Abstract
Purpose
Fertility preservation is an important issue for young cancer patients. Random‐start controlled ovarian stimulation and double ovarian stimulation have been proposed for efficient oocyte retrieval within the limited time before cancer therapy. We aimed to clarify the efficacy of these new protocols within the Japanese population.
Methods
We performed a retrospective observational study at a multicenter from February 2012 to August 2017. The study entailed 50 cycles with 34 patients who underwent fertility preservation due to breast cancer. Follicular phase or luteal phase ovarian stimulation with aromatase inhibitor was performed. A second ovarian stimulation was started with or without waiting until the next menstruation. We measured the number of retrieved oocytes and cryopreserved oocytes/embryos, the ratio of mature oocytes, and the fertilization rate.
Results
The numbers of retrieved oocytes and frozen oocytes/embryos were not significantly different between follicular phase and luteal phase ovarian stimulation. The number of retrieved oocytes was not reduced at the second ovum pick up compared to the first ovum pick up in the double ovarian stimulation.
Conclusions
Random‐start controlled ovarian stimulation and double ovarian stimulation with aromatase inhibitor for breast cancer patients were effective protocols for retrieving a greater number of oocytes within the limited time.
Keywords: aromatase inhibitor, breast cancer, double ovarian stimulation, fertility preservation, random-start
1. INTRODUCTION
Breast cancer is the most common type of cancer among young adult women.1, 2 Quality of life for cancer survivors is important issue because of the high survival rate due to early diagnosis and improvements in cancer treatment. Many breast cancer survivors face diminished ovarian reserves and infertility after gonadotoxic chemotherapy and long‐lasting adjuvant therapies such as tamoxifen. Therefore, fertility preservation (FP) prior to cancer treatment is important for young adults. The American Society of Clinical Oncology (ASCO) guidelines recommend that health care providers should inform cancer patients about the possibility of infertility and should also be prepared to discuss FP options and/or to refer all potential patients to appropriate reproductive specialists.3
The American Society for Reproductive Medicine and the Society of Assisted Reproductive Technology have stated that oocyte vitrification and warming should no longer be considered experimental.4 Thus, in addition to embryo freezing, oocyte freezing has become a common method of FP. However, oocyte and embryo cryopreservation need ovarian stimulation, which results in increased serum estradiol levels that may accelerate breast cancer growth. In order to avoid estradiol elevation, the protocol of ovarian stimulation with aromatase inhibitor (AI) was proposed,5 as the protocol is unlikely to increase recurrence risk in breast cancer.6
Normally, ovarian stimulation begins in the early follicular phase. Because there is often the need to start cancer treatment at the earliest convenience, random‐start ovarian stimulation, in which ovarian stimulation begins in the luteal phase, has been proposed in order to avoid waiting until the next menstruation.7, 8, 9
To harvest more oocytes efficiently within the limited time available before starting cancer treatment, double ovarian stimulation (DuoStim) within the same menstrual cycle was proposed.10, 11 DuoStim provides a greater opportunity for retrieving oocytes within a short period. Oocytes from follicular phase ovarian stimulation (FPS) and luteal phase ovarian stimulation (LPS) have similar developmental potential, and subsequent frozen embryo transfer provides optimal pregnancy outcomes.11, 12
As there are no published reports concerning random‐start ovarian stimulation and DuoStim among the Japanese population, here we examined the efficacy of these protocols in Japan.
2. MATERIALS AND METHODS
2.1. Study patients
We performed a multicenter retrospective observational study among patients who underwent FP from February 2012 to August 2017. The breast cancer patients were referred by the oncologist for consults on FP. Follow‐up information concerning cancer recurrence was collected from the medical records of oncologists or from patient interviews.
2.2. Breast cancer subtypes
Intrinsic subtypes were classified by hormone receptor (HR; estrogen receptor and/or progesterone receptor) and human epidermal receptor 2 (HER2) status. Luminal A, luminal B, and triple negative were defined as HR+/HER2−, HR+/HER2+, and HR−/HER2−, respectively.13
2.3. Ovarian stimulation protocol
We performed a short or GnRH antagonist protocol with AI (letrozole 2.5 or 5 mg/d) for ovarian stimulation. Briefly, GnRH agonist and FSH/HMG were co‐administered until the final trigger for the short protocol, and GnRH antagonist was administered after the leading follicle reached 14‐16 mm diameter with FSH/HMG oocyte stimulation in the GnRH antagonist protocol. Buserelin acetate or human chorionic gonadotropin (hCG) 5000 or 10 000 U was administered for final oocyte maturation.
