Abstract
Background
The pregnancy and delivery rates following assisted reproductive technology (ART) start to decrease and that the miscarriage rate increases rapidly from 35 years old. The miscarriage rate exceeds 50% at 43 years old. The number of aneuploid fetuses in miscarriages increases according to female age, reaching more than 90% when women are over 40 years old.
Methods
Different cytoplasmic donation technologies used to rescue aged oocytes with high percentage of aneuploidy were analyzed, and their efficacy compared.
Main findings (Results)
Germinal vesicle transfer (GVT) might be superior to spindle chromosome transfer (ST) theoretically from the point of higher capability of rescuing the disjunction at meiosis I which cannot be helped by ST. However, actually, in vitro maturation (IVM) of oocyte after GVT has not yet been totally completed. ST among other nuclear donations showed the higher possibility to rescue them, due to the fact it does not require in vitro maturation and it has an ethical advantage over pronuclear transfer (PNT) which requires the destruction of an embryo.
Conclusion
Spindle chromosome transfer has the potential to rescue aged oocytes to some extent, but we have to continue the basic study further to establish the clinical application of cytoplasmic donation to rescue aged oocytes.
Keywords: aged oocytes, cytoplasmic donation, germinal vesicle transfer (GVT), mitochondrial DNA (mtDNA), spindle chromosome transfer (ST)
1. INTRODUCTION
It is well known that the pregnancy and delivery rates following ART for women under 34 years old are over 40%. However, these rates start to decrease rapidly among patients from around 37 years old and the former becomes less than 15% per embryo transfer and the latter is almost zero in patients over 43 years old. On the other hand, the miscarriage rate increases rapidly from 35 years old and rapidly exceeds 50% at 43 years old (80% at 48 years old) (Figure 1). This clarifies the direct relationship between human fecundity and patients age.1
Figure 1.
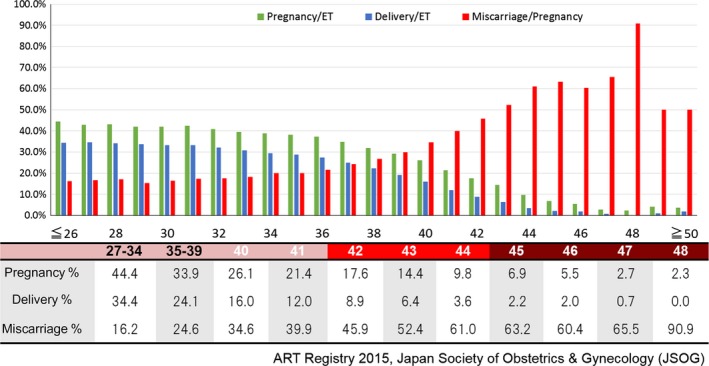
Pregnancy and delivery rates decrease but miscarriage rate increases as female patients grow older
The frequency of fetal cytogenetic abnormalities in miscarriages has been reported to be between 46.3% and 76.7%2, 3 and increases according to female age, surpassing 90% in women over 40 years old. Almost all of the cases are autosomal trisomy,4, 5 this is because monosomy embryos disappear at the early developmental stage. Such aneuploidy is mostly produced by the chromosomal pre‐division or nondisjunction, whereby homologous chromosomes fail to pair or separate appropriately at the meiotic metaphase, resulting in disomic and nullisomic gametes.6, 7, 8, 9 All living organisms age and eventually die. When aging occurs in an ovary, both nuclear and cytoplasmic functions of all the cells contained decrease resulting in ovarian dysfunction and lower fecundity. Nothing can stop this unavoidable process, aging. However, scientists continue to pursue the dream to rejuvenate the aged oocytes. For age‐related decreasing fecundity, the novel treatment of ooplasmic transfer (OT) was introduced in 1997 by Cohen et al10 to rescue the aged oocyte for the first time in the world and was then followed by germinal vesicle transfer (GVT), pronuclear transfer (PNT), and spindle chromosome transfer (ST). There have been many reports which supported these procedures, but some scientists remain skeptical.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 In this manuscript, we would like to review whether the different cytoplasmic donation methods (OT, GVT, PNT, ST) are effective options to rejuvenate the aged oocytes or not.
2. CHARACTERISTICS OF OOCYTE AGING
We would like to analyze the mechanism of oocyte aging from the following three points of view, chromosomal abnormality (aneuploidy), mitochondrial dysfunction, and epigenetic alteration.
2.1. The origin of human aneuploidy
2.1.1. Age‐associated increase in aneuploidy
Aneuploid oocyte results from meiotic chromosome mis‐segregation, which might be caused by impaired regulating mechanisms for maintaining the sister chromatid cohesion or defective regulators of chromosome distribution. The regulators mentioned above might suffer from deterioration caused by numerous factors during the storage period of the immature GV oocytes until they are released into the reproductive cycles in humans. In mammalian GV oocytes, the most remarkable features of the termer regulators are that the bivalent chromosomes form crossover by chiasmata between homologous arms, and that their cohesion is also maintained with physical linkage rings.24 In the latter process, the cohesin protein subunits play a crucial role25, 26 localizing at the chromosome centromeres and arms and holding sister chromatids together. Therefore, it is easy to suppose that sister chromatids tend to separate prematurely when cohesion ring joints are dislocated with advanced age.
In fact, cohesin deficiencies result in loss of chromosomal cohesion and increased chromosome mis‐segregation during maternal aging.27, 28 Furthermore, some studies showed that the aging‐associated chromosome mis‐segregation is followed by a decrease in Shugoshin 2,29, 30 which plays a role to protect cohesin dissociation at the centromeric region until sister chromatid separation.31, 32 Cohesin loading for the chiasmata maintenance and meiotic divisions starts in the initial meiotic stages of oocytes,33, 34 and its deterioration without replenishment33, 35 due to maternal aging is accountable for the increase in chromosomal abnormalities. Similarly, some researchers have reported that cohesion between centromeres of sister chromatids becomes fragile in human oocytes during maternal aging.36 Spindle assembly checkpoint proteins regulate meiotic segregation of homologous chromosomes in mouse oocyte37, 38, 39, 40, 41, 42, 43 and control mitosis upon fertilization.41, 44 Gene expression in young and aged human oocytes indicates that there is a difference in gene products related to cell‐cycle regulation, spindle formation, and organelle integrity, thus contributing to frequent chromosomal segregation errors in meiosis. The observations suggest that mitotic centromere‐associated protein is necessary for spindle formation, chromosome assembly, and cell‐cycle progression. Its mRNA and protein reductions in a context of permissive spindle assembly checkpoint are a risk factor of aneuploidy.
