectiveness of Vi-TT conjugate vaccines could be significantly higher than is estimated in challenge studies.
Current models that predict the potential impact of Vi-conjugate vaccines account for this potential indirect protection by assuming that transmission of S. Typhi is reduced in a manner that is proportional to the vaccine efficacy (ie, by preventing infection and hence shedding among a proportion of vaccinated individuals). The results presented here support such assumptions. The protection afforded by Vi-TT against typhoid infection is similar to its effect against stool shedding. When both are expressed as ORs, the effects of Vi-TT against typhoid diagnosis and shedding have largely overlapping CIs (typhoid diagnosis: OR, 0.16; 95% CI, 0.05–0.46 and shedding: OR, 0.41; 95% CI, 0.19–0.91). However, more analysis is needed to examine the relationships between vaccination, stool shedding, and the development of clinical typhoid and how this may vary between the human challenge model and field settings. Additionally, the mechanism by which anti-Vi antibodies prevent stool shedding requires further investigation.
The nonsignificant reductions in shedding with Ty21a vaccine contrast with data from early challenge studies, where Ty21a was associated with a reduction in any stool shedding of S. Typhi from between day 4 and day 30 post-challenge (OR, 0.08; 95% CI, 0.02–0.29) [9]. The differences may result from methodological differences between the Oxford and Maryland challenge studies, including mode of administration, pretreatment with sodium bicarbonate instead of milk, differences in challenge dose (Maryland, 105 organisms; Oxford, 104), and criteria for initiating antibiotics. Of note, Ty21a appeared to provide herd immunity in field studies conducted in Chile [10].
As was observed in early typhoid challenge studies, early shedding increased the likelihood of subsequent development of typhoid fever [15]. Interestingly, we observed an increase in shedding in the undiagnosed group from day 10 onward. It is possible that late shedding represents a harbinger of subsequent bacteremia or fever, such that a proportion of the “undiagnosed” group may have developed enteric fever had the infection not been halted by commencing antibiotics at day 14. Conversely, late, asymptomatic shedding has been described in undiagnosed participants from early challenge studies, where shedding peaked in the second week post-challenge before clearing spontaneously by 6 weeks (in the absence of clinical disease or antibiotic treatment) [15]. These data emphasize that a proportion of individuals exposed to typhoidal Salmonella will act as asymptomatic short-term carriers who transiently shed in the absence of overt clinical disease; this is a factor that should be considered when determining the target population for vaccination campaigns.
In individuals previously challenged with S. Typhi, we detected a significant reduction in the rate of stool shedding compared with naive controls. A single episode of typhoid exposure is thought to confer moderate protection against subsequent clinical disease (estimated at approximately 23% from historical challenge studies [30]), and modeling studies assume that multiple episodes of typhoid exposure are required to induce functional immunity [31]. These are the first data to suggest that prior typhoid exposure significantly affects the pattern of shedding following rechallenge, albeit from a small sample size (n = 27 rechallenged). Interestingly, we observed that individuals previously exposed to S. Paratyphi A had substantially higher rates of shedding when rechallenged with S. Typhi. The reasons for the increased risk of typhoid shedding on heterotypic rechallenge are unclear and are the subject of ongoing studies focusing on the role of secretory IgA [32].
When shedding rates were compared between typhoid and paratyphoid challenge studies, rates of shedding following S. Typhi challenge were twice as high as those following S. Paratyphi A challenge. The higher rate of shedding following typhoid challenge may reflect the higher challenge dose administered in the typhoid model (104 vs 103 colony-forming units), or differences may exist between these serovars in host–pathogen interactions and transmission mechanisms. Several studies in areas coendemic for S. Typhi and S. Paratyphi A have suggested that transmission dynamics and risk factors may differ between the 2 serovars [7, 33, 34]. For example, S. Paratyphi A cases appear to be more spatially dispersed than S. Typhi cases in an urban area [33] and may be particularly associated with foodborne transmission [7, 34]. Improved understanding of paratyphoid shedding and transmission dynamics will be an important consideration in the development of vaccines for paratyphoid fever [35].