The patients were divided into two groups according to their menstruation phase at the start of ovarian stimulation. FPS and LPS were defined as the initiation of gonadotropins in the follicular phase and luteal phase of the menstruation cycle, respectively (Figure 1). The luteal phase was defined as detection of the corpus luteum by ultrasound sonography or elevated serum progesterone (>2 ng/mL). We performed sequential second ovarian stimulation after the first ovum pick up without waiting until the next menstruation in the DuoStim protocol (Figure 1).
Figure 1.
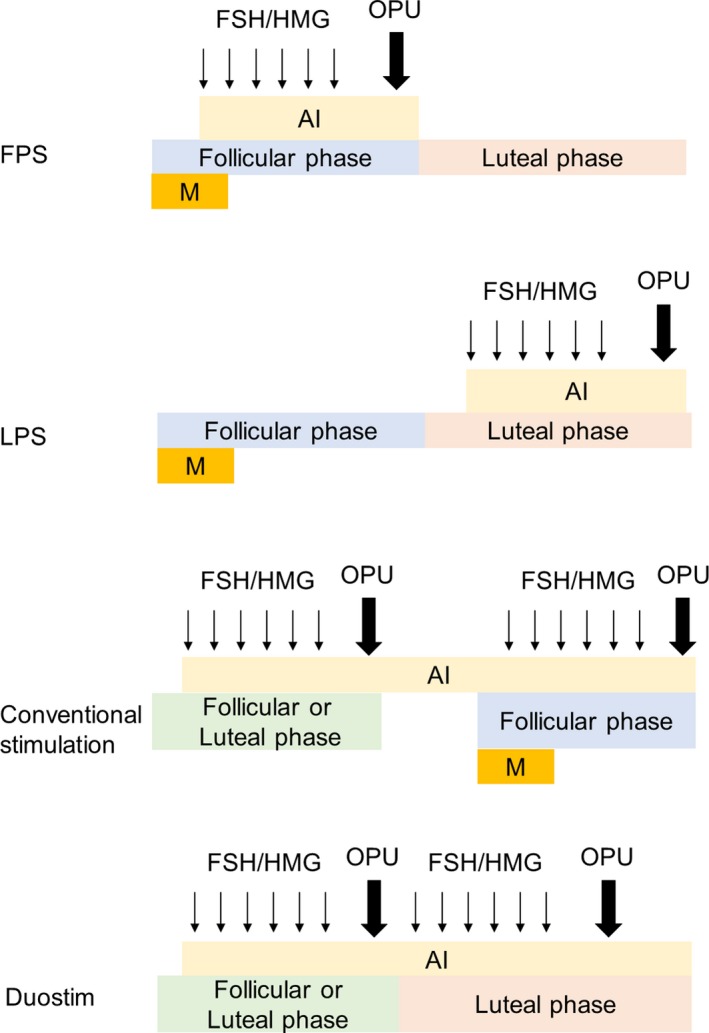
Schemata of ovarian stimulation protocols. AI, aromatase inhibitor; DuoStim, double ovarian stimulation; FPS, follicular phase ovarian stimulation; LPS, luteal phase ovarian stimulation; M, menstruation; OPU, ovum pick up
2.4. Oocytes and embryo cryopreservation
Oocyte cryopreservation was performed if the patients did not have a partner, and embryo cryopreservation was performed if the patient had a partner. For oocyte cryopreservation, MII oocytes were cryopreserved by the vitrification method at the day of ovum pick up or one day after ovum pick up. Mature oocytes were defined as MII oocytes at the day of ovum pick up in cases of oocyte cryopreservation. For embryo cryopreservation, intracytoplasmic sperm injection (ICSI) or in vitro fertilization (IVF)‐ICSI split insemination was performed if medically indicated (n = 9, 7, respectively); otherwise, oocytes were fertilized by IVF (n = 9). Embryos were cryopreserved by the vitrification method at the cleavage or blastocyst stage. Embryos developed to blastocyst stage were graded according to the criteria proposed by Gardner and Schoolcraft.14 Embryos with ≥4 cells on day 2, ≥7 cells on day 3, and better than grade 3CC on day 5 or day 6 were vitrified.
2.5. Statistical analysis
Paired and unpaired t tests and Fisher's exact test were performed to compare differences between two groups. Statistically significant difference was defined as P < 0.05.