2.1.2. Non–age‐associated increase in aneuploidy
In older women, the production of aneuploidy is accelerated by chromosomal pre‐division that results from age‐related deterioration of cohesin localized on meiotic chromosomes, as described above.45 However, about 60% of human aneuploidy appears to be trisomy which is caused by nondisjunction.46 Three main characteristics of human nondisjunction have been identified: The first one is that in all somatic and sex chromosomes, most trisomies originate during oogenesis. The second one indicates that maternal meiosis I errors contribute more commonly to trisomy than maternal meiosis II errors. This thought is based on the phenomenon that the oocyte first meiotic division commences in the early fetal ovary and it is arrested at the prophase for more than 10 years until the time of ovulation. The third characteristic is that there also appear to be mechanisms that differentially influence specific chromosome groups. For example, nondisjunction patterns are similar among the acrocentric chromosomes (chromosomes 13, 14, 15, 21, and 22) that contain long heterochromatin region on the short arm. Furthermore, some patterns of nondisjunction appear to be chromosome‐specific. All trisomy 16 cases are derived from maternal MI errors, while MII errors are significantly dominant in trisomy 18. However, there is a possibility that the cases may result in selective survival or abortion with disomic homogeneity. Besides the three characteristics mentioned, altered recombination is also a known important causal factor on the human trisomy. This is mostly due to failure of crossover between homologous chromosomes, and it results in random segregation at metaphase I and an increase of 50% in the incidence of nondisjunction.47 However, some homologues are in a more complicated situation whereby crossovers for nondisjunction are formed on unusual positions of chromosomes. Trisomy 21 is a compelling evidence of this, though it also typically increases with the age of the mother.48, 49 Among younger women, telomeric exchanges dominantly contribute to these aneuploidy cases derived at maternal MI. However, these exchanges are not serious among older women. For MII trisomies, pericentromeric exchanges happen commonly in older women. In addition, other types of abnormal recombination account for about 50% of maternal MI errors in both young and old women. Therefore, it might be concluded that non–age‐associated factors become more important than advanced maternal age.
2.2. The connection between human aneuploidy and mitochondrial dysfunction
One of the changes recognized in mature oocytes is the appearance of the meiotic spindle that is formed with microtubules. Microtubule organizing centers (MTOCs) are necessary for the assembly and disassembly of the spindle microtubules. The microtubule motor proteins depend on the association with microtubules between the chromosomal kinetochore50 and MTOC or centrosomes at the MII or the first cleavage metaphase, respectively.51 These motor proteins actively move on the microtubules and participate in the arrangement and stability of the spindle structure. In aging human oocytes, lack of integrity in the microtubule network has been reported.52 The process of chromosomal disjunction, whether in meiosis I or meiosis II, is dependent on ATP energy to pull and separate the tetrad (in MI) and diad chromatids (in MII) to the opposite ends of the spindle.53, 54 The mitochondrial DNA (mtDNA) mutations accumulated during aging could harmfully influence the potency of ATP production in the oocyte. In addition, the mitochondrial respiratory is the main source of free radicals in the body. Therefore, defects in mtDNA integrity could be associated with damage by reactive oxygen species.
2.3. Mitochondria dysfunction during oocytes aging
Hamatani et al11 compared the mRNA expression profiles between MII oocytes from young (5‐ to 6‐week‐old) and aged (42‐ to 45‐week‐old) C57BL/6 female mice using microarray analysis, and found that out of about 11 000 gene transcripts detected in mature oocytes, only 5% (530) were affected by maternal aging. The results showed that gene expression is not likely to be affected so much by oocyte aging. Therefore, organelle dysfunction seems to be an important factor for the problems associated with aged oocytes. It is well established that the development of oocytes requires synchronous coordination between nucleus and cytoplasm. In the oocyte cytoplasm, there are numerous organelles and molecules that are used for early development. Any dysfunction of organelles and biochemical reactions, for example, mitochondrial malfunctions, mtDNA mutation, insufficient protein synthesis or untimely protein resolution, and oocyte plasma membrane degradation will decrease the oocyte developmental potency, resulting in a detrimental effect on embryo quality.55, 56
Relationship between mitochondria malfunctions and advanced maternal age has been reported in oocytes. Variations of ATP content imply distinct oocyte quality.57, 58 Moreover, mitochondria seem to be responsible for aberrations in spindle assembly, chromosome segregation, and cell‐cycle regulation, as shown in oocytes from aged women and mice.59, 60 In addition, Keefe et al61 reported that 93% of oocytes from patients aged >37 years undergoing IVF treatment contained detectable mtDNA deletion, compared with only 28% of oocytes from younger women,61, 62, 63 suggesting that oocytes obtained from older women may contain a reduced number of mtDNA copies than those in oocytes from younger women. Hence, it is considered that a bioenergetic deficit caused by mitochondrial dysfunction is a major factor leading to reduced IVF outcomes, and in older women in particular.
2.4. Epigenetic changes in aged oocytes
Oocyte quality is dependent on both genomic and epigenetic changes during oocyte storage in the ovary. Epigenetic changes are nonheritable phenotypic changes that result from alteration of gene expression, accompanied by genomic mutation. There are at least three systems including DNA methylation, histone modification and noncoding RNA‐associated gene silencing that are known to initiate epigenetic changes.
DNA methylation is the main cause of genomic imprinting, and it is a necessary process for proper oocyte maturation and embryonic development in humans and other mammalian species. Reprogramming of DNA methylation starts at different growth stages in the male and female germlines, and the differences in their reprogramming pattern cause the distinct gene expression pattern between the maternal and paternal genome in embryos. In the mouse female germline, methylation reprogramming begins in growing oocytes after birth and finishes before entry into the first meiotic metaphase. DNA methylation is mainly catalyzed by DNA methyltransferase 3 s (DNMT3s),64 which are allowed to bind the amino‐terminal tail of histone H3 with histone modification before interaction with the associated DNA strand. In mammalian oocytes, the reprogramming of histone modification also occurs during oogenesis.
Genomewide analysis has shown that global genomic methylation is altered with aging. Small noncoding RNA (miRNA), whose expression is also regulated by DNA methylation,65 negatively interferes in gene expression through binding to target gene mRNA. The function of miRNAs is post‐transcriptional regulation of gene expression through recruitment into miRNA protein complexes,66 although suppressed altogether during oogenesis. Recently, altered expression of miRNAs in aged mice and human organs has been found.67, 68, 69 Therefore, age‐related difference in miRNA expression seems to affect epigenetic process. The facts indicate that severe spindle and chromosomal segregation defects resulted from miRNA dysfunction in mouse oocytes.70
An alteration of mRNA expression in human mature oocytes has also been widely confirmed with female aging.71, 72 In addition, mouse oocyte aging changes the mRNA and protein expression. Dysfunctions of the aged ovary may be responsible for the changes.73, 74 For maintenance of DNA methylation, DNMT1 is expressed in mouse oocytes, but its defect disturbs the expression of imprinted genes during early embryonic development.75, 76 The change of DNMTs expression in oocytes from individuals of advanced maternal age11, 71 might be the direct reason for causing the DNA methylation alterations. These findings appear to conclude that alteration of the epigenetic modifications in oocytes with maternal age appears to cause an increase in the miscarriage rate.
