Abstract
Background
Klinefelter syndrome (KS) is one of the major causes of nonobstructive azoospermia (NOA). Microdissection testicular sperm extraction (micro‐TESE) is often performed to retrieve sperm. Infertility specialists have to care for KS patients on a lifelong basis.
Methods
Based on a literature review and our own experience, male infertility treatment and KS pathophysiology were considered on a lifelong basis.
Main findings
Patients diagnosed early often have an increased number of aberrant X chromosomes. Cryptorchidism and hypospadias are often found, and surgical correction is required. Cryopreservation of testicular sperm during adolescence is an issue of debate because the sperm retrieval rate (SRR) in KS patients decreases with age. The SRR in adult KS patients is higher than that in other patients with NOA; however, low testosterone levels after micro‐TESE will lower the general health and quality of life. KS men face a number of comorbidities, such as malignancies, metabolic syndrome, diabetes, cardiovascular disease, bone disease, and immune diseases, which ultimately results in increased mortality rates.
Conclusion
A deeper understanding of the pathophysiology of KS and the histories of KS patients before they seek infertility treatment, during which discussions with multidisciplinary teams are sometimes needed, will help to properly treat these patients.
Keywords: comorbidity, Klinefelter syndrome, microdissection testicular sperm extraction, nonobstructive azoospermia, pediatrics, testosterone
1. INTRODUCTION
In 1942, Klinefelter and Albright reported that nine men had a “Syndrome characterized by gynecomastia, aspermatogenesis without Leydigism and increased excretion of follicle‐stimulating hormone”.1 Karyotype analysis elucidated that this syndrome was caused by an extra X chromosome (XXY). The classic form (nonmosaic type) of Klinefelter syndrome (KS) is observed in one per 660 births, with up to 3%‐4% occurring in infertile men and 10%‐12% occurring in nonobstructive azoospermia (NOA). A study on the KS prevalence in Japan found a lower prevalence at 60 per 100 000,2 indicating the presence of racial differences. KS is the most common sex chromosome abnormality associated with male infertility,3, 4 and men with KS have been shown to be potentially fertile by using a testicular sperm and intracytoplasmic sperm injection (ICSI) protocol.6 Approximately 85% of KS cases are due to a single aberrant X chromosome (47,XXY), whereas the remaining 15% display multiple aneuploidies (48,XXXY; 48,XXYY; 49,XXXXY), causing the development of more severe phenotypes, which are usually diagnosed during prenatal and infantile periods as abnormalities of the external genitalia.7 A structurally abnormal X chromosome (eg, 47,iXq,Y) or mosaicisms (eg, 47,XXY/46,XY) are also included in KS. The origin of the aberrant X chromosome in KS, of either paternal or maternal origin, and how the presence of a supernumerary X chromosome causes the phenotype and morbidity in KS are not fully understood.
The multiple aneuploidies causing the abnormalities of external genitalia and children with KS with severe intellectual and behavioral problems are relatively rare compared with other cases of male infertility, usually NOA. These are symptoms often present among men with KS, and in most of the cases, lead to its diagnosis during the workup for infertility. Less than 10% of these patients are diagnosed before puberty.3 On the other hand, we should not forget that KS is also responsible for general comorbidities related to metabolic and psychological disorders and risks of specific malignancies, which, in part, are caused by low testosterone in KS patients. As microdissection testicular sperm extraction (micro‐TESE) has been shown to be an ideal sperm retrieval method8 used by many urologists and, in Japan, gynecologists, the chances of low‐testosterone‐related symptoms and comorbidities are increasing. The testicular size is significantly smaller and serum testosterone levels are lower in KS patients compared to those in other NOA cases; thus, a post‐micro‐TESE decline in testosterone is enhanced in KS patients.9 Unfortunately, we often see many KS patients who claim to have impaired sexual function, general fatigue, and comorbidities after micro‐TESE, and they are not properly followed up after micro‐TESE irrespective of the results of sperm retrieval. Diagnosing KS should be the beginning of a strict follow‐up of general health; however, reproductive medicine has caused new and severely low levels of testosterone in men with KS in the era of assisted reproductive technologies (ART).
Data related to infertility treatment are apt to be focused on the sperm retrieval of KS. The purposes of this review are to understand the background pathophysiology of KS patients, including how they grew up, which symptoms occurred before they were treated for infertility, what information is available after their infertility treatment, and finally how they should be managed for lifelong basis.
2. KS AND INFERTILITY TREATMENT
For a long time, KS was considered to be an absolute infertility condition once diagnosed. In 1996, Tournaye et al10 reported a successful testicular sperm retrieval by conventional TESE in men with KS. One year later, Palermo et al6 reported the first pregnancy and live birth resulting from a KS father after TESE/ICSI. A recent review reported by Majzoub et al11 indicated that publications from many different centers documented sperm retrieval rates (SRRs) in KS adults of approximately 50%‐70%, which are higher than those of general NOA cases, and reported excellent pregnancy rates and healthy 46,XY or 46,XX offspring. A recent meta‐analysis has shown an overall SRR of approximately 40%, ranging from 1% to 50%, in both conventional TESE and micro‐TESE.12 These results indicate that micro‐TESE, but not conventional TESE, is an ideal method to retrieve sperm from men with KS. A survey conducted on Dutch patients reported that the majority of KS patients and their partners would like to have a positive attitude toward TESE/ICSI to conceive a child.13
The age at micro‐TESE is the only parameter to predict successful sperm retrieval. Okada et al14 reported that the SRR was 81% in patients 25‐29 years of age, 73% in patients 30‐34 years of age, 25% in patients 35‐39 years of age, and 22% in men 40‐44 years of age, and proposed that micro‐TESE should be performed before 35 years of age in men with KS. Data from the Cornell group showed that the SRR in men with KS was 71% in the 22‐ to 30‐year age‐group, 86% in the 31‐ to 35‐year age‐group, and 50% in the 36‐ to 50‐year age‐group. In fact, age affects the SRR, but the data are not bleak.
The hormonal pattern and testicular volume have been advocated as possible prognostic factors for successful SRR in KS patients.5, 15, 16 Rohayem et al18 reported that the combination of total serum testosterone above 7.5 nmol/L and LH levels below 17.5 U/L resulted in higher retrieval rates of spermatozoa by micro‐TESE in both adolescents and adults with KS. Similar results were more recently reported by Cissen et al19 Interestingly, Rohayem et al18 did not document any clinical difference in nonmosaic KS patients with or without spermatozoa in their seminal fluid. The lack of prognostic value of the FSH levels might be related to the low inhibin B levels (which is almost undetectable during early puberty) in all patients with KS, which does not allow for the negative feedback of FSH secretion.20
It should be recognized that postoperative testicular damage leading to a decrease in testicular function has been described as a complication of micro‐TESE. The transient but statistically significant decrease in testosterone levels indicates that men are at risk of developing hypogonadism after micro‐TESE, but there is insufficient evidence for whether patients actually experience clinical symptoms in the case of decreased serum testosterone levels. One apparent parameter to predict post‐micro‐TESE hypogonadism is KS,9 and a recent meta‐analysis has shown that men with KS and men with NOA had the strongest decreases in total testosterone levels at 6 months after TESE.21 The largest disaster regarding this issue is that a majority of men with KS are not properly followed up after micro‐TESE, which will be addressed in another section in this review.
3. PRENATAL, INFANTILE, AND PEDIATRIC PERIODS
The opportunity for physicians to diagnose and manage KS may occur even during the prenatal period, as the diagnosis is sometimes established by prenatal genetic information. The recent development of noninvasive prenatal screening is anticipated to increase the number of couples seeking counseling; however, a discussion on whether to abort has never occurred in KS cases.
Studies in KS infants have found that the postnataltemporary surge in gonadotropins (“mini‐puberty”) may be accompanied by low testosterone levels22, 23 or normal testosterone levels.24, 25 Simultaneously, FSH levels increase at 2‐3 months after birth, followed by a subsequent rapid decline.25 This early exposure of androgens is severely disturbed with an increase in the number of X chromosomes. Genital anomalies (micropenis, undescended testis, bifid scrotum, and hypospadia) might present at birth, but whether they are due to the effects of supernumerary X chromosome(s) or androgen deficiency during the fetal period remains undetermined. Figure 1 shows a case of a KS baby with 49,XXXXY that was referred to us for the evaluation of sex development disorders. He underwent bilateral orchiopexy and two‐stage hypospadia repair, and he can now void at standing position. On the other hand, individuals with mosaic KS tend to present with milder symptoms.