3. RESULTS
Fifty cycles of ovum pick up among 34 patients were analyzed (Figure 2). Twenty patients underwent one cycle of ovum pick up, 12 patients underwent two cycles, and two patients underwent three cycles. Twenty‐nine cycles were FPS, and 21 cycles were LPS. Both oocyte and embryo cryopreservation were performed due to the patient's desire in three cases. Thirty patients (88.2%) were nullipara, and four patients (11.8%) were para 1. Characteristics of breast cancer are shown in Figure 3. Stage I or II patients constituted 76.6% of the study subjects, and 94.1% of the patients were HR positive (luminal A or B).
Figure 2.
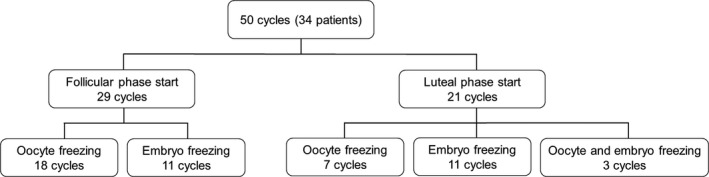
Flowchart of fertility preservation for breast cancer patients. Oocyte and embryo freezing together were performed in three cycles
Figure 3.
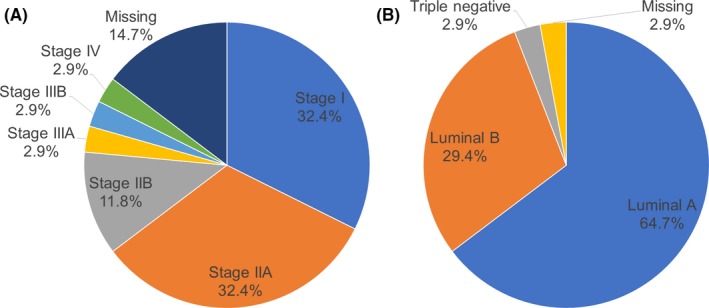
Characteristics of breast cancer. A, Stages of breast cancer. B, Intrinsic subtypes of breast cancer
Characteristics of FPS and LPS are shown in Table 1. The two groups were comparable in mean age at OPU, AMH level, and baseline FSH. Although the number of retrieved oocytes and rate of mature oocytes were not different between the two groups, number of days stimulated and total FSH/HMG dose were higher in the luteal phase group (9.0 ± 1.9 days vs 11.3 ± 2.6 days, 1290.5 ± 586.2 IU vs 1957.1 ± 1030.2 IU, respectively). Fertilization rates using IVF and ICSI were not significantly different between the two groups.
Table 1.
Characteristics of FPS and LPS
| FPS (n = 29) | LPS (n = 21) | P | |
|---|---|---|---|
| Age at OPU (y) | 35.3 ± 4.0 | 37 ± 3.1 | 0.12 |
| AMH (ng/mL) | 3.8 ± 2.5 | 3.5 ± 2.5 | 0.66 |
| Baseline FSH (mIU/mL) | 8.0 ± 3.5 | 7.0 ± 3.2 | 0.28 |
| Peak E2 (pg/mL) | 595.2 ± 491.1 | 530.9 ± 538.1 | 0.66 |
| Endometrial thickness (mm) | 10.0 ± 2.0 | 11.3 ± 3.3 | 0.089 |
| No. of follicles > 17 mm | 2.6 ± 2.0 | 2.7 ± 2.6 | 0.84 |
| Peak follicle size (mm) | 19.2 ± 2.8 | 18.9 ± 2.6 | 0.74 |
| No. of days stimulated | 9.0 ± 1.9 | 11.3 ± 2.6 | <0.001 |
| Total FSH/HMG dose (IU) | 1290.5 ± 586.2 | 1957.1 ± 1030.2 | 0.0056 |
| No. of retrieved oocytes | 8.7 ± 6.0 | 10.0 ± 6.8 | 0.48 |
| Mature (MII) oocytes (%) | 83.3 (125/150) | 81.7 (49/60) | 0.84 |
| MI oocytes (%) | 4.0 (6/150) | 6.7 (4/60) | 0.48 |
| GV oocytes (%) | 11.3 (17/150) | 8.3 (5/60) | 0.62 |
| Degenerated oocytes (%) | 1.3 (2/150) | 3.3 (2/60) | 0.32 |
| Fertilization rate (%) | |||
| IVF | 62.5 (35/56) | 47.1 (48/102) | 0.069 |
| ICSI | 70.3 (26/37) | 90.0 (36/40) | 1 |
FPS, follicular phase ovarian stimulation; GV, germinal vesicle; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; LPS, luteal phase ovarian stimulation; MI, metaphase I; MII, metaphase II; OPU, ovum pick up. Data are presented as mean ± SD. Mature oocytes were calculated in cases of oocyte cryopreservation (n = 28). Fertilization rate was calculated in cases of embryo cryopreservation (n = 25)
The numbers of frozen oocytes and embryos in FPS and LPS are shown in Table 2. There was no significant difference between the two groups.