There are several limitations to assessing stool shedding using data from human challenge studies. The population enrolled comprises adults from a nonendemic country; shedding dynamics may differ in individuals from endemic countries with prior immune priming or in children, who represent the majority of enteric fever cases (but not necessarily the majority of shedders). In this analysis, data were pooled across 6 studies conducted over 6 years. While all samples were processed in the same laboratory using consistent protocols, study-to-study variation may still exist in the sensitivity of stool testing; thus, “study” was adjusted for in all models. Furthermore, challenge was conducted using only a single strain of S. Typhi (Quailes) or S. Paratyphi A (NVGH308) at a single dose, which may not mirror the shedding dynamics of contemporary circulating strains in Asia and Africa, such as the multidrug-resistant associated H58 (genotype 4.3.1) strain of S. Typhi [36]. These limitations primarily reflect safety considerations required for controlled human challenge, and these data should be interpreted alongside emerging data from surveillance studies [37].
In summary, we have performed the first detailed model of shedding dynamics in the context of controlled typhoid and paratyphoid challenge and provide evidence for efficacy of new and existing typhoid vaccines (Vi-PS and Vi-TT) in reducing rates of shedding. These studies illustrate the value of closely monitored experimental human challenge studies in obtaining novel insights into host–pathogen interactions and microbial dynamics, which can directly inform the disease control efforts for priority pathogens.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. M. M. G., C. J., and T. D. coordinated the clinical studies. M. M. G., C. J., T. D., H. T. B., E. J., P. B., and M. M. collected the data. M. V. conceived the study design and analyzed the data. B. A. provided clinical oversight to all studies. M. M. G. and M. V. wrote the manuscript. A. J. P., M. A. G., A. S., and V. C. designed, oversaw, and obtained funding for study OVG2014/01. A. J. P. supervised the work and obtained funding for the 6 studies. All authors reviewed and edited the manuscript and approved the final version.
Acknowledgments. The authors acknowledge the contribution of all participants who took part in the studies described herein. In addition, the authors acknowledge the following persons and groups: all members of the microbiology laboratory at the Oxford University Hospital NHS Foundation Trust; the University of Maryland School of Medicine for provision of S. Typhi Hd and S. Paratyphi Hd antigens; Dr Laura Martin (GSK Vaccines for Global Health) for provision of the S. Paratyphi NVGH308 challenge strain and O:2 antigen; and Prof William E. Woodward for permission to publish data from his 1975 report on the Maryland challenge studies.
Disclaimer. M. V. and M. M. G. had full access to all study data and had final responsibility for the decision to submit the manuscript. The funders had no involvement in the design and conduct of the studies; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed in this manuscript are those of the authors and do not necessarily reflect the views of the Joint Committee on Vaccination and Immunisation (JCVI), NHS, NIHR, UK the Department of Health, European Medicines Agency (EMA), or the World Health Organization (WHO).
Funding. M. V. is funded by the National Institute for Health Research (NIHR) Doctoral Research Fellowship (DRF-2015-08-048). Studies OVG2009/10 and OVG2011/12 were supported by a Wellcome Trust Strategic Translational Award (grant 092661) and Emergent BioSolutions (challenge strain manufacture, vaccine/placebo supply, and labeling). Study OVG 2013/07 was supported by the Bill & Melinda Gates Foundation (OPP1084259) and the European Vaccine Initiative. Study 2014/08 was supported by the Bill & Melinda Gates Foundation (OPP1084259) and European Commission FP7 grant Advanced Immunization Technologies. Study OVG 2016/03 was funded by the Bill & Melinda Gates Foundation (OPP1126235). Study 2014/01 was funded by the Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement and is also part of the The European & Developing Countries Clinical Trials Partnership (EDCTP2) programme supported by the European Union. The authors acknowledge the support of the NIHR Oxford Biomedical Research Centre and the NIHR Thames Valley and South Midlands Clinical Research Network. V. E. P. is supported by the Bill & Melinda Gates Foundation (OPP1116967, OPP1151153) and the Wellcome Trust (106158/Z/14/Z). A. J. P. is an NIHR senior investigator.