Figure 1.
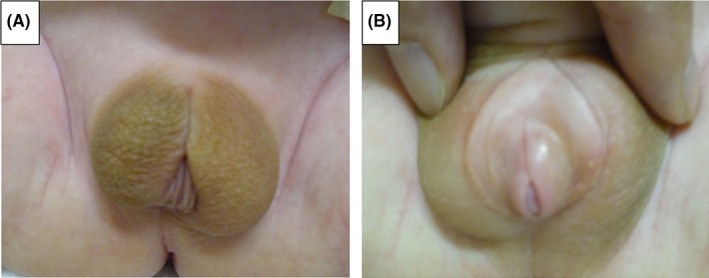
Photographs of proximal hypospadias and penoscrotal disposition in a newborn with a 49,XXXXY karyotype are shown (A). The scrotum is raised, and a small penis is observed (B). This patient was managed with bilateral orchiopexy and a 2‐stage hypospadias repair with a simultaneous correction of a penoscrotal transposition repair
Basically, testosterone‐related symptoms do not occur during infancy or early pediatric periods. Signs and symptoms appearing during this period, such as longer legs, disturbance of speech and cognition,adaptation problems, attention deficits, and social skill impairments,26, 27 have been attributed to the genetic abnormality rather than to hypogonadism. As a result, men with KS are prone to learning difficulties, and their academic and professional status achievements are reported to be inferior to those without KS28; however, this finding has not been fully investigated, and men with KS who visit our infertility clinic, of course they are only limited cases of KS, are always highly educated and talented. The management of children with KS warrants the collaboration of a multidisciplinary team consisting of pediatricians as well as behavioral specialists to ameliorate the developmental defects in early life.29 In addition, an echocardiographic study should be performed to reveal congenital cardiovascular abnormalities.30
Although multidisciplinary treatment for each child with KS should be administered and testosterone may not play a major role in the treatment of KS during this period, there are some anticipated benefits of testosterone replacement therapy (TRT) on the improvement of the energy level, attention span, mood, and cardiac health of young KS patients.31, 32 Recently, two reports have indicated that early TRT may be beneficial for developmental and behavioral issues in boys with KS without serious adverse effects.33, 34 However, there is concern that TRT might cause obstructive sleep apnea and venous thromboembolism.35, 36 In addition, there is evidence that TRT in boys with KS is not as effective as that observed in males with a normal karyotype, at least regarding body proportions and bone mineral density (BMD).37, 38 Studies that apply population‐based genetic screening should be performed to investigate a proper evaluation of the costs and benefits of such an approach in KS patients.39 Randomized controlled trials are also needed to evaluate the efficacy of TRT on different aspects of the symptoms, to determine optimal dose regimens and, most importantly, to prevent the impairment of spermatogenesis or to stimulate future spermatogenesis in boys with KS.40 In this situation, psychological aspects regarding their future reproductive function should be taken into consideration to avoid negative impacts on reproductive health, and follow‐up should be performed by a specialized team consisting of pediatricians, adult endocrinologists/andrologists, and psychologists during the transition from pediatric and adolescent periods to adulthood.
4. ADOLESCENT PERIOD
Although the onset of puberty in KS patients occurs at the same time as that in normal boys, hypogonadism remains silent until pubertal onset because testosterone levels are usually sufficient for satisfactory development of the body and genitalia.41, 42 Additionally, the severity of low‐testosterone‐related symptoms is mild in KS patients during the adolescent period, unlike in male hypogonadotropic hypogonadism. Furthermore, the negative feedback elevation of LH activates aromatase, resulting in an increase in estradiol, which may contribute to the development of gynecomastia.43 At puberty, only a few patients notice overt hypogonadism signs, such as poor muscle development, a short penis, and a lack of or sparse pubic, axillary, and facial hair. Low‐to‐normal testosterone levels contribute to the development of tall stature and disturb the ratio between the upper and lower skeletal segments.44 On the other hand, Bojesen et al reported that 65%‐85% of KS patients present overt hypogonadism after the age of 25,45 and this number might differ among countries and according to the circumstances surrounding KS patients.
Along with LH, FSH is increased via the negative feedback mechanism of testosterone, estradiol, and inhibin B, whichultimately cause the hypergonadotropic levels observed in adult KS patients.46 Additionally, we suspect that some disturbances in testicular development occur during this period. The testes of KS adolescents can develop to a volume of 6 mL due to the proliferation and maturation of Sertoli and Leydig cells,47 which is considered to be normal initial testicular development. Rising intratesticular testosterone levels are subsequently followed by an accelerating decline in germ cells, hyalinization of the tubules, degeneration of Sertoli cells, and hyperplasia of Leydig cells,47 resulting in the loss of testicular volume and a decrease in serum testosterone levels. It is still unclear why the rise in serum or intratesticular testosterone during puberty is associated with accelerated destruction of the seminiferous tubules during this period.48 Lue et al49 suggested that germ cell defects occurred secondary to a defect in spermatogonial migration during the postnatal period and not the prenatal period in XXY mice. On the other hand, normal spermatogenesis in KS can potentially be explained by the following three mechanisms: (a) Occult intratesticular mosaicism in which 46,XY spermatogonia are able to differentiate normally into mature sperm50; (b) Spermatogenesis arising from 47,XXY spermatogonia. It has previously been demonstrated that 47,XXY spermatogonia and spermatocytes are able to enter the spermatogenic process, leading to the formation of mature sperm51; and (c) Active repair of testicular stem cell renewal via the silencing or loss of the extra X chromosomes.52 This phenomenon causes the presence of ejaculate sperm during the adolescent period in some KS patients, which leads to the hypothesis that SRR by TESE might be superior during the adolescent period rather than in the adult period to preserve future fertility potential.