Table 2.
No. of frozen oocytes and embryos
| FPS (n = 29) | LPS (n = 18) | P | |
|---|---|---|---|
| No. of frozen oocytes | 7.4 ± 5.1 (n = 18) | 5.0 ± 5.4 (n = 7) | 0.31 |
| No. of frozen embryos | 3.2 ± 1.9 (n = 11) | 4.4 ± 2.9 (n = 11) | 0.27 |
FPS, follicular phase ovarian stimulation; LPS, luteal phase ovarian stimulation. Data are presented as mean ± SD. Three cases in which both oocytes and embryos cryopreservation were performed were excluded.
We divided the cases into two groups in terms of letrozole dose in a subgroup analysis (Table 3). Although there was no significant difference, there was a trend in that serum peak E2 level was lower in the 5 mg group. The number of retrieved oocytes was not different between the two groups. Although total FSH/HMG dose was higher in the 5 mg group, it could be related that there were more LPS cycles in the 5 mg group.
Table 3.
Characteristics and numbers of retrieved oocytes for each letrozole dose
| Letrozole dose | P | ||
|---|---|---|---|
| 2.5 mg (n = 34) | 5 mg (n = 16) | ||
| No. of FPS cycles (%) | 23 (67.6) | 6 (37.5) | |
| No. of LPS cycles (%) | 11 (32.4) | 10 (62.5) | |
| Age at OPU (y) | 34.9 ± 3.4 | 38.4 ± 3.4 | 0.001 |
| Peak E2 (pg/mL) | 625.4 ± 545.0 | 446.6 ± 403.7 | 0.25 |
| Total FSH/HMG dose | 1341.1 ± 626.3 | 2057.8 ± 1087.2 | 0.005 |
| No. of retrieved oocytes | 8.9 ± 6.6 | 9.9 ± 5.7 | 0.58 |
FPS, follicular phase ovarian stimulation; LPS, luteal phase ovarian stimulation; OPU, ovum pick up. Data are presented as mean ± SD.
We divided the cases into two groups depending on whether menstruation occurred between first ovum pick up and the start of second ovarian stimulation among the patients who underwent two or more cycles of ovum pick up. Conventional stimulation was defined as first ovum pick up in FPS or LPS and second ovum pick up in FPS, and there is at least one menstruation during first ovum pick up and the start of second ovarian stimulation. DuoStim was defined as first ovum pick up in FPS or LPS and second ovum pick up in LPS, and there is no menstruation during first ovum pick up and the start of second ovarian stimulation (Figure 1).
The number of first‐ and second‐retrieved oocytes was not significantly different between Conventional stimulation and DuoStim. The number of second‐retrieved oocytes was not decreased compared to the number of first‐retrieved oocytes in either the Conventional stimulation or DuoStim (Table 4).
Table 4.
No. of retrieved oocytes at first and second ovum pick up
| Conventional stimulation (n = 7) | DuoStim (n = 7) | P | |
|---|---|---|---|
| Age at OPU (y) | 35.9 ± 4.8 | 37.3 ± 3.2 | 0.36 |
| No. of first‐retrieved oocytes | 8.1 ± 4.8 | 7.1 ± 4.1 | 0.68 |
| No. of second‐retrieved oocytes | 9.3 ± 5.3 | 7.6 ± 7.1 | 0.62 |
Conventional stimulation, first ovum pick up in follicular or luteal phase ovarian stimulation and second ovum pick up in follicular phase ovarian stimulation, and there is at least one menstruation during first ovum pick up and the start of second ovarian stimulation. Duostim, first ovum pick up in follicular or luteal phase ovarian stimulation and second ovum pick up in luteal phase ovarian stimulation, and there is no menstruation during first ovum pick up and the start of second ovarian stimulation. OPU, ovum pick up. Data are presented as mean ± SD.
There were no cases of cancer recurrence among the 28 patients for whom we had follow‐up data. Mean follow‐up period after ovum pick up was 459 days. Although four embryos among two patients were transferred, there were no clinical pregnancies.