Potential conflicts of interest. A. J. P. has previously conducted studies on behalf of Oxford University funded by vaccine manufacturers, but currently does not undertake industry-funded clinical trials. A. J. P. chairs the UK Department of Health’s Joint Committee on Vaccination and Immunisation and the EMA scientific advisory group on vaccines, is a member of the World Health Organization’s (WHO) Strategic Advisory Group of Experts. He has received grants from the Wellcome Trust, Bill & Melinda Gates Foundation, Medical Research Council, and NIHR Oxford Biomedical Research Centre during the conduct of the study and grants from Okairos and Pfizer outside the submitted work. All other authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ 2004; 82:346–53. [PMC free article] [PubMed] [Google Scholar]
- 2. Crump JA, Mintz ED. Global trends in typhoid and paratyphoid fever. Clin Infect Dis 2010; 50:241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buckle GC, Walker CLF, Black RE. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health 2012; 2:10401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mogasale V, Maskery B, Ochiai RL, et al. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health 2014; 2:e570–80. [DOI] [PubMed] [Google Scholar]
- 5. Antillón M, Warren JL, Crawford FW, et al. The burden of typhoid fever in low- and middle-income countries: a meta-regression approach. PLoS Negl Trop Dis 2017; 11:e0005376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saad NJ, Bowles CC, Grenfell BT, et al. The impact of migration and antimicrobial resistance on the transmission dynamics of typhoid fever in Kathmandu, Nepal: a mathematical modelling study. PLoS Negl Trop Dis 2017; 11:e0005547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karkey A, Thompson CN, Tran Vu Thieu N, et al. Differential epidemiology of Salmonella Typhi and Paratyphi A in Kathmandu, Nepal: a matched case control investigation in a highly endemic enteric fever setting. PLoS Negl Trop Dis 2013; 7:e2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO. Typhoid vaccines: WHO position paper–March 2018. Wkly Epidemiol Rec 2018; 93:477–500. [Google Scholar]
- 9. Gilman RH, Hornick RB, Woodard WE, et al. Evaluation of a UDP-glucose-4-epimeraseless mutant of Salmonella Typhi as a liver oral vaccine. J Infect Dis 1977; 136:717–23. [DOI] [PubMed] [Google Scholar]
- 10. Levine MM, Ferreccio C, Black RE, Tacket CO, Germanier R. Progress in vaccines against typhoid fever. Rev Infect Dis 1989; 11(Suppl 3):S552–67. [DOI] [PubMed] [Google Scholar]
- 11. Sur D, Ochiai RL, Bhattacharya SK, et al. A cluster-randomized effectiveness trial of Vi typhoid vaccine in India. N Engl J Med 2009; 361:335–44. [DOI] [PubMed] [Google Scholar]
- 12. Khan MI, Soofi SB, Ochiai RL, et al. ; DOMI Typhoid Karachi Vi Effectiveness Study Group Effectiveness of Vi capsular polysaccharide typhoid vaccine among children: a cluster randomized trial in Karachi, Pakistan. Vaccine 2012; 30:5389–95. [DOI] [PubMed] [Google Scholar]
- 13. Jin C, Gibani MM, Moore M, et al. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: a randomised controlled, phase 2b trial. Lancet 2017. doi: 10.1016/S0140-6736(17)32149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dobinson HC, Gibani MM, Jones C, et al. Evaluation of the clinical and microbiological response to Salmonella Paratyphi A infection in the first paratyphoid human challenge model. Clin Infect Dis 2017; 64:1066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hornick RB, Greisman SE, Woodward TE, DuPont HL, Dawkins AT, Snyder MJ. Typhoid fever: pathogenesis and immunologic control. 2. N Engl J Med 1970; 283:739–46. [DOI] [PubMed] [Google Scholar]
- 16. Pitzer VE, Bowles CC, Baker S, et al. Predicting the impact of vaccination on the transmission dynamics of typhoid in South Asia: a mathematical modeling study. PLoS Negl Trop Dis 2014; 8:e2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Watson CH, Edmunds WJ. A review of typhoid fever transmission dynamic models and economic evaluations of vaccination. Vaccine 2015; 33(Suppl 3):C42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waddington CS, Darton TC, Jones C, et al. An outpatient, ambulant-design, controlled human infection model using escalating doses of Salmonella Typhi challenge delivered in sodium bicarbonate solution. Clin Infect Dis 2014; 58:1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Darton TC, Jones C, Blohmke CJ, et al. Using a human challenge model of infection to measure vaccine efficacy: a randomised, controlled trial comparing the typhoid vaccines M01ZH09 with placebo and Ty21a. PLoS Negl Trop Dis 2016; 10:e0004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gibani M, Jin C, Thomaides-Brears H, et al. Investigating systemic immunity to typhoid and paratyphoid fever: characterising the response to re-challenge in a controlled human infection model. Open Forum Infect Dis 2017; 4:S227–8. [Google Scholar]
- 21. ClinicalTrials.gov Identifier: NCT03067961. Investigating typhoid fever pathogenesis-full text view-ClinicalTrials.gov Available at: https://clinicaltrials.gov/ct2/show/NCT03067961?term=TYGER&rank=1. accessed 17 March 2017.