5. IS TESE DURING ADOLESCENCE EFFECTIVE IN KS PATIENTS?
Limited data suggest that sperm production in men with KS, as determined by the results of TESE, is rapidly impaired after a certain age (approximately 35 years of age), and it might be better to retrieve sperm as soon as the diagnosis is made. This suggestion was generated from data reported by Okada et al.14 In their study, the SRR in 25 patients >35 years of age was only 23%. On the other hand, data from Cornell University showed that the SRR in men with KS was 71% in the 22‐ to 30‐year age‐group, 86% in the 31‐ to 35‐year age‐group, and 50% in the 36‐ to 50‐year age‐group. In our experience, the SRR in 84 men with KS was 61% (11/18) in the 21‐ to 30‐year age‐group, 55% (21/38) in the 31‐ to 35‐year age‐group, 52% (14/27) in the 36‐ to 50‐year age‐group, and 100% (1/1) in 52‐year‐old men. Based on these results, spermatogenesis in men with KS declines gradually and not rapidly. Taking these results together with the biological information that progressive hyalinization of seminiferous tubules is observed after puberty in men with KS,47, 48 it has been suggested that performing earlier TESE procedures might result in better SRR results.17 A meta‐analysis has shown that successful SRR in KS patients is independent of age.12
Several studies have reported the presence of sperm by ejaculation in adolescents and young adults with KS,15, 53, 54 and these sperm should be cryopreserved for future treatment. However, problems arise in KS adolescents who have never performed masturbation or whose semen analysis results in azoospermia. In this situation, TESE is an option for evaluating spermatogenesis and sperm retrieval. In 2001, Damani et al56 first reported the results of TESE in a 15‐year‐old adolescent to preserve fertility before TRT for the symptoms of hypogonadism. In 2004, Wikstrom et al47 performed a single‐site testis biopsy for histologic analysis in 14 adolescents with KS, which is the same procedure used for conventional TESE, and reported that no mature sperm were observed in any tissue sections. In this study, a few types of Ap and Ad spermatogonia were found in seven boys aged 10‐12.5 years, whereas no spermatogonia of any type were detected in the other seven boys aged 11.7‐14 years, and they concluded that diploid germ cells vanished in early puberty in boys with KS.47 In 2012, Gies et al54 reported data from testicular biopsy in seven boys aged 10.2‐15.6 years, and they could not find any mature sperm; however, they could identify spermatogonia upon histologic analysis in four of the boys. In 2013, Rives et al57 published their results in five adolescents with KS who underwent bilateral conventional TESE and noted that sperm were found in a 16‐year‐old boy, elongated spermatids and spermatocytes were found in a 15.5‐year‐old boy, and no germ cells were found in the remaining three boys aged 15‐16.5 years. In the same year, Van Saen et al reported that no mature sperm were found in adolescents boys with KS, but 72% (5/7) of the patients, boys with KS from 13 to 16 years of age, presented with spermatogonia. The overall number of spermatogonia in the boys with KS was significantly reduced compared with that in normal adolescent boys.58, 59
The micro‐TESE procedure performed in adolescent boys with KS was first performed by a Cornell group and reported by Mehta et al in 2013. Ten KS patients aged 14‐22 years underwent micro‐TESE, and mature sperm were found in seven patients (SRR: 70%).60 A subsequent publication from the same institution showed a 65% retrieval rate in 127 KS adult men, which was the same rate of success for adolescents and adults.61 Plotten et al62 investigated the largest series to date comparing SRRs at the same institution for young KS patients aged 15‐23 years and adult KS patients more than 23 years of age and found SRRs of 52% and 62%, respectively, which were not significantly different. In the same year, Rohayem et al18 reported the results of micro‐TESE in 50 adolescents from 13 to 19 years of age, and the SRR was 38% (19/50). Their result was interesting because the SRR was 10% (1/10) in the subgroup of adolescents between 11 and 14 years of age, whereas in the adolescents between 15 and 19 years of age, the SRR was much higher at 45% (18/40).18 Most recently, Nahata et al55 reported a 50% SRR in 10 adolescent boys with KS between 15 and 23 years of age and indicated no distinct pattern or prediction based on the clinical data. The accumulated data from these publications showed that total SRRs by conventional TESE were 30% (15/50) and 42.6% (29/68) by micro‐TESE, which is summarized in Figure 2. It is clear that micro‐TESE has a substantial advantage for retrieving sperm, but the risks and benefits of the procedure must be considered. Information related to reproductive outcomes is still lacking; for example, the ICSI results related to using frozen sperm, how many KS patients have successful sperm retrieval, how many KS patients are married, and how many KS patients could have a child born live have not been determined. Experimentally, there are several options for cryopreservation of spermatogenic stem cells from either a testicular cell suspension or testicular tissue. However, there are no reports of inducing haploid cells in vitro using 47,XXY spermatogonia. In summary, fertility preservation techniques for adolescent boys with KS are still experimental, and use of the cryopreserved material when the boys reach adulthood cannot be guaranteed.
Figure 2.
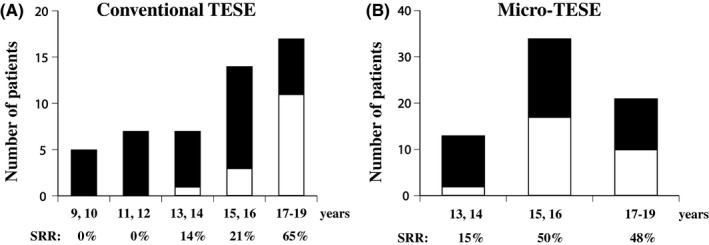
The numbers of patients and sperm retrieval rates in adolescents who underwent conventional (A) and micro‐testicular sperm extraction (B) procedures are shown. The closed bar indicates no sperm retrieval, and the open bar indicates successful sperm retrieval
One dilemma in the management of adolescent KS patients is the negative effect of TRT on spermatogenesis.TRT has previously been reported to have a negative impact on the future fertility of KS patients, as an SRR of 20% was observed in five adults with KS.63 In contrast, Plotton et al62 found no negative influence of TRT on sperm retrieval in 41 KS patients and reported an SRR by micro‐TESE in 9/17 (52.9%) men with previous TRT and a positive TESE in 14/24 (59.1%) men with KS who never had TRT. TRT was stopped 9 months prior to micro‐TESE. Another study included 10 KS adolescents who received continuous topical testosterone replacement and/or an aromatase inhibitor and found mature sperm by micro‐TESE in seven of 10 participants (70%).60 Considering these results together, TRT is unlikely to have a permanent negative impact on spermatogenesis, but larger studies and other medical treatment (eg, gonadotropins, clomiphene, other aromatase inhibitors) are warranted to confirm these findings.
Most importantly, we must consider how patients and their families feel about fertility preservation, especially in cases in which micro‐TESE needs to be performed. A psychosocial investigation clearly demonstrates a high incidence of negative feelings toward infertility and its effect on overall life satisfaction and well‐being.64 Two other studies have shown that reproductive function is not an issue of awareness in adolescent boys with KS, whereas their parents and physicians appreciate fertility preservation at a young age.57, 65 The influence of a TESE procedure on the psychological health of adolescents with KS has not been investigated, and further studies are warranted.
6. POSTINFERTILITY MANAGEMENT: DIAGNOSIS OF KS IS A PROXY OF GENERAL HEALTH
A 26‐year‐old man with KS was referred to our clinic and complained of decreased libido and general fatigue. His testicular size was 0.5 mL bilaterally, and his total testosterone concentration was 15 ng/dL. He underwent micro‐TESE at an IVF clinic 6 months prior to visit at our clinic, and his preoperative total testosterone level was 276 ng/dL. Unfortunately, no sperm were found in his testes, and his management was stopped at the clinic. At our hospital, he started TRT every 3 weeks, and his symptoms were dramatically improved. This type of case is not rare but rather very common in our urology department. What are the problems in this case?
In addition to reproductive medicine, many urologists in Japan are engaged in general urology as well as nephrology, hemodialysis, and renal transplant, which enables a quick start to the management of comorbidities, such as hypertension, diabetes, dyslipidemia, and hyperuricemia, in most men with KS after infertility treatment (Figure 3). If micro‐TESE is performed at an IVF clinic by either a urologist or gynecologist, patients are recommended to visit the urology/andrology or endocrinology department as soon as possible for follow‐up for an extended period. In other words, micro‐TESE induces new and severely low testosterone levels in recipients. Furthermore, we must make every effort to improve our ability to diagnose KS. The high frequency of mild KS phenotypes explains, at least in part, why a majority of the patients remain undiagnosed.66 A distinct racial difference in the prevalence of KS and the patterns of symptoms during the pediatric and adolescent periods are still unclear; in our experience, the BMIs of men with KS who visited an infertility clinic are obviously low (approximately 23) compared with those described in data from the United States and Europe (approximately 24‐30). Investigations regarding the patterns of comorbidity and the TRT treatment regimen for KS patients from Japan and Asian countries are warranted.
Figure 3.
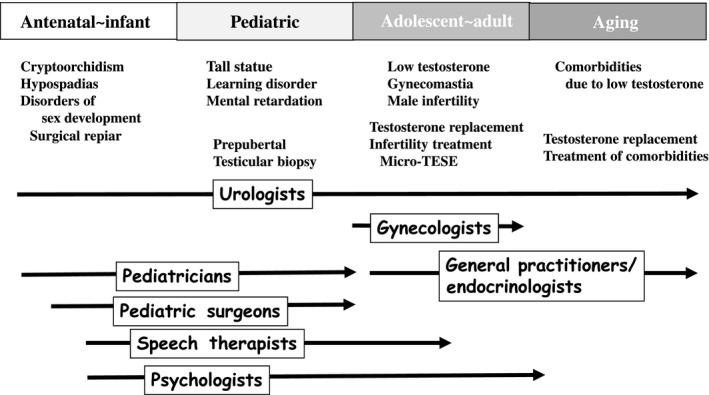
The symptoms of and strategies for managing Klinefelter syndrome on a lifelong basis and the roles of those who should participate in the management
Based on data from epidemiological studies, KS is associated with increased morbidity and mortality althoughall the information collected regarding KS is derived from diagnosed cases comprising only approximately 25% of the expected affected number of patients.3, 66 In other words, the diagnosis of KS at the time of infertility treatment is a special opportunity to evaluate their general health, and patients and medical providers should begin follow‐up and the management of comorbidities. Multidisciplinary management is needed for lifelong follow‐up (Figure 3).