4. DISCUSSION
In the present study, we found that nearly same number of oocytes/embryos were cryopreserved between FPS and LPS using the letrozole protocol. Furthermore, the number of second oocytes retrieved in DuoStim was not decreased compared to the first oocyte retrieval. Although the observational time was relatively short, cancer recurrence was not observed during the study period.
The number of retrieved oocytes in the LPS was similar to that of the FPS. This result was consistent with the findings reported in previousstudies.15, 16 A previous study showed that there was no elevated rate of abnormality at birth after LPS compared to FPS.17 Another study showed that euploid blastocyst rate calculated either per biopsied blastocyst or injected MII oocyte was not significantly different between FPS and LPS groups.10 A previous study showed that 35‐ to 37‐year‐old women needed to cryopreserve about 20 mature oocytes to have an 80% chance of obtaining at least one successful pregnancy.18 In the present study, the mean numbers of frozen oocytes were 7.4 and 5.1 per ovum pick up in FPS and LPS, respectively. Therefore, about three cycles of ovum pick up were needed to have an 80% chance of at least one successful pregnancy. Random‐start and DuoStim were efficient strategies for obtaining more oocytes within the limited time available.
Although an association between the use of letrozole for infertility treatment and congenital anomalies was reported in a relatively small number of pregnancies,19 such an association was rejected in recent studies.20, 21, 22 In general, letrozole 2.5 or 5 mg per day was used for ovarian stimulation.5, 7 Although the difference in serum peak E2 level did not reach statistical significance, there was a trend in that serum peak E2 level was lower in the 5 mg compared to 2.5 mg group. Serum peak E2 level in the 5 mg per day‐ letrozole group was controlled to about 400 pg/mL, which is comparable to the natural ovulatory cycle.23 The number of retrieved oocytes was similar in spite of the lower serum E2 level and higher average age of patients in the 5 mg group. These results were probably due to the fact that we could administer enough FSH/HMG without causing elevation of serum E2. Therefore, administration of 5 mg/d letrozole was effective, particularly in the estrogen receptor‐positive breast cancer patients.
Several breast cancer risk factors have been identified, and estrogen exposure is one example.24, 25
Although our study involved a relatively short observational period, we note that there were no recurrent breast cancer patients among patients given FP with letrozole. This result suggested that ovarian stimulation with letrozole does not have a great influence on the recurrence rate for breast cancer during at least short periods; this is consistent with the results of a previous study that followed patients for a longer time period.6 In this study, we used letrozole during ovarian stimulation regardless of the existence of estrogen receptor. As estrogen signaling occurs not only via estrogen receptor but also via non‐estrogen receptor‐related proteins,26 use of letrozole for FP of breast cancer patients seems reasonable regardless of the existence of estrogen receptors.
This study demonstrated that the number of retrieved oocytes was not different between FPS and LPS. As there were very few frozen and then thawed and transferred embryos, we could not analyze clinical pregnancy rate, live birth rate, or prognosis for children.
In the present study, we have demonstrated the efficacy of LPS and DuoStim with the AI protocol among Japanese breast cancer patients in regard to increasing the number of oocytes harvested within the limited time available before starting cancer treatment.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human rights statements and informed consent: The study was approved by the Tokyo Medical and Dental University (M2000‐2036), Kameda Medical Center, and Kameda IVF Clinic Makuhari Review Board (17‐018). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all patients for being included in the study.
ACKNOWLEDGEMENT
We thank all other members of our institution.
Nakasuji T, Kawai K, Ishikawa T, et al. Random‐start ovarian stimulation with aromatase inhibitor for fertility preservation in women with Japanese breast cancer. Reprod Med Biol. 2019;18:167–172. 10.1002/rmb2.12263
Takashi Nakasuji and Kiyotaka Kawai contributed equally to this work.