In mammalian oocytes, histones are widely deacetylated during meiosis. Aoki et al found that an inhibition of meiotic histone deacetylation is followed by an increase of aneuploidy in mice embryo. Histones that remained acetylated in the oocytes from older (10‐month‐old) female mice were responsible for embryonic death, suggesting that histone deacetylation is needed for normal embryonic development. Histone deacetylation may be involved in the distribution of meiotic chromosomes. This means that an increase of aneuploidy in the human embryos may be dependent on inadequate histone deacetylation during meiosis.77
Furthermore, a new study has reported that an abnormal phenotype of CD9‐deficient mouse oocytes is rescued by injection of mouse CD9, human CD9, or mouse CD81 miRNA. The phenotype observed in CD9‐deficient mice was a defect in the sperm‐egg fusion process. This result suggests that the heterogeneous mRNA transferred to the nucleus produces functional protein. It also means that transferred cytoplasm with healthy and abundant mRNA compensate normal embryonic development in the oocyte reconstructed with different nucleus and cytoplasm.78
3. HISTORY OF CYTOPLAST DONATION
3.1. Cytoplasmic Transfer (CT)
Cohen et al10 reported the first case of cytoplast donation into human oocyte to rescue women having repeated implantation failure (Table 1). A 39‐year‐old woman, with a history of 6.5 years of infertility and failed IVF treatments, was able to conceive by cytoplast donation from a 27‐year‐old egg donor. They placed the aspirated donor ooplasm and husband sperm in the patient's egg close to its metaphase chromosome. Then, a baby girl was born at term weighing 4356 g. By 2004, more than 30 children had been born following the cytoplast injection of young, donor oocytes into recipient oocytes with an ICSI technique .10, 79, 80, 81 About 5%‐15% of cytoplast from a young donor oocyte is transferred into a recipient one directly to improve pregnancy rate in advanced‐age women. The precise components of cytoplasm are most likely to involve the healthier mitochondria, mRNAs, and proteins.81, 82 In addition, injecting healthy mitochondria into recipient oocytes increases cytoplasmic ATP content and avoids apoptosis in aged oocytes.83, 84 This newly developed treatment was highly expected to rescue the maternal infertility because of aging. However, CT was prohibited in the United States by the FDA due to mitochondrial heteroplasmy with two types of mtDNA. Cohen et al reported that 2 of 15 babies born following CT showed the heteroplasmy of two kinds of mtDNA85 resulted in three different DNA: nuclear DNA, recipient mtDNA and donor mtDNA. Heteroplasmy is likely to cause mitochondrial maturation and transmit to the next generation.86, 87 Furthermore, we should be warned about the heterogeneous mtDNA potential to induce epigenetic changes upon maternal and paternal genomes.81, 88
Table 1.
History of cytoplast donation
| 1997 | Cohen J. et al reported the first birth after ooplasmic transfer in the Lancet 1997 |
| 1999 | Zhang J. et al reported the in vitro maturation of human preovulatory oocytes reconstructed by germinal vesicle transfer in the Fertil. Steril 1999 |
| 2001 | Takeuchi T. et al reported the preliminary findings in germinal vesicle transplantation of immature human oocytes in the Hum. Reprod 1999 |
| 2001 | FDA (Food and Drug Administration)bans cytoplasmic donation in 2001 |
| 2003 | Zhang J. et al reported the first human successful case after nuclear transfer at the pronuclear stage in American Society for Reproductive Medicine (ASRM) |
| 2006 | ZHAO‐DAI BAI et al reported the developmental potential of aged oocyte rescued by nuclear transfer following parthenogenetic activation and in vitro fertilization in Mol Reprod 2006 |
| 2008 | Newcastle university team was authorized to perform the nuclear transfer for treating Mitochondrial disease by HFEA (Human Fertilisation Embryology Authority) in the UK in the Nature 2010 |
| 2009 | Tachibana M. et al reported the first successful birth of a primate following the nuclear transfer at M‐II stage in the Nature 2009 |
| 2009 | Tanaka A. et al reported the human nuclear transfer at M‐II stage for rescuing aged oocytes in the Reproductive BioMedicine Online 2009 |
| 2010 | Newcastle team reported the successful human nuclear transfer at PN stage in the Nature 2010 |
| 2013 | Paull D. et al reported the nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants in the Nature 2013 |
| 2013 | Tachibana M. et al reported the toward germline gene therapy of inherited mitochondrial diseases in the Nature 2013 |
| 2015 | UK approves laws to allow the clinical application of nuclear transfer in 2015 |
| 2017 | J. Zhang reported the first successful birth of human being following the nuclear transfer at M‐II stage in the ASRM 2017 |
3.2. Germinal vesicle transfer (GVT)
Theoretically, the transfer of a germinal vesicle (GV) of an aged woman to another enucleated oocyte of a young woman makes it possible to rescue aneuploidy caused by aging. This technique was first reported by Zhang et al in 1999, and human GV oocytes from ICSI cycles were collected after consent from ICSI patients who participated in a study.89 Newly constructed age‐related oocytes were in vitro matured, but a successful maturation has not been achieved to this date. On the other hand, this method showed more successful results in mice.90, 91
The strong point of GVT is that it can be carried out before the start of M‐I. A large number of aneuploidies derive from nondisjunction, and chromosome misalignment – during M‐I.8, 46, 51, 92, 93, 94 The chromosomal misalignment at M‐I could induce nondisjunction, due to a decreased number of chiasmata or incomplete separation of univalents in aged oocytes.95, 96 An obvious relationship between oocyte aging and malsegregation due to the nondisjunction of bivalents during M‐I was reported.93 So, GVT may be a promising treatment to correct abnormal nondisjunction at M‐I or M‐II.
One of the benefits of GVT is related to the interaction with mitochondria. It is reported that mitochondrial damage has a detrimental effect on oocyte maturation, chromosomal segregation, and spindle formation.97 This damage was overcome by GVT and chromosomal analysis showed that almost all of these reconstructed oocytes had a normal number of chromosomes, and they regained the former reproductive capability.
Palermo et al showed that a healthy mouse ooplasm could rescue the damaged mitochondrial function of GV stage caused by photoirradiation and that 62% of these reconstructed oocytes matured to metaphase II.98
On the other hand, there is a report that indicates that ooplasm from young mice could not rescue aging‐related chromosomal abnormalities.15 This might have been caused by noncytoplasmic factors in GV stage that affect chromosome segregation. This objection is based on the results with a mouse experiment exchanging GVs and ooplasms of varying ages.15 The chromosomal abnormality rate in newly reconstructed oocytes was found to be much higher (57.1%) when the GV of an aged mouse was transferred to the enucleated oocyte of a younger one. On the other hand, it was 16.7% when the GV of a younger mouse was transferred to enucleated aged oocytes. Whether GVT could rescue chromosomal abnormalities in aged oocytes needs further examination.