Comorbidities associated with KS, such as metabolic syndrome, diabetes, osteoporosis, and cardiovascular diseases, often appear in adulthood, with many of the patients being of reproductive age; these comorbidities increase with age,67 which is ultimately related to a significant increase in mortality risk. Actually, men with KS require more frequent hospitalization, and the life span of KS patients appears to be shorter by approximately 2.1 years compared to that of the general population.68 The main comorbidity associated with increased mortality risk is the increased prevalence of type 2 diabetes and thrombosis/embolism disorders, both of which are risk factors of cardiovascular events.69 Other conditions increasing the morbidity of men with KS are osteoporosis and bone fractures as well as the higher prevalence of specific types of cancer and immunological diseases (Table 1). The reason for this impaired mortality is not well understood, and further investigations are needed to clarify whether it is due to the syndrome per se or is mainly a consequence of low testosterone levels. A number of reports show that low testosterone is a biomarker for increased mortality in normal healthy men.70
Table 1.
Comorbidity of Klinefelter syndrome (KS)
| Findings | References | |
|---|---|---|
| Cancer | ||
| Extragonadal germ cell tumors | Nonseminomatous subtype | Nichols (1987)103 |
| Younger than non‐KS | ||
| Breast cancer | 4‐ to 60‐fold compared with non‐KS |
Swerdlow et al (2005)94
Brinton (2011)75 Sasco et al (1993)74 Gomez‐Raposo et al (2010)76 |
| Younger than non‐KS | ||
| Metabolism | ||
| Obesity, metabolic syndrome | Abdominal fat is increased |
Bojesen et al (2006)90
Aksglaede (2008)104 Laaksonen (2014)105 Pasquali et al (2013)38 |
| 4‐ to 5‐fold compared with non‐KS | ||
| Odds ratio of 2.3 with low testosterone | ||
| Effect of TRT on BMI is controversial | ||
| Diabetes | 8%‐50% in Western countries |
Takeuchi (1999)106
Lichiardopol (2004)107 Swerdlow et al (2005)94 Bojesen et al (2006)90 Pasquali et al (2013)38 |
| 3.9%‐4.1% in Japan | ||
| HR of 2.21 for T1D and 3.71 for T2D | ||
| Prediabetes is more frequent | ||
| No effect of TRT | ||
| Cardiovascular disease | ||
| Congenital abnormalities | Mitral valve prolapse |
Fricke (1981, 1984)108, 109
Pasquali et al (2013)38 |
| Diastolic dysfunction | ||
| Conduction defects | Short QTc interval |
Jorgensen (2015)93
Liu (2010)110 Lai (2009)111 |
| Atrial fibrillation | ||
| Thrombosis | Hazard ratio of 3.6 to 5.7 for pulmonary thrombosis |
Bojesen et al (2006)90
Swerdlow et al (2005)94 Campbell et al (1981)96 |
| Hazard ratio of 6.6 to 7.9 for deep vein thrombosis | ||
| Bone disease | ||
| Osteoporosis/fracture | Decreased bone mineral density |
Bojesen et al (2006)90
Swerdlow et al (2005)94 Bojesen and Gravholt (2011)68 Ferlin et al (2015)98 |
| Femur fracture | ||
| 8‐fold increase compared with non‐KS | ||
| Low levels of 25‐OH vitamin D | ||
| Immunological diseases | ||
| Autoimmune diseases | 13‐fold increase in systemic lupus erythematosus compared with non‐KS |
Scofield (2008)112
El‐Mansoury (2005)113 Sawalha et al (2009)100 Rovensky et al (2010)101 Seminog et al (2015)102 |
| Rheumatic diseases | ||
| Addison's disease | ||
| Diabetes mellitus type 1 | ||
| Multiple sclerosis | ||
| Acquired hypothyroidism | ||
| Sjogren's syndrome | ||
TRT, testosterone replacement therapy.
In addition to the comorbidities mentioned above, the loss of muscle and a depressed mood due to low testosterone are not directly related to mortality but are associated with a markedly decreased quality of life.71 Of note, from observational studies, some positive effects of TRT in KS have been reported, including decreased fatigue, less irritability, and improved libido, muscle strength, and sleep.72, 73 The general consensus indicates that most men with KS should be offered TRT around the time of puberty with a target testosterone level in the high normal range.45 However, no randomized placebo‐controlled trials have been performed to date to verify this recommendation, and data on future spermatogenesis remain unknown. As a result, the timing of the initiation and the dose of TRT in KS patients remain topics for further investigation. Specifically, a study on treatment during the pediatric period showed promising results for improving behavior and neurodevelopment,33 and this treatment could have an overall positive effect on social integration during the pediatric and adolescent periods and, presumably, also in adult life.
6.1. KS and cancer
Breast cancer andgerm cell tumors, especially extragonadal tumors, occur more frequently in KS patients than in the general population (Table 1). Several meta‐analyses concerning the prevalence of male breast cancer have shown the incidence of breast cancer in KS to be increased 4‐ to 30‐fold compared with that in normal men,74, 75 which supports that KS is the strongest independent risk factor for breast cancer in males.76 The mean age of breast cancer onset in KS patients is 58 years,77 which is earlier than that of normal healthy men (67 years of age).78 The early diagnosis of breast cancer requires monthly breast self‐examination and a periodic physical examination by a specialized physician.75 A markedly increased prevalence of germ cell tumors in KS patients has been confirmed in pathology‐based studies,79 and nonseminomatous tumors are the major pathological type. Additionally, the diagnosis occurs at a younger age in KS patients than in normal healthy men. A recent Italian consensus80 suggests a biannual chest X‐ray to address the risk of extragonadal germ cell tumors.
On the other hand, the prevalence of prostate cancer and the associated mortality have been reported to be lower in KS patients than in normal healthy men.79, 81 The reason for these lower rates may be because the presence of hypogonadism does not stimulate the growth of prostate cancer cells, whereas recent studies cast doubt on the correlation between intraprostatic testosterone levels and the carcinogenesis and progression of prostate cancer.82 Interestingly, the level of prostate‐specific antigen, a marker of prostate cancer, stays below the normal range regardless of the presence of TRT.83
6.2. KS and diabetes
In the general population, a clear relationship between the serum testosterone concentration and insulin sensitivity has been reported.84, 85 Low testosterone levels are associated with increased insulin resistance,86 and the onset of insulin resistance may be influenced by testosterone levels.87, 88 Serum testosterone levels are independently associated with insulin resistance, suggesting that low testosterone might be responsible for diabetes (in this review, type 2 diabetes),89 and a 5‐fold increase in metabolic syndrome38 has been observed in men with KS. After a diabetes diagnosis, atherosclerosis progressively follows, requiring the evaluation of endothelial function.
We expect and hope for a high efficacy of TRT on glucose metabolism. Some studies have reported that the actual treatment for low testosterone improves metabolic risk factors and insulin resistance in some individuals, but not all studies have shown positive results.90 Unfortunately, KS is a condition in which TRT does not have positive effects on glucose metabolism.91 In a study reported by Pasquali et al38 that included 48 men with KS and 21 men with hypogonadotropic hypogonadism who were all treated with TRT for 3 years, KS patients were more insulin resistant, had increased body fat and lower levels of HDL, and showed an increased prevalence of metabolic syndrome than those with hypogonadotropic hypogonadism treated with TRT. Compared to other types of hypogonadism, TRT has not been shown to improve metabolic syndrome or diabetes in KS patients,38, 91 indicating either a potential hormonal resistance, consistent with the accompanying elevated gonadotropins,80 an increased aromatization,92 or more simply, that testosterone is not involved in the glucose metabolism of KS, and more complex and unrevealed mechanisms exist that are associated with KS and glucose metabolism. However, information regarding the effect of TRT on glucose metabolism in Japanese men with KS is lacking, and investigations are needed to determine optimal therapeutic regimens and follow‐up schedules for Japanese patients.