REFERENCES
- 1. Narod SA. Breast cancer in young women. Nat Rev Clin Oncol. 2012;9(8):460‐470. [DOI] [PubMed] [Google Scholar]
- 2. Bleyer A, Barr R, Hayes‐Lattin B, et al. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8(4):288‐298. [DOI] [PubMed] [Google Scholar]
- 3. Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500‐2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Practice Committees of American Society for Reproductive Medicine , Society for Assisted Reproductive Technology . Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99(1):37‐43. [DOI] [PubMed] [Google Scholar]
- 5. Oktay K, Hourvitz A, Sahin G, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91(10):3885‐3890. [DOI] [PubMed] [Google Scholar]
- 6. Kim J, Turan V, Oktay K. Long‐term safety of letrozole and gonadotropin stimulation for fertility preservation in women with breast cancer. J Clin Endocrinol Metab. 2016;101(4):1364‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: random‐start controlled ovarian stimulation. Fertil Steril. 2013;100(6):1673‐1680. [DOI] [PubMed] [Google Scholar]
- 8. Bedoschi GM, de Albuquerque FO, Ferriani RA, Navarro PA. Ovarian stimulation during the luteal phase for fertility preservation of cancer patients: case reports and review of the literature. J Assist Reprod Genet. 2010;27(8):491‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. von Wolff M, Thaler CJ, Frambach T, et al. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil Steril. 2009;92(4):1360‐1365. [DOI] [PubMed] [Google Scholar]
- 10. Ubaldi FM, Capalbo A, Vaiarelli A, et al. Follicular versus luteal phase ovarian stimulation during the same menstrual cycle (DuoStim) in a reduced ovarian reserve population results in a similar euploid blastocyst formation rate: new insight in ovarian reserve exploitation. Fertil Steril. 2016;105(6):1488‐1495.e1. [DOI] [PubMed] [Google Scholar]
- 11. Kuang Y, Chen Q, Hong Q, et al. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol). Reprod Biomed Online. 2014;29(6):684‐691. [DOI] [PubMed] [Google Scholar]
- 12. Kuang Y, Hong Q, Chen Q, et al. Luteal‐phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen‐thawed embryo transfer cycles. Fertil Steril. 2014;101(1):105‐111. [DOI] [PubMed] [Google Scholar]
- 13. Anderson WF, Rosenberg PS, Katki HA. Tracking and evaluating molecular tumor markers with cancer registry data: HER2 and breast cancer. J Natl Cancer Inst. 2014;106(5):dju093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155‐1158. [DOI] [PubMed] [Google Scholar]
- 15. Boots CE, Meister M, Cooper AR, Hardi A, Jungheim ES. Ovarian stimulation in the luteal phase: systematic review and meta‐analysis. J Assist Reprod Genet. 2016;33(8):971‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pereira N, Voskuilen‐Gonzalez A, Hancock K, Lekovich JP, Schattman GL, Rosenwaks Z. Random‐start ovarian stimulation in women desiring elective cryopreservation of oocytes. Reprod Biomed Online. 2017;35(4):400‐406. [DOI] [PubMed] [Google Scholar]
- 17. Chen H, Wang Y, Lyu Q, et al. Comparison of live‐birth defects after luteal‐phase ovarian stimulation vs. conventional ovarian stimulation for in vitro fertilization and vitrified embryo transfer cycles. Fertil Steril. 2015;103(5):1194‐1201.e2. [DOI] [PubMed] [Google Scholar]
- 18. Doyle JO, Richter KS, Lim J, Stillman RJ, Graham JR, Tucker MJ. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertil Steril. 2016;105(2):459‐466. [DOI] [PubMed] [Google Scholar]
- 19. Biljan MM, Hemmings R, Brassard N. The outcome of 150 babies following the treatment with letrozole or letrozole and gonadotropins. Fertil Steril. 2005;84:S95. [Google Scholar]
- 20. Tatsumi T, Jwa SC, Kuwahara A, Irahara M, Kubota T, Saito H. No increased risk of major congenital anomalies or adverse pregnancy or neonatal outcomes following letrozole use in assisted reproductive technology. Hum Reprod. 2017;32(1):125‐132. [DOI] [PubMed] [Google Scholar]
- 21. Tulandi T, Martin J, Al‐Fadhli R, et al. Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate. Fertil Steril. 2006;85(6):1761‐1765. [DOI] [PubMed] [Google Scholar]
- 22. Diamond MP, Legro RS, Coutifaris C, et al. Letrozole, gonadotropin, or clomiphene for unexplained infertility. N Engl J Med. 2015;373(13):1230‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shechter A, Boivin DB. Sleep, hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder. Int J Endocrinol. 2010;2010:259345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moreno‐Aspitia A, Advani P. Current strategies for the prevention of breast cancer. Breast Cancer Targets Ther. 2014;6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou W, Slingerland JM. Links between oestrogen receptor activation and proteolysis: relevance to hormone‐regulated cancer therapy. Nat Rev Cancer. 2014;14(1):26‐38. [DOI] [PubMed] [Google Scholar]
- 26. Nilsson S, Gustafsson J‐A. Estrogen receptor action. Crit Rev Eukaryot Gene Expr. 2002;12(4):237‐257. [DOI] [PubMed] [Google Scholar]