3.3. Pronuclear transfer
Pronuclear transfer (PNT) is essentially the same procedure as GVT, except for the removal of pronucleus after fertilization and it has had some successful normal births.17, 99, 100, 101 Craven et al 102 performed human PNT and reported that the volume of carryover of donor mtDNA was minimal and the reconstructed embryo developed to blastocysts.
There are two advantages in PNT. First, the PN is easily visualized, so the extraction of PN is easier than ST.102 It is difficult to extract the metaphase chromosome intactly as the M‐II chromosomes are not clearly visible to the naked eyes.51 Second, PN has superior embryonic development potentiality. If oocyte dysfunction is derived from cytoplasmic factor, PNT will have higher potentiality to develop normally than the other alternative cytoplasmic donations: CT, GVT, and ST. On the other hand, PNT has some shortcomings. First, the exchange and fusion of two PN is accompanied by technical difficulties due to large volume. Second, the volume of mtDNA carried over into the recipient oocyte is the largest among all alternatives, resulting in the densest mitochondrial heteroplasmy. Lastly, this procedure requires the destruction of an embryo, which makes it difficult to apply clinically because of ethical concerns.
In 2003, Zhang et al103 reported the first clinical application of PNT. A 30‐year‐old nulligravida female had two failed IVF cycles, and 7 out of 20 2PN zygotes were successfully reconstructed by PNT with recipient 2PN oocytes. Out of the seven reconstructed, five zygotes were transferred to the patient's uterus. A triplet pregnancy was then achieved and resulted in immature births after fetal reduction. Nuclear genetic fingerprinting showed that the nuclear DNA was identical to that of the patient's. mtDNA profiles in fetuses were similar to those from donor cytoplasm with no detection of patient's mtDNA.
3.4. Spindle chromosome transfer (ST)
Cytoplasmic transfer (CT) and germinal vesicle transfer (GVT) both drew attention at one time. However, in the former, it is difficult to verify whether a small volume of injected cytoplasm has improved the quality of cytoplasmic organelles (mtDNA, mRNA, cytoskeleton, etc). Concerning the latter, theoretically the newly reconstructed oocyte by GVT has higher potentiality to normalize the quality of cytoplasm than ST, but it has not yet been successful in vitro culture to M‐II oocyte .104
In 2006, Zhao‐Dai‐Bai et al105 reported the higher embryonic developmental potential of aged oocyte rescued by nuclear transfer in mice. In their investigation, blast formation percentage of reconstructed oocytes with young nucleus and aged cytoplasm was low (15.0%). However, blastocyst development was surprisingly higher (86.2%) with aged nucleus and young cytoplasm and three viable pups have been obtained after embryo transfer. These observations validated that cytoplasm plays a more determinant role than the nucleus in improving the quality of aged oocyte and might partly rescue nucleus apoptosis from aging.
In 2009, Yoshizawa et al106 reported the higher embryonic development and production of pups by transferring karyoplasts at the stage of M‐II of senescent mouse oocytes into cytoplasm of healthy mouse oocytes. They investigated the effects of reciprocal transplantation of M‐II karyoplasts between senescent and healthy mouse oocytes and evaluated the effectiveness of ST by the rate of blastocyst development. The reconstructed oocytes that consisted of aged karyoplasts and healthy cytoplasts showed significantly improved embryonic development and development to term as compared with the oocytes reconstructed with young karyoplasts and aged cytoplasm. That study showed successful rejuvenation for age‐related infertility using exchange of M‐II karyoplasts in mouse models. They reported that no genetic or epigenetic abnormalities were found in their study and suspected an exchange of M‐II karyoplast accompanied by very small volume of mtDNA heteroplasmy.107 They concluded that their study showed successful rejuvenation of age‐related infertility in mouse model using M‐II karyoplasts exchange.
In 2009, Tanaka et al108 reported the usefulness of Metaphase II karyoplast transfer in humans to rescue aged oocytes. It is well known that in vitro culture of immature oocytes from IVF patients developed to M‐II oocyte after a one‐night in vitro culture. They found the similarity of chromosomal karyotype of in vitro M‐II oocytes and aged oocytes.109 Both of them show high incidence of premature splitting of chromosomes (PSC). They performed ST between donor fresh M‐II oocyte collected from ICSI patients who consented to participate in that study and recipient patient's in vitro matured M‐II oocyte. Karyoplast fusion was performed by electrical stimulation, and they reported that fertilization, cleavage, and blastocyst formation rates following ICSI were 76.0%, 64.0%, and 28.0% respectively for reconstructed oocytes and significantly lower rates respectively for control oocytes (Figure 2). Five embryos developed after ST and ICSI showed normal diploid sets of 46 chromosomes without PSC (Figure 3).
Figure 2.
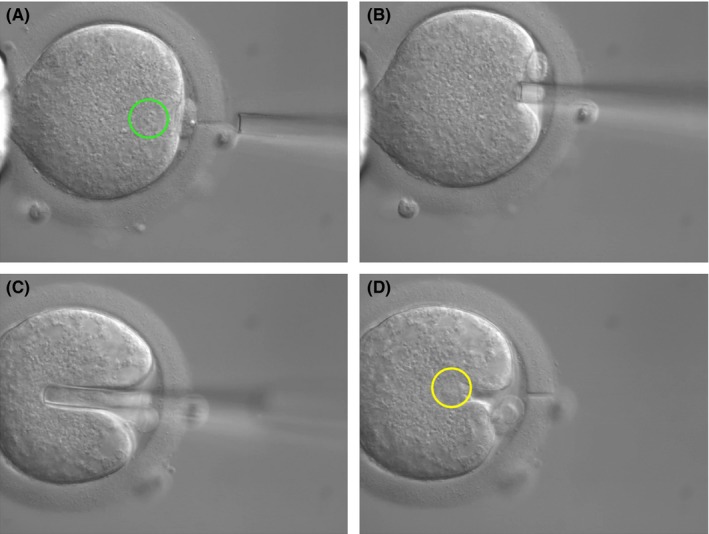
A, Zona cutting with laser, spindle chromosome is visible in green circle. B, Aspiration of spindle chromosome. C, Insertion of spindle chromosome of aged oocyte, after immersion into inactivated Sendai virus, into enucleated donor cytoplasm. D, Inserted karyoplast of aged oocyte in yellow circle
Figure 3.
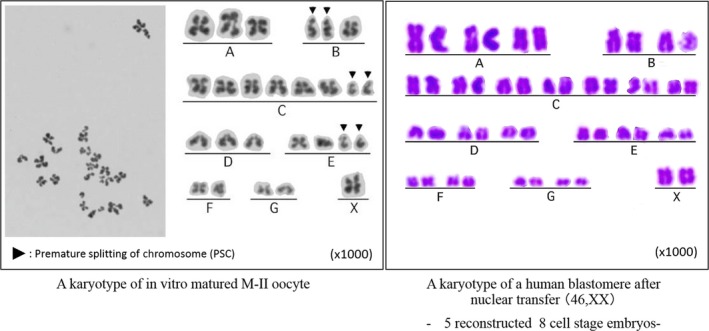
Chromosomal analysis of in vitro matured M‐II oocyte and blastomere after karyoplast transfer. A karyotype of in vitro matured M‐II oocyte showed high frequency of premature splitting of chromosomes (PSC) before spindle chromosome transfer (ST) but showed normal frequency after a karyoplast transfer into enucleated fresh oocyte
The first successful report of ST in humans was delayed for 3 years by Tanaka et al due to the difficulty to confirm the existence of metaphase II chromosome, though it was easily recognized as a chromosomal bump in mice. A polarized light (POL) microscope showed an obvious view of meiotic spindles,110 but identification of the M‐II spindle in living human oocytes was possible only in at most 80%. In addition, the micro‐manipulation of oocytes with POL was not easy because the procedure was carried out watching a video screen and not under a microscope. Therefore, a new method to remove M‐II karyoplast under an inverted microscope with Normarski differential interference contrast system without any special devices on staining was developed111 (Figure 4). On the other hand, Tachibana et al continued to develop the original POL system and several years later after the first report of POL by Oldenbourg; they established the complete system to find and remove spindle‐chromosomal complex. This system's disadvantages are that a Laser apparatus is necessary to cut the envelope and that the identification of spindle is susceptible to room temperature and not always perfect. However, this method is believed to be the best procedure for ST.
Figure 4.
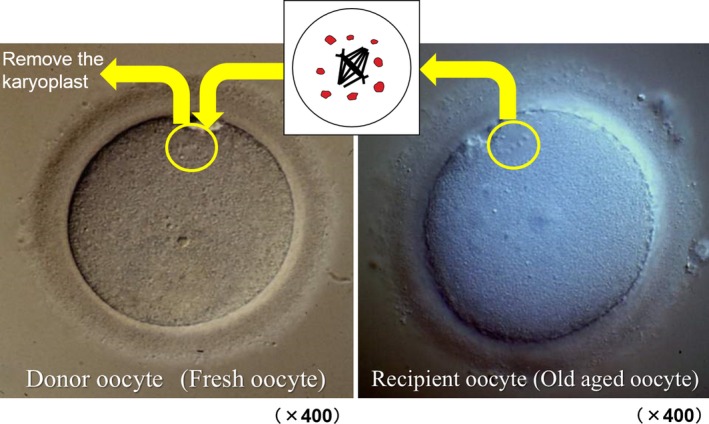
Spindle chromosome of aged oocyte was transferred into the enucleated fresh oocyte
In 2008, a Newcastle University team in the UK reported the possibility of PNT for treatment of mitochondria disease, in an article titled “Pronuclear transfer in human embryos to prevent transmission of mtDNA disease.” However, blastocyst formation after PNT was less than 1%.102
In 2009, Tachibana et al86 reported the first successful ST using a POL microscope in nonhuman prime oocyte (Macaca mulatta). They demonstrated that the mtDNA could be effectively exchanged in Macaca mulatta oocytes by ST from one egg to an enucleated egg. The reconstructed oocytes showed normal embryonic development with fertilization: 95%, 8‐cell: 93%, Morula: 78%, Blastocyst: 61%, and healthy offspring was born. No spindle donor mtDNA was detected in offspring or in newly generated embryonic stem cell lines. This method proposed a new way to prevent mutant mtDNA transmission and to save aged oocytes.
In 2013, Tachibana et al112 reported the results of reciprocal exchange of human ST. Fertilization rate was 73%, almost the same as the controls, but about a half of fertilization was 3PN. Among 2PN embryos, blastocyst rate was 62% and embryonic stem cell isolation (38%) rates were comparable to controls. All embryonic stem cell had exclusively donor mtDNA. The high percentage of abnormal fertilization might be derived from the lack of synchronization between nucleus and cytoplasm. Human M‐II oocytes seem to be more sensitive to spindle manipulations.
In 2013, Paull et al113 reported that nuclear genome transfer did not reduce developmental efficiency to the blastocyst stage. Transferred mtDNA at ST was initially detected at levels below 1%, decreasing in blastocysts and stem cell lines to undetectable levels, and remained undetectable after more than one year. In this study, they also reported no significant differences of respiratory chain enzyme activities and basal oxygen consumption were found among stem cell line‐derived fibroblasts and oocyte donor skin fibroblasts.
In 2015, the UK parliament approved the clinical application of ST but only for the treatment of mitochondrial disease. However, the actual road map for clinical application for the treatment of mitochondrial disease has not proceeded due to insufficient consensus (personal correspondence).
In 2016, Zhang et al114 reported the first successful birth of human baby following the nuclear transfer at M‐II stage. The patient was a 36‐year‐old with a history of four pregnancy losses and two deceased children at age 8 months and 6 years from Leigh syndrome as confirmed by >95% mutation load. Four out of five collected oocytes were fertilized with ICSI after ST developed to blastocyst, and then, only one euploid blastocyst was transferred resulting in the birth of a healthy baby. The level of transmitted mtDNA in several neonatal tissues was <1.60% ± 0.92%.
4. DISCUSSION POINTS
4.1. Effect of coexistence of multiple wild‐type mitochondrial genomes
It is still unknown whether oocyte heteroplasmy with two different wild‐type mtDNA has a detrimental effect to the offspring or not. Sequence differences between native and “foreign” mtDNA can produce proteins with altered amino acid sequences. This has been proved in both cattle20 and pigs,21 and there might be unexpected interaction between the different mtDNA originated from different cytoplasm. This would reduce capacity of energy production, show symptoms similar to mitochondrial disease, and then influence embryonic fetal development. However, the issue argued is not the case of cloning using somatic cell but the mixture of two wild types of M‐II mitochondria. Tachibana et al demonstrated that the mtDNA can be efficiently exchanged in mature Macaca mulatta and human oocytes that were reconstructed by ST. Genetic results showed that the cells of the three offspring born contained spindle donor nuclear DNA and the cytoplast donor mtDNA. Donor mtDNA was undetectable in offspring. These results might lead to the speculation that the mixture of two different wild types of mtDNA does not affect the embryonic potentiality. This study indicated that the mitochondrial exchange by nuclear transfer was capable of producing normal embryonic development resulted in healthy offspring.
4.2. Interaction between nuclear genomes and mitochondrial ones
The importance of intergenomic communication for efficient cellular function seems to be explained by the interaction that occurs between proteins encoded by nuclear genomes and those encoded by the mtDNA genome.115 The electron transfer chain (ETC) requires nuclear‐encoded proteins to be transported to the mitochondria.116 Failure of mitochondrial transcription factor A (TFAM) and mitochondrial transcription factor B (TFBM) to co‐ordinate transcription would also have serious effects for the activity of ETC.117
However, other experimental results suggest that there is considerable flexibility in nuclear/mitochondrial interaction. A recent study showed that mitochondrial function in cell hybrids between mtDNA‐less Mus musculus domesticus cells and Mus spretus cells was normal.118 A similar study indicated more flexibility in primates, chimpanzee, and gorilla mitochondria could functionally replace human mitochondria.119 In conclusion, the embryological and cell hybrid experiments would argue that there is potentially considerable flexibility in mitochondrial/nuclear interaction. Tachibana et al completed a 5‐year follow‐up study on monkey spindle transfer offspring born in 200986 and reported that no significant differences in body weight could be found between ST juvenile monkeys and age‐matched controls. They also confirmed that ATP levels in skin fibroblasts were similar to those of controls. During those 5 years, there were no significant changes in mtDNA carryover and heteroplasmy in blood and skin samples with age. We may speculate that nuclear‐mtDNA interactions keep harmony and co‐ordinate well, judging from that follow‐up study.
5. CONCLUDING SUMMARY AND FUTURE PROSPECTS
With the world's trends of late marriage, more females joining the workforce to get better jobs, longevity, and low birth rates, how to rescue the aged oocytes has become a worldwide urgent issue to help the childless advanced‐age couples who prefer not to opt for oocyte donation.
Oocyte quality, controlled by nucleus and cytoplasm, decreases mostly due to aging. A great number of trials in ART for rescuing aged oocytes continue development of ovarian stimulation for decreased ovarian reserve, preimplantation genetic aneuploidy test (PGA‐T), and cryopreservation before aging. However, the results are still far from being satisfactory.
The advent of new technology of cytoplasmic transfer (CT), germinal vesicle transfer (GVT), pronuclear transfer (PNT), and spindle chromosome transfer (ST) might have the potential to partly resolve those difficult tasks. The biggest advantage is a supply of abundant and healthy mitochondria, mRNA, microRNA, and epigenetic factors (DNA methyl transferase, histone deacetylase). With these supplementations, some aneuploidy caused by nondisjunction at meiosis II, premature splitting chromosome, mitochondrial malfunction, decreased mRNA, and microRNA could be corrected to euploid.
Not all causes of miscarriages are chromosomally abnormalities; 40%‐50% of all aged embryos are euploid but have cytoplasmic dysfunction.32 So, all aged oocytes could be rescued by ST to some extent.
Judging from the fact that all oocytes have completed oogenesis by the prophase of metaphase I, GVT might be superior to ST theoretically from the point of higher capability of rescuing the nondisfunction at meiosis I which cannot be helped by ST. GVT seems to be more effective to correct cohesin and mitochondrial dysfunction and epigenetic disorders than other methods. However, actually, IVM of oocyte after GVT has not yet been totally completed. Compared to GV transfer and zygote pronuclear transfer, ST has several advantages. First, MII oocytes do not require in vitro maturation, compared to GV oocytes, which have not reached mature MII oocyte in vitro.91 Second, compared to PNT, MII oocytes do not require destruction of human pronucleus embryos, which is controversial from an ethical point of view .101 Lastly, the volume of mtDNA carried over into ST oocyte is much less than that of GVT or PNT.120
On the other hand, opinions critical of cytoplasmic donation remain. They insist that nuclear DNA in old oocytes is already affected through the long wait until ovulation. So, CT cannot return the nuclear DNA to a young status even after the donation of healthy cytoplasm that contains factors which help epigenetic activity.
The full implications of mixing nuclear DNA and mtDNA from two different sources remain unknown. We, clinicians, should have a thorough discussion about merits and risks involved in ST with patients. Furthermore, we have to, as much as possible, collect recent information concerning ST before entering clinical applications while at the same time continue the basic study further to establish the clinical application of cytoplasmic donation to rescue aged oocyte.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interests. Human and Animal rights: This study was approved by the Institutional Review Boards of the Saint Mother Obstetrics and Gynecology Clinic (August 2004) and the Japanese Organization of Obstetrics and Gynecology (April 2006). The authors obtained signed consents from the patients to publish the information. This article does not contain any animal studies that have been performed by any of the authors.
ACKNOWLEDGMENTS
The author thanks Mr. Roberto Rodriguez, for his advice in the preparation of this manuscript.
Tanaka A, Watanabe S. Can cytoplasmic donation rescue aged oocytes? Reprod Med Biol. 2019;18:128–139. 10.1002/rmb2.12252
REFERENCES
- 1. Comparison in pregnancy, delivery and miscarriage rate according to patients age with ART Registry 2015. Japan Society of Obstetrics & Gynecology (JSOG). https://plaza.umin.ac.jp/~jsogart/2015data_201709.pdf
- 2. Hassold T, Chen N, Funkhouser I et al. A cryogenetic study of 1000 abortions. Ann Hum Genet. 1980;44:151‐164. [DOI] [PubMed] [Google Scholar]
- 3. Guerneri S, Bettio D, Simoni G, Brambati B, Lanzani A, Fraccaro M. Prevalence and distribution of chromosome abnormalities in a sample of first trimester internal abortions. Hum Reprod. 1987;2:735‐739. [DOI] [PubMed] [Google Scholar]
- 4. Hassold T, Chiu D. Maternal age‐specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet. 1985;70:11‐17. [DOI] [PubMed] [Google Scholar]
- 5. Fukuyama K, Nakaoka Y, Endo K, Koike A, Mizuno S. Chromosome analysis was performed on concepti from patients who had early spontaneous abortion after infertility treatment. J Fertil Implant (Tokyo). 2005;22:134‐137. [Google Scholar]
- 6. Angell R. Predivision in human oocytes at meiosis I: a mechanism for trisomy formation in man. Hum Genet. 1991;86:383‐387. [DOI] [PubMed] [Google Scholar]
- 7. Angell R. Mechanism of chromosome nondisjunction in human oocytes. Prog Clin Biol Res. 1995;393:13‐26. [PubMed] [Google Scholar]
- 8. Angell R. First‐meiotic‐division nondisjunction in human oocytes. Am J Hum Genet. 1997;61:23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vialard F, Petit C, Bergere M et al. Evidence of a high proportion of unbalanced premature sister chromatid separation in the first polar bodies of women of advanced age. Hum Reprod. 2006;21:1172‐1178. [DOI] [PubMed] [Google Scholar]
- 10. Cohen J, Scott R, Schimmel T, Levron J, Willadsen S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet. 1997;350:186‐187. [DOI] [PubMed] [Google Scholar]
- 11. Hamatani T, Falco G, Carter MG et al. Age‐associated alteration of gene expression patterns in mouse oocytes. Hum. Mol. Genet. 2004;13:2263‐2278. [DOI] [PubMed] [Google Scholar]
- 12. Hawes SM, Sapienza C, Latham KE. Ooplasmic donation in humans: the potential for epigenic modifications. Hum Reprod. 2002;17:850‐852. [DOI] [PubMed] [Google Scholar]
- 13. Spikings EC, Alderson J, St John JC. Transmission of mitochondrial DNA following assisted reproduction and nuclear transfer. Hum Reprod Update. 2006;12:401‐415. [DOI] [PubMed] [Google Scholar]
- 14. Barritt JA, Brenner CA, Malter HE, Cohen J. Rebuttal: interooplasmic transfers in humans. Reprod Biomed Online. 2001;3:47‐48. [DOI] [PubMed] [Google Scholar]
- 15. Cui LB, Huang XY, Sun FZ. Transfer of germinal vesicle to ooplasm of young mice could not rescue ageing‐associated chromosome misalignment in meiosis of oocytes from aged mice. Hum Reprod. 2005;20:1624‐1631. [DOI] [PubMed] [Google Scholar]
- 16. Fulka H. Distribution of mitochondria in reconstructed mouse oocytes. Reproduction. 2004;127:195‐200. [DOI] [PubMed] [Google Scholar]
- 17. Meirelles FV, Smith LC. Mitochondrial genotype segregation during preimplantation development in mouse heteroplasmic embryos. Genetics. 1998;148:877‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCreath KJ, Howcroft J, Campbell KH, Colman A, Schnieke AE, Kind AJ. Production of gene‐targeted sheep by nuclear transfer from cultured somatic cells. Nature. 2000;405:1066‐1069. [DOI] [PubMed] [Google Scholar]
- 19. Cibelli JB, Campbell KH, Seidel GE, West MD, Lanza RP. The health profile of cloned animals. Nat Biotechnol. 2002;20:13‐14. [DOI] [PubMed] [Google Scholar]
- 20. Steinborn R, Schinogl P, Wells DN, Bergthaler A, Muller M, Brem G. Coexistence of Bos taurus and B. indicus mitochondrial DNAs in nuclear transfer‐derived somatic cattle clones. Genetics. 2002;162:823‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. St. John J, Moffatt O, D’Souza N. Aberrant heteroplasmic transmission of mtDNA in cloned pigs arising from double nuclear transfer. Mol Reprod Dev. 2005;72:450‐460. [DOI] [PubMed] [Google Scholar]
- 22. ElShourbagy SH, Spikings EC, Freitas M, St. John JC. Mitochondria directly influence fertilisation outcome in the pig. Reproduction. 2006;131:233‐245. [DOI] [PubMed] [Google Scholar]
- 23. Lin DP, Huang CC, Wu HM, Cheng TC, Chen CI, Lee MS. Comparison of mitochondrial DNA contents in human embryos with good or poor morphology at the 8‐cell stage. Fertil Steril. 2004;81:73‐79. [DOI] [PubMed] [Google Scholar]
- 24. Qiao J, Wang ZB, Feng HL et al. The root of reduced fertility in aged women and possible therapentic options: current status and future perspects. Mol Aspects Med. 2014;38:54‐85. [DOI] [PubMed] [Google Scholar]
- 25. Prieto I, Tease C, Pezzi N et al. Cohesin component dynamics during meiotic prophase I in mammalian oocytes. Chromosome Res. 2004;12:197‐213. [DOI] [PubMed] [Google Scholar]
- 26. Revenkova E, Jessberger R. Keeping sister chromatids together: cohesins in meiosis. Reproduction. 2005;130:783‐790. [DOI] [PubMed] [Google Scholar]
- 27. Hodges CA, Revenkova E, Jessberger R, Hassold TJ, Hunt PA. SMC1beta‐deficient female mice provide evidence that cohesins are a missing link in age‐related nondisjunction. Nat. Genet. 2005;37:1351‐1355. [DOI] [PubMed] [Google Scholar]
- 28. Subramanian VV, Bickel SE. Aging predisposes oocytes to meiotic nondisjunction when the cohesin subunit SMC1 is reduced. PLoS Genet. 2008;4:e1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age‐related aneuploidy in oocytes. Curr. Biol. 2010;20:1522‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lister LM, Kouznetsova A, Hyslop LA et al. Age‐related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr. Biol. 2010;20:1511‐1521. [DOI] [PubMed] [Google Scholar]
- 31. Lee J, Kitajima TS, Tanno Y et al. Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat. Cell Biol. 2008;10:42‐52. [DOI] [PubMed] [Google Scholar]
- 32. Llano E, Gomez R, Gutierrez‐Caballero C et al. Shugoshin‐2 is essential for the completion of meiosis but not for mitotic cell division in mice. Genes Dev. 2008;22:2400‐2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jessberger R. Deterioration without replenishment–the misery of oocyte cohesin. Genes Dev. 2010;24:2587‐2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Revenkova E, Herrmann K, Adelfalk C, Jessberger R. Oocyte cohesin expression restricted to predictyate stages provides full fertility and prevents aneuploidy. Curr. Biol. 2010;20:1529‐1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tachibana‐Konwalski K, Godwin J, van der Weyden L et al. Rec8‐containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev. 2010;24:2505‐2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duncan FE, Hornick JE, Lampson MA, Schultz RM, Shea LD, Woodruff TK. Chromosome cohesion decreases in human eggs with advanced maternal age. Aging Cell. 2012;11:1121‐1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hached K, Xie SZ, Buffin E et al. Mps1 at kinetochores is essential for female mouse meiosis I. Development. 2011;138:2261‐2271. [DOI] [PubMed] [Google Scholar]
- 38. Homer H, Gui L, Carroll J. A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression. Science. 2009;326:991‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Homer HA, McDougall A, Levasseur M, Yallop K, Murdoch AP, Herbert M. Mad2 prevents aneuploidy and premature proteolysis of cyclin B and securin during meiosis I in mouse oocytes. Genes Dev. 2005;19:202‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leland S, Nagarajan P, Polyzos A et al. Heterozygosity for a Bub1 mutation causes female‐specific germ cell aneuploidy in mice. Proc Nat Acad Sci USA. 2009;106:12776‐12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li M, Li S, Yuan J et al. Bub3 is a spindle assembly checkpoint protein regulating chromosome segregation during mouse oocyte meiosis. PLoS One. 2009;4:e7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wassmann K, Niault T, Maro B. Metaphase I arrest upon activation of the Mad2‐dependent spindle checkpoint in mouse oocytes. Curr. Biol. 2003;13:1596‐1608. [DOI] [PubMed] [Google Scholar]