6.3. KS and cardiovascular disease
A number of reports indicate that KS is closely associated with CVD and abnormalities in electrocardiography and cardiopulmonary exercise tests (reviewed in Salzano et al30). There are no standardized guidelines for the follow‐up of men with KS. In addition to the current guidelines of TRT for hypogonadism, including T, PSA, and hematocrit evaluation,93 KS patients should be followed up for related comorbidities, especially for CVD. Because CVD is the most life‐threatening comorbidity, the initial evaluation has been proposed to include risk assessment for metabolic syndrome and thromboembolic disease as well as echocardiography.30 AS structural and functional cardiovascular abnormalities, a shorter QTc interval in KS patients compared with that in controls,94 and a 55% prevalence of mitral valve prolapse was found in 22 patients with KS. Data from large registry‐based studies95 indicate that men with KS are at an increased risk of thromboembolic events. One reason for this increased risk is that the viscosity of blood in KS patients is high, causing the formation of venous thromboembolism.96 In addition, Campbell et al found that the risks of pulmonary embolism and deep venous thrombosis were 5‐20 times higher in KS patients than in normal healthy men.97 The updated AUA guideline for the evaluation and management of testosterone deficiency indicates that whether TRT increases or decreases the risk of cardiovascular events cannot be definitively stated. This guideline also indicates that there is no definitive evidence linking TRT to a higher incidence of venous thromboembolic events.98 Obviously, this guideline is for general hypogonadal men, and data on men with KS are needed for future investigations.
6.4. KS and bone disease
Klinefelter syndrome patients also face an increased prevalence of bone disorders, particularly reduced BMD. A study found that 42.5% of KS patients had a combination of osteoporosis and osteopenia, which was 8‐fold higher than the incidence in men with a normal karyotype.99 Reduced BMD is caused by increased bone turnover and is accompanied by an increased risk of bone fractures, especially in the femoral area. Unlike fractures that occur in aging males, men with KS are more active, and the influence of fractures on their body and socioeconomic aspects are more serious. Furthermore, decreased BMD and fractures affecting morbidity and mortality 68, 95 have long been associated with KS. The mechanism of decreased BMD changes upon normal aging because the hypogonadism that occurs during the critical pubertal stages of bone development accompanying low physical exercise capacity and muscle strength is present in KS patients. TRT has been shown to improve BMD,100 but vitamin D repletion has been demonstrated to be superior to TRT for improving BMD; KS patients are more prone to 25‐OH vitamin D fluctuations than normal men.99 Practically, biannual assessments of BMD and 25‐OH vitamin D levels to evaluate the risk of osteoporosis are recommended.80
6.5. KS and immune diseases
The association between KS and immune diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), idiopathic juvenile arthritis, psoriatic arthritis, polymyositis/dermatomyositis, systemic sclerosis, and mixed connective tissue disease, has long been described101, 102; however, there has been no systematic evidence or rationale to account for the link. A retrospective study has demonstrated that compared to that of normal healthy men, men with KS have a significantly increased risk of developing Sjogren's syndrome (risk ratio: 19.3), SLE (risk ratio: 18.1), Addison's disease (risk ratio: 11.7), type 1 diabetes mellitus (risk ratio: 6.1), multiple sclerosis (risk ratio: 4.3), RA (risk ratio: 3.3), and acquired hypothyroidism (risk ratio: 2.7) .
7. CONCLUSION
The incidence of KS syndrome is 1:600, and the majority of patients in Japan are diagnosed upon a male infertility evaluation by medical providers, especially gynecologists and urologists. Finding sperm via micro‐TESE is only one consideration for men with KS among a number of comorbidities that are found during adulthood, especially after infertility treatment. In other words, patients need lifelong care and often need a multidisciplinary team that includes general practitioners, psychologists, speech therapists, and endocrinologists in addition to urologists and gynecologists. Additionally, infertility specialists should understand the pathophysiology and previous histories of KS patients before they visit the IVF clinic, and discussions with general practitioners and pediatricians are sometimes needed to properly treat these patients. Again, KS should be managed from the infantile and pediatric periods to the geriatric period.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human rights statement and informed consent: This article does not contain any study with human participants that have been performed by any of the authors. Animal studies: This article does not contain any study with animal participants that have been performed by any of the authors.
Shiraishi K, Matsuyama H. Klinefelter syndrome: From pediatrics to geriatrics. Reprod Med Biol. 2019;18:140–150. 10.1111/rmb2.12261
REFERENCES
- 1. Klinefelter H, Albright F. Syndrome characterized by gynecomastia, aspermatogenesis without A‐Leydigism, and increased excretion of follicle stimulating hormone. J Clin Endocrinol. 1942;2:615‐627. [Google Scholar]
- 2. Higurashi M, Iijima K, Ishikawa N, Hoshina H, Watanabe N. Incidence of major chromosome aberrations in 12,319 newborn infants in Tokyo. Hum Genet. 1979;46:163‐172. [DOI] [PubMed] [Google Scholar]
- 3. Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. 2003;88:622‐626. [DOI] [PubMed] [Google Scholar]
- 4. Morris JK, Alberman E, Scott C, Jacobs P. Is the prevalence of Klinefelter syndrome increasing? Eur J Hum Genet. 2008;16:163‐170. [DOI] [PubMed] [Google Scholar]
- 5. Forti G, Corona G, Vignozzi L, Krausz C, Maggi M. Klinefelter’s syndrome: a clinical and therapeutical update. Sex Dev. 2010;4:249‐258. [DOI] [PubMed] [Google Scholar]
- 6. Palermo GD, Schlegel PN, Sills ES, et al. Births after intracytoplasmic injection of sperm obtained by testicular extraction from men with nonmosaic Klinefelter’s syndrome. N Engl J Med. 1998;338:588‐590. [DOI] [PubMed] [Google Scholar]
- 7. Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter’s syndrome. Lancet. 2004;364:273‐283. [DOI] [PubMed] [Google Scholar]
- 8. Ramasamy R, Ricci JA, Palermo GD, Gosden LV, Rosenwaks Z, Schlegel PN. Successful fertility treatment for Klinefelter's syndrome. J Urol. 2009;182:1108‐1113. [DOI] [PubMed] [Google Scholar]
- 9. Takada S, Tsujimura A, Ueda T, et al. Androgen decline in patients with nonobstructive azoospemia after microdissection testicular sperm extraction. Urology. 2008;72:114‐118. [DOI] [PubMed] [Google Scholar]
- 10. Tournaye H, Staessen C, Liebaers I, et al. Testicular sperm recovery in nine 47,XXY Klinefelter patients. XXY Klinefelter patients. Hum Reprod. 1996;11:1644‐1649. [DOI] [PubMed] [Google Scholar]
- 11. Majzoub A, Arafa M, Al Said S, et al. Outcome of testicular sperm extraction in nonmosaic Klinefelter syndrome patients: what is the best approach? Andrologia. 2016;48:171‐176. [DOI] [PubMed] [Google Scholar]