- 22. McCullagh D, Dobinson HC, Darton T, et al. Understanding paratyphoid infection: study protocol for the development of a human model of Salmonella enterica serovar Paratyphi A challenge in healthy adult volunteers. BMJ Open 2015; 5:e007481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wong VK, Baker S, Connor TR, et al. ; International Typhoid Consortium An extended genotyping framework for Salmonella enterica serovar Typhi, the cause of human typhoid. Nat Commun 2016; 7:12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Standards Unit, Microbiology Services PHE. UK standards for microbiology investigations–investigation of faecal specimens for enteric pathogens. Bacteriology 2014; B30:1–41. [Google Scholar]
- 25. Micoli F, Rondini S, Gavini M, et al. A scalable method for O-antigen purification applied to various Salmonella serovars. Anal Biochem 2013; 434:136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meiring JE, Gibani M; TyVAC Consortium Meeting Group: The Typhoid Vaccine Acceleration Consortium (TyVAC): vaccine effectiveness study designs: accelerating the introduction of typhoid conjugate vaccines and reducing the global burden of enteric fever. Report from a meeting held on 26–27 October 2016, Oxford, UK. Vaccine 2017; 35:5081–8. [DOI] [PubMed] [Google Scholar]
- 27. Lin FY, Ho VA, Khiem HB, et al. The efficacy of a Salmonella Typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med 2001; 344:1263–9. [DOI] [PubMed] [Google Scholar]
- 28. Mohan VK, Varanasi V, Singh A, et al. Safety and immunogenicity of a Vi polysaccharide-tetanus toxoid conjugate vaccine (Typbar-TCV) in healthy infants, children, and adults in typhoid endemic areas: a multicenter, 2-cohort, open-label, double-blind, randomized controlled phase 3 study. Clin Infect Dis 2015; 61:393–402. [DOI] [PubMed] [Google Scholar]
- 29. Voysey M, Pollard AJ. Seroefficacy of Vi polysaccharide-tetanus toxoid typhoid conjugate vaccine (Typbar TCV). Clin Infect Dis 2018; 67:18–24. [DOI] [PubMed] [Google Scholar]
- 30. Dupont HL, Hornick RB, Snyder MJ, Dawkins AT, Heiner GG, Woodward TE. Studies of immunity in typhoid fever. Protection induced by killed oral antigens or by primary infection. Bull World Health Organ 1971; 44:667–72. [PMC free article] [PubMed] [Google Scholar]
- 31. Saul A, Smith T, Maire N. Stochastic simulation of endemic Salmonella enterica serovar Typhi: the importance of long lasting immunity and the carrier state. PLoS One 2013; 8:e74097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moor K, Diard M, Sellin ME, et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature 2017; 544:498–502. [DOI] [PubMed] [Google Scholar]
- 33. Baker S, Holt KE, Clements AC, et al. Combined high-resolution genotyping and geospatial analysis reveals modes of endemic urban typhoid fever transmission. Open Biol 2011; 1:110008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vollaard AM, Ali S, van Asten HA, et al. Risk factors for typhoid and paratyphoid fever in Jakarta, Indonesia. JAMA 2004; 291:2607–15. [DOI] [PubMed] [Google Scholar]
- 35. Martin LB, Simon R, MacLennan CA, Tennant SM, Sahastrabuddhe S, Khan MI. Status of paratyphoid fever vaccine research and development. Vaccine 2016; 34:2900–2. [DOI] [PubMed] [Google Scholar]
- 36. Wong VK, Baker S, Pickard DJ, et al. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat Genet 2015; 47:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Darton TC, Meiring JE, Tonks S, et al. ; STRATAA Study Consortium The STRATAA study protocol: a programme to assess the burden of enteric fever in Bangladesh, Malawi and Nepal using prospective population census, passive surveillance, serological studies and healthcare utilisation surveys. BMJ Open 2017; 7:e016283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.