- 43. Wei L, Liang XW, Zhang QH et al. BubR1 is a spindle assembly checkpoint protein regulating meiotic cell cycle progression of mouse oocyte. Cell Cycle. 2010;9:1112‐1121. [DOI] [PubMed] [Google Scholar]
- 44. Kallio M, Eriksson JE, Gorbsky GJ. Differences in spindle association of the mitotic checkpoint protein Mad2 in mammalian spermatogenesis and oogenesis. Dev Biol. 2000;225:112‐123. [DOI] [PubMed] [Google Scholar]
- 45. Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2001;2:280‐291. [DOI] [PubMed] [Google Scholar]
- 46. Kajii T, Ferrier A, Niikawa N, Takahara H, Ohama K, Avirachan S. Anatomic and chromosomal anomalies in 639 spontaneous abortuses. Hum Genet. 1980;55:87‐98. [DOI] [PubMed] [Google Scholar]
- 47. Hassold T, Hall H, Hunt P. The origin of human aneuploidy: where we have been, where we are going. Hum Mol Genet. 2007;16:R203–R208. [DOI] [PubMed] [Google Scholar]
- 48. Lamb NE, Yu K, Shaffer J, Feingold E, Sherman SL. Association between maternal age and meiotic recombination for trisomy 21. Am J Hum Genet. 2005;76:91‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sherman SL, Lamb NE, Feingold E. Relationship of recombination patterns and maternal age among non‐disjoined chromosomes 21. Biochem Soc Trans. 2006;34:578‐580. [DOI] [PubMed] [Google Scholar]
- 50. de Pennart H, Cibert C, Petzelt C, Maro B. Microtubule tracks can be detected in mouse oocytes with an antibody directed against a calcium transporter. J Cell Set. 1994;107:1899‐1908. [DOI] [PubMed] [Google Scholar]
- 51. Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11:2217‐2222. [DOI] [PubMed] [Google Scholar]
- 52. Battaglia DE, Klein NA, Soules MR. Changes in centrosomal domains during meiotic maturation in the human oocyte. Mol Hum Reprod. 1996;2:845‐851. [DOI] [PubMed] [Google Scholar]
- 53. Eichenlaub‐Ritter U, Baart E, Yin H, Betzendahl I. Mechanisms of spontaneous and chemically induced aneuploidy in mammalian oogenesis: basis of sex‐specific differences in response to aneugens and the necessity for further tests. Mutat Res. 1996;372:279‐294. [DOI] [PubMed] [Google Scholar]
- 54. Eichenlaub‐Ritter U. Genetics of oocyte ageing. Maturitas. 1998;30:143‐169. [DOI] [PubMed] [Google Scholar]
- 55. Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009;15:573‐585. [DOI] [PubMed] [Google Scholar]
- 56. Wang Q, Sun QY. Evaluation of oocyte quality: morphological, cellular and molecular predictors. Reprod Fertil Dev. 2007;19:128‐139. [DOI] [PubMed] [Google Scholar]
- 57. Van Blerkom J, Davis PW, Lee J. ATP content of human oocytes and developmental potential and outcome after in‐vitro fertilization and embryo transfer. Hum. Reprod. 1995;10:415‐424. [DOI] [PubMed] [Google Scholar]
- 58. Quinn P, Wales RG. The relationships between the ATP content of preimplantation mouse embryos and their development in vitro during culture. J Reprod Fertil. 1973;35:301‐309. [DOI] [PubMed] [Google Scholar]
- 59. Eichenlaub‐Ritter U. Reproductive semi‐cloning respecting biparental origin. Reconstitution of gametes for assisted reproduction. Hum Reprod. 2003;18:473‐475. [DOI] [PubMed] [Google Scholar]
- 60. Eichenlaub‐Ritter U, Wieczorek M, Luke S, Seidel T. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion. 2011;11:783‐796. [DOI] [PubMed] [Google Scholar]
- 61. Keefe DL, Niven‐Fairchild T, Powell S, Buradagunta S. Mitochondrial deoxyribonucleic acid deletions in oocytes and reproductive aging in women. Fertil Steril. 1995;64:577‐583. [PubMed] [Google Scholar]
- 62. Chen X, Prosser R, Simonetti S, Sadlock J, Jagiello G, Schon EA. Rearranged mitochondrial genomes are present in human oocytes. Am J Hum Genet. 1995;57:239‐247. [PMC free article] [PubMed] [Google Scholar]
- 63. Chan CC, Liu VW, Lau EY, Yeung WS, Ng EH, Ho PC. Mitochondrial DNA content and 4977 bp deletion in unfertilized oocytes. Mol. Hum Reprod. 2005;11:843‐846. [DOI] [PubMed] [Google Scholar]
- 64. Tomizawa S, Nowacka‐Woszuk J, Kelsey G. DNA methylation establishment during oocyte growth: mechanisms and significance. Int J Dev Biol. 2012;56:867‐875. [DOI] [PubMed] [Google Scholar]
- 65. Anwar SL, Lehmann U. DNA methylation, microRNAs, and their crosstalk as potential biomarkers in hepatocellular carcinoma. World J Gastroenterol. 2014;20:7894–7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Truesdell SS, Mortensen RD, Seo M et al. MicroRNA‐mediated mRNA translation activation in quiescent cells and oocytes involves recruitment of a nuclear microRNP. Sci Rep. 2012;2:842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pincus Z, Smith‐Vikos T, Slack FJ. MicroRNA predictors of longevity in Caenorhabditis elegans. PLoS Genet. 2011;7:e1002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Inukai S, de Lencastre A, Turner M, Slack F. Novel microRNAs differentially expressed during aging in the mouse brain. PLoS ONE. 2012;7:e40028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ye Y, Li D, Ouyang D et al. MicroRNA expression in the aging mouse thymus. Gene. 2014;547:218–225. [DOI] [PubMed] [Google Scholar]
- 70. Murchison EP, Stein P, Xuan Z et al. Critical roles for Dicer in the female germline. Genes Dev. 2007;21:682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Grondahl ML, Yding Andersen C, Bogstad J, Nielsen FC, Meinertz H, Borup R. Gene expression profiles of single human mature oocytes in relation to age. Hum Reprod. 2010;25:957–968. [DOI] [PubMed] [Google Scholar]
- 72. Santonocito M, Guglielmino MR, Vento M et al. The apoptotic transcriptome of the human MII oocyte: characterization and age‐related changes. Apoptosis. 2013;18:201–211. [DOI] [PubMed] [Google Scholar]
- 73. Schwarzer C, Siatkowski M, Pfeiffer MJ et al. Maternal age effect on mouse oocytes: new biological insight from proteomic analysis. Reproduction. 2014;148:55–72. [DOI] [PubMed] [Google Scholar]
- 74. Tatone C, Eichenlaub‐Ritter U, Amicarelli F. Dicarbonyl stress and glyoxalases in ovarian function. Biochem Soc Trans. 2014;42:433–438. [DOI] [PubMed] [Google Scholar]
- 75. Hirasawa R, Chiba H, Kaneda M et al. Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes Dev. 2008;22:1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kurihara Y, Kawamura Y, Uchijima Y et al. Maintenance of genomic methylation patterns during preimplantation development requires the somatic form of DNA methyltransferase 1. Dev Biol. 2008;313:335–346. [DOI] [PubMed] [Google Scholar]
- 77. Akiyama T, Nagata M, Aoki F. Inadequate histone deacetylation during oocyte meiosis causes aneuploidy and embryo death in mice. Proc Natl Acad Sci USA. 2006;103:7339–7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kaji K, Oda S, Miyazaki S, Kudo A. Infertility of CD9‐