- 12. Corona G, Pizzocaro A, Lanfranco F, et al. Sperm recovery and ICSI outcomes in Klinefelter syndrome: a systematic review and meta‐analysis. Hum Reprod Update. 2017;23:265‐275. [DOI] [PubMed] [Google Scholar]
- 13. Maiburg MC, Hoppenbrouwers AC, van Stel HF, Giltay JC. Attitudes of Klinefelter men and their relatives toward TESE‐ICSI. J Assist Reprod Genet. 2011;28:809‐8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okada H, Goda K, Yamamoto Y, et al. Age as a limiting factor for successful sperm retrieval in patients with nonmosaic Klinefelter’s syndrome. Fertil Steril. 2005;84:1662‐1664. [DOI] [PubMed] [Google Scholar]
- 15. Aksglaede L, Juul A. Testicular function and fertility in men with Klinefelter syndrome: a review. Eur J Endocrinol. 2013;168:67‐76. [DOI] [PubMed] [Google Scholar]
- 16. Franik S, Hoeijmakers Y, D'Hauwers K, et al. Klinefelter syndrome and fertility: sperm preservation should not be offered to children with Klinefelter syndrome. Hum Reprod. 2016;31:1952‐1959. [DOI] [PubMed] [Google Scholar]
- 17. Gies I, Oates R, De Schepper J, Tournaye H. Testicular biopsy and cryopreservation for fertility preservation of prepubertal boys with Klinefelter syndrome: a pro/con debate. Fertil Steril. 2016;105:249‐255. [DOI] [PubMed] [Google Scholar]
- 18. Rohayem J, Fricke R, Czeloth K, et al. Age and markers of Leydig cell function, but not of Sertoli cell function predict the success of sperm retrieval in adolescents and adults with Klinefelter’s syndrome. Andrology. 2015;3:868‐875. [DOI] [PubMed] [Google Scholar]
- 19. Cissen M, Meijerink AM, D'Hauwers KW, et al. Prediction model for obtaining spermatozoa with testicular sperm extraction in men with non‐obstructive azoospermia. Hum Reprod. 2016;31:1934‐1941. [DOI] [PubMed] [Google Scholar]
- 20. Aksglaede L, Skakkebaek NE, Almstrup K, et al. Clinical and biological parameters in 166 boys, adolescents and adults with nonmosaic Klinefelter syndrome: a Copenhagen experience. Acta Paediatr. 2011;100:793‐806. [DOI] [PubMed] [Google Scholar]
- 21. Eliveld J, van Wely M, Meisner A, Repping S, van der Veen F, van Pelt A. The risk of TESE‐induced hypogonadism: a systematic review and meta‐analysis. Hum Reprod Update. 2018;24:442‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lahlou N, Fennoy I, Carel JC, Roger M. Inhibin B and anti‐Mullerian hormone, but not testosterone levels, are normal in infants with nonmosaic Klinefelter syndrome. J Clin Endocrinol Metab. 2004;89:1864‐1868. [DOI] [PubMed] [Google Scholar]
- 23. Ross JL, Samango‐Sprouse C, Lahlou N, Kowal K, Elder FF, Zinn A. Early androgen deficiency in infants and young boys with 47,XXY Klinefelter Syndrome. Horm Res Paediatr. 2005;64:39‐45. [DOI] [PubMed] [Google Scholar]
- 24. Aksglaede L, Petersen JH, Main KM, Skakkebaek NE, Juul A. High normal testosterone levels in infants with non‐mosaic Klinefelter’s syndrome. Eur J Endocrinol. 2007;57:345‐350. [DOI] [PubMed] [Google Scholar]
- 25. Cabrol S, Ross JL, Fennoy I, Bouvattier C, Roger M, Lahlou N. Assessment of Leydig and Sertoli cell functions in infants with nonmosaic Klinefelter syndrome: insulin‐like peptide 3 levels are normal and positively correlated with LH levels. J Clin Endocrinol Metab. 2011;96:746‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boada R, Janusz J, Hutaff‐Lee C, Tartaglia N. The cognitive phenotype in Klinefelter syndrome: a review of the literature including genetic and hormonal factors. Dev Disabil Res Rev. 2009;15:284‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Geschwind DH, Boone KB, Miller BL, Swerdloff RS. Neurobehavioral phenotype of Klinefelter syndrome. Ment Retard Dev Disabil Res Rev. 2000;6:107‐116. [DOI] [PubMed] [Google Scholar]
- 28. Simm PJ, Zacharin MR. The psychosocial impact of Klinefelter syndrome–a 10 year review. J Pediatr Endocrinol Metab. 2006;19:499‐505. [PubMed] [Google Scholar]
- 29. Tartaglia N, Cordeiro L, Howell S, Wilson R, Janusz J. The spectrum of the behavioral phenotype in boys and adolescents 47, XXY (Klinefelter syndrome). Pediatr Endocrinol Rev. 2010;8(Suppl. 1):151‐159. [PMC free article] [PubMed] [Google Scholar]
- 30. Salzano A, Arcopinto M, Marra AM, et al. Klinefelter syndrome, cardiovascular system, and thromboembolic disease: review of literature and clinical perspectives. Eur J Endocrinol. 2016;175:27‐40. [DOI] [PubMed] [Google Scholar]
- 31. Rogol AD, Tartaglia N. Considerations for androgen therapy in children and adolescents with Klinefelter syndrome (47, XXY). Pediatr Endocrinol Rev. 2010;8(Suppl. 1):145‐150. [PubMed] [Google Scholar]
- 32. Davis SM, Cox‐Martin MG, Bardsley MZ, Kowal K, Zeitler PS, Ross JL. Effects of oxandrolone on cardiometabolic health in boys with klinefelter syndrome: a randomized controlled trial. J Clin Endocrinol Metab. 2017;102:176‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Samango‐Sprouse C, Stapleton EJ, Lawson P, et al. Positive effects of early androgen therapy on the behavioral phenotype of boys with 47, XXY. Am J Med Genet C Semin Med Genet. 2015;169:150‐157. [DOI] [PubMed] [Google Scholar]
- 34. Mehta A, Clearman T, Paduch DA. Safety and efficacy of testosterone replacement therapy in adolescents with Klinefelter syndrome. J Urol. 2014;191(Suppl. 5):1527‐1531. [DOI] [PubMed] [Google Scholar]
- 35. Tufik SB, Berro LF, Andersen ML, Tufik S. Re: safety and efficacy of testosterone replacement therapy in adolescents with klinefelter syndrome. J Urol. 2014;192(192):1300‐1301. [DOI] [PubMed] [Google Scholar]
- 36. Martinez C, Suissa S, Rietbrock S, et al. Testosterone treatment and risk of venous thromboembolism: population based case‐control study. BMJ. 2016;355; 10.1136/bmj.i5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kübler A, Schulz G, Cordes U, Beyer J, Krause U. The influence of testosterone substitution on bone mineral density in patients with Klinefelter's syndrome. Exp Clin Endocrinol Diabetes. 1992;100:129‐132. [DOI] [PubMed] [Google Scholar]
- 38. Pasquali D, Arcopinto M, Renzullo A, et al. Cittadini, Cardiovascular abnormalities in Klinefelter syndrome. Int J Cardiol. 2013;168:754‐759. [DOI] [PubMed] [Google Scholar]
- 39. Herlihy AS, McLachlan RI. Screening for Klinefelter syndrome. Curr Opin Endocrinol Diabetes Obes. 2015;22:224‐229. [DOI] [PubMed] [Google Scholar]
- 40. Nieschlag E, Ferlin A, Gravholt CH, et al. The Klinefelter syndrome: current management and research challenges. Andrology. 2016;4:545‐549. [DOI] [PubMed] [Google Scholar]
- 41. Topper E, Dickerman Z, Prager‐Lewin R, Kaufman H, Maimon Z, Laron Z. Puberty in 24 patients with Klinefelter syndrome. Eur J Pediatr. 1982;139:8‐12. [DOI] [PubMed] [Google Scholar]
- 42. Bonomi M, Rochira V, Pasquali D, et al. Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism. J Endocrinol Investig. 2017;40:123‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Forest MG, Lecoq A, Saez JM. Kinetics of human chorionic gonadotropin‐induced steroidogenic response of the human testis. II. Plasma 17 alphahydroxyprogesterone, delta4‐androstenedione, estrone, and 17 beta‐estradiol: evidence for the action of human chorionic gonadotropin on intermediate enzymes implicated in steroid biosynthesis. J Clin Endocrinol Metab. 1979;49:284‐291. [DOI] [PubMed] [Google Scholar]
- 44. Chang S, Skakkebaek A, Trolle C, et al. Anthropometry in Klinefelter syndrome—multifactorial influences due to CAG length, testosterone treatment and possibly intrauterine hypogonadism. J Clin Endocrinol Metab. 2015;100:508‐517. [DOI] [PubMed] [Google Scholar]
- 45. Høst C, Skakkebæk A, Groth KA, Bojesen A. The role of hypogonadism in Klinefelter syndrome. Asian J Androl. 2014;16:185‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aksglaede L, Wikstrom AM, Rajpert‐De Meyts E, Dunkel L, Skakkebaek NE, Juul A. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum Reprod Update. 2006;12:39‐48. [DOI] [PubMed] [Google Scholar]
- 47. Wikström AM, Raivio T, Hadziselimovic F, et al. Klinefelter syndrome in adolescence: onset of puberty is associated with accelerated germ cell depletion. J Clin Endocrinol Metab. 2004;89:2263‐2270. [DOI] [PubMed] [Google Scholar]
- 48. Davis SM, Rogol AD, Ross JL. Testis development and fertility potential in boys with Klinefelter syndrome. Endocrinol Metab Clin North Am. 2015;44:843‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lue Y, Rao PN, Sinha Hikim AP, et al. XXY male mice: an experimental model for Klinefelter syndrome. Endocrinology. 2001;142:1461‐1470. [DOI] [PubMed] [Google Scholar]
- 50. Paduch DA, Bolyakov A, Cohen P, Travis A. Reproduction in men with Klinefelter syndrome: the past, the present and the future. Semin Reprod Med. 2009;27:137‐148. [DOI] [PubMed] [Google Scholar]
- 51. Skakkebaek NE. Two types of tubules containing only Sertoli cells in adults with Klinefelter's syndrome. Nature. 1969;223:643‐645. [DOI] [PubMed] [Google Scholar]
- 52. Bergere M, Wainer R, Nataf V, et al. Biopsied testis cells of four 47, XXY patients: fluorescence in‐situ hybridization and ICSI results. Hum Reprod. 2002;17:32‐37. [DOI] [PubMed] [Google Scholar]
- 53. Gies I, Schepper JD, Goossens E, Saen DV, Pennings G, Tournaye H. Spermatogonial stem cell preservation in boys with Klinefelter syndrome: to bank or not to bank, that’s the question. Fertil Steril. 2012;98:284‐289. [DOI] [PubMed] [Google Scholar]
- 54. Gies I, de Schepper J, van Saen D, et al. Failure of a combined clinical‐ and hormonal‐based strategy to detect early spermatogenesis and retrieve spermatogonial stem cells in 47, XXY boys by single testicular biopsy. Hum Reprod. 2012;27:998‐1004. [DOI] [PubMed] [Google Scholar]
- 55. Nahata L, Yu RN, Paltiel HJ, et al. Sperm retrieval in adolescents and young adults with klinefelter syndrome: a prospective. Pilot Study. J Pediatr. 2016;170:260‐265. [DOI] [PubMed] [Google Scholar]
- 56. Damani MN, Mittal R, Oates RD. Testicular tissue extraction in a young male with 47, XXY Klinefelter’s syndrome: potential strategy for preservation of fertility. Fertil Steril. 2001;76:1054‐1056. [DOI] [PubMed] [Google Scholar]
- 57. Rives N, Milazzo JP, Perdrix A, et al. The feasibility of fertility preservation in adolescents with Klinefelter syndrome. Hum Reprod. 2013;28:1468‐1479. [DOI] [PubMed] [Google Scholar]
- 58. Van Saen D, Tournaye H, Goossens E. Presence of spermatogonia in 47, XXY men with no spermatozoa recovered after testicular sperm extraction. Fertil Steril. 2012;97:319‐323. [DOI] [PubMed] [Google Scholar]
- 59. Van Saen D, Gies I, De Schepper J, Tournaye H, Goossens E. Can pubertal boys with Klinefelter syndrome benefit from spermatogonial stem cell banking? Hum Reprod. 2012;27:323‐330. [DOI] [PubMed] [Google Scholar]
- 60. Mehta A, Bolyakov A, Roosma J, et al. Successful testicular sperm retrieval in adolescents with Klinefelter syndrome treated with at least 1 year of topical testosterone and aromatase inhibitor. Fertil Steril. 2013;100:970‐974. [DOI] [PubMed] [Google Scholar]
- 61. Dabaja AA, Schlegel PN. Microdissection testicular sperm extraction: an update. Asian J Androl. 2013;15:35‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Plotton I, Giscard d'Estaing S, Cuzin B, et al. Preliminary results of a prospective study of testicular sperm extraction in young versus adult patients with nonmosaic 47, XXY Klinefelter syndrome. J Clin Endocrinol Metab. 2015;100:961‐967. [DOI] [PubMed] [Google Scholar]
- 63. Schiff JD, Palermo GD, Veeck LL, Goldstein M, Rosenwaks Z, Schlegel PN. Success of testicular sperm injection and intracytoplasmic sperm injection in men with Klinefelter syndrome. J Clin Endocrinol Metab. 2005;90:6263‐6267. [DOI] [PubMed] [Google Scholar]
- 64. Boivin J, Takefman J, Braverman A. The fertility quality of life (FertiQoL) tool: development and general psychometric properties. Hum Reprod. 2011;26:2084‐2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gies I, Tournaye H, De Schepper J. Attitudes of parents of Klinefelter boys and pediatricians towards neonatal screening and fertility preservation techniques in Klinefelter syndrome. Eur J Pediatr. 2016;175:399‐404. [DOI] [PubMed] [Google Scholar]
- 66. Groth KA, Skakkebaek A, Host C, Gravholt CH, Bojesen A. Clinical review: Klinefelter syndrome—a clinical update. J Clin Endocrinol Metab. 2013;98:20‐30. [DOI] [PubMed] [Google Scholar]
- 67. Wikstrom AM, Dunkel L. Klinefelter syndrome. Best Pract Res Clin Endocrinol Metab. 2011;25:239‐250. [DOI] [PubMed] [Google Scholar]
- 68. Bojesen A, Gravholt CH. Morbidity and mortality in Klinefelter syndrome (47, XXY). Acta Paediatr. 2011;100:807‐813. [DOI] [PubMed] [Google Scholar]
- 69. Price WH, Clayton JF, Wilson J, Collyer S, DeMey R. Causes of death in X chromatin positive males (Klinefelter's syndrome). J Epidemiol Community Health. 1985;39:330‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Muraleedharan V, Jones TH. Testosterone and mortality. Clin Endocrinol. 2014;81:477‐487. [DOI] [PubMed] [Google Scholar]
- 71. Bojesen A, Gravholt CH. Klinefelter syndrome in clinical practice. Nat Clin Pract Urol. 2007;4:192‐204. [DOI] [PubMed] [Google Scholar]
- 72. Nielsen J, Pelsen B, Sorensen K. Follow‐up of 30 Klinefelter males treated with testosterone. Clin Genet. 1988;33:262‐269. [DOI] [PubMed] [Google Scholar]
- 73. Meikle AW, Dobs AS, Arver S, Caramelli KE, Sanders SW, Mazer NA. Androgen replacement in the treatment of Klinefelter’s syndrome: efficacy and safety of a nonscrotal permeation‐enhanced testosterone transdermal system. Endocr Pract. 1998;4:17‐22. [DOI] [PubMed] [Google Scholar]
- 74. Sasco AJ, Lowenfels AB, Pasker‐de JP. Review article: epidemiology of male breast cancer. A meta‐analysis of published case‐control studies and discussion of selected aetiological factors. Int J Cancer. 1993;53:538‐549. [DOI] [PubMed] [Google Scholar]
- 75. Brinton LA. Breast cancer risk among patients with Klinefelter syndrome. Acta Paediatr. 2011;100:814‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gomez‐Raposo C, Zambrana Tevar F, Sereno Moyano M, Lopez Gomez M, Casado E. Male breast cancer. Cancer Treat Rev. 2010;36:451‐457. [DOI] [PubMed] [Google Scholar]
- 77. Eliassen AH, Missmer SA, Tworoger SS, et al. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98:1406‐1415. [DOI] [PubMed] [Google Scholar]
- 78. Giordano SH, Buzdar AU, Hortobagyi GN. Breast cancer in men. Ann Intern Med. 2002;137:678‐687. [DOI] [PubMed] [Google Scholar]
- 79. De Sanctis V, Fiscina B, Soliman A, Giovannini M, Yassin M. Klinefelter syndrome and cancer: from childhood to adulthood. Pediatr Endocrinol Rev. 2013;11:44‐50. [PubMed] [Google Scholar]
- 80. Radicioni AF, Ferlin A, Balercia G, et al. Consensus statement on diagnosis and clinical management of Klinefelter syndrome. J Endocrinol Investig. 2010;33:839‐850. [DOI] [PubMed] [Google Scholar]
- 81. Ji J, Zöller B, Sundquist J, Sundquist K. Risk of solid tumors and hematological malignancy in persons with Turner and Klinefelter syndromes: a national cohort study. Int J Cancer. 2016;139:754‐758. [DOI] [PubMed] [Google Scholar]
- 82. Debruyne F, Behre HM, Roehrborn CG, et al. Testosterone treatment is not associated with increased risk of prostate cancer or worsening of lower urinary tract symptoms: prostate health outcomes in the registry of hypogonadism in men. BJU Int. 2017;119:216‐224. [DOI] [PubMed] [Google Scholar]
- 83. Selice R, Mambro AD, Garolla A, et al. Testosterone, sex hormone‐binding globulin and the metabolic syndrome: a systematic review and meta‐analysis of observational studies. Int J Epidemiol. 2011;40:189‐207. [DOI] [PubMed] [Google Scholar]
- 84. Kelly DM, Akhtar S, Sellers DJ, Muraleedharan V, Channer KS, Jones TH. Testosterone differentially regulates targets of lipid and glucose metabolism in liver, muscle and adipose tissues of the testicular feminised mouse. Endocrine. 2016;54:504‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mitsuhashi K, Senmaru T, Fukuda T, et al. Testosterone stimulates glucose uptake and GLUT4 translocation through LKB1/AMPK signaling in 3T3‐L1 adipocytes. Endocrine. 2016;51:174‐184. [DOI] [PubMed] [Google Scholar]
- 86. Camacho EM, Huhtaniemi IT, O’Neill TW, et al. Age‐associated changes in hypothalamic‐pituitary‐testicular function in middle‐aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168:445‐455. [DOI] [PubMed] [Google Scholar]
- 87. Landry D, Pare A, Jean S, Martin LJ. Adiponectin influences progesterone production from MA‐10 Leydig cells in a dose‐dependent manner. Endocrine. 2015;48:957‐967. [DOI] [PubMed] [Google Scholar]
- 88. Yesilova Z, Oktenli C, Sanisoglu SY, et al. Evaluation of insulin sensitivity in patients with Klinefelter’s syndrome: a hyperinsulinemic euglycemic clamp study. Endocrine. 2005;27:11‐15. [DOI] [PubMed] [Google Scholar]
- 89. Isidori AM, Balercia G, Calogero AE, et al. Outcomes of androgen replacement therapy in adult male hypogonadism: recommendations from the Italian society of endocrinology. J Endocrinol Invest. 2015;38:103‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bojesen A, Kristensen K, Birkebaek NH, et al. The metabolic syndrome is frequent in Klinefelter's syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care. 2006;29:1591‐1598. [DOI] [PubMed] [Google Scholar]
- 91. Bekaert M, Van Nieuwenhove Y, Calders P, et al. Determinants of testosterone levels in human male obesity. Endocrine. 2015;50:202‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cunningham GR, Stephens‐Shields AJ, Rosen RC, et al. Testosterone treatment and sexual function in older men with low testosterone levels. J Clin Endocrinol Metab. 2016;101:3096‐3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jorgensen IN, Skakkebaek A, Andersen NH, et al. Short QTc interval in males with klinefelter syndrome‐influence of CAG repeat length, body composition, and testosterone replacement therapy. Pacing Clin Electrophysiol. 2015;38:472‐482. [DOI] [PubMed] [Google Scholar]
- 94. Swerdlow AJ, Higgins CD, Schoemaker MJ, Wright AF, Jacobs PA; United Kingdom Clinical Cytogenetics Group . Mortality in patients with Klinefelter syndrome in Britain: a cohort study. J Clin Endocrinol Metab. 2005;90:6516‐6522. [DOI] [PubMed] [Google Scholar]
- 95. Zoller B, Ji J, Sundquist J, Sundquist K. High risk of venous thromboembolism in Klinefelter syndrome. J Am Heart Assoc. 2016;5:e003567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Campbell WA, Price WH. Venous thromboembolic disease in Klinefelter’s syndrome. Clin Genet. 1981;19:275‐280. [DOI] [PubMed] [Google Scholar]
- 97. Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423‐432. [DOI] [PubMed] [Google Scholar]
- 98. Ferlin A, Selice R, Di Mambro A, et al. Role of vitamin D levels and vitamin D supplementation on bone mineral density in Klinefelter syndrome. Osteoporos Int. 2015;26:2193‐2202. [DOI] [PubMed] [Google Scholar]
- 99. Behre HM, Kliesch S, Leifke E, Link TM, Nieschlag E. Longterm effect of testosterone therapy on bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 1997;82:2386‐2390. [DOI] [PubMed] [Google Scholar]
- 100. Sawalha AH, Harley JB, Scofield RH. Autoimmunity and Klinefelter's syndrome: when men have two X chromosomes. J Autoimmun. 2009;33:31‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rovenský J, Imrich R, Lazúrová I, Payer J. Rheumatic diseases and Klinefelter's syndrome: annals of the New York Academy of Sciences. Ann N Y Acad Sci. 2010;1193:140‐9. [DOI] [PubMed] [Google Scholar]
- 102. Seminog OO, Seminog AB, Yeates D, Goldacre MJ. Associations between Klinefelter's syndrome and autoimmune diseases: English national record linkage studies. Autoimmunity. 2015;48:125‐128. [DOI] [PubMed] [Google Scholar]
- 103. Nichols CR, Heerema NA, Palmer C, et al. Klinefelter's syndrome associated with mediastinal germ cell neoplasms. J Clin Oncol 1987;5:1290‐1294. [DOI] [PubMed] [Google Scholar]
- 104. Aksglaede L, Skakkebaek NE, Juul A. Abnormal sex chromosome constitution and longitudinal growth: serum levels of insulin‐like growth factor (IGF)‐I, IGF binding protein‐3, luteinizing hormone, and testosterone in 109 males with 47, XXY, 47, XYY, or sex‐determining region of the Y chromosome (SRY)‐positive 46, XX karyotypes. J Clin Endocrinol Metab. 2008;93:169‐176. [DOI] [PubMed] [Google Scholar]
- 105. Laaksonen DE, Niskanen L, Punnonen K, et al. Estosterone and sex hormone‐binding globulin predict he metabolic syndrome and diabetes in middle‐aged men. Diabetes Care. 2014;27:1036‐1041. [DOI] [PubMed] [Google Scholar]
- 106. Takeuchi Y, Murata Y, Sintani J, et al. Klinefelter's syndrome accompanied by mixed connective tissue disease and diabetes mellitus. Intern Med. 1999;38:875‐881. [DOI] [PubMed] [Google Scholar]
- 107. Lichiardopol C, Mota M, Pănuş C. Metabolic changes in Klinefelter syndrome. Rom J Intern Med. 2004;42:415‐422. [PubMed] [Google Scholar]
- 108. Fricke GR, Mattern HJ, Schweikert HU. Mitral valve prolapse in Klinefelter syndrome. Lancet. 1981;2:8260‐8261. [DOI] [PubMed] [Google Scholar]
- 109. Fricke GR, Mattern HJ, Schweikert HU, et al. Klinefelter's syndrome and mitral valve prolapse. an echocardiographic study in twenty‐two patients. Biomed Pharmacother. 1984;38:88‐97. [PubMed] [Google Scholar]
- 110. Liu T, Shehata M, Li G, Wang X. Androgens and atrial fibrillation: friends or foes? Int J Cardiol. 2010;145:365‐367. [DOI] [PubMed] [Google Scholar]
- 111. Lai J, Zhou D, Xia S, et al. Reduced testosterone levels in males with lone atrial fibrillation. Clin Cardiol. 2009;2:43‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Scofield RH, Bruner GR, Namjou B, et al. Klinefelter's syndrome (47, XXY) in male systemic lupus erythematosus patients: support for the notion of a gene‐dose effect from the X chromosome. Arthritis Rheum. 2008;58:2511‐2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. El‐Mansoury M, Bryman I, Berntorp K, et al. Hypothyroidism is common in Turner syndrome: results of a five‐year follow‐up. J Clin Endocrinol Metab. 2005;90:2131‐2135. [DOI] [PubMed] [Google Scholar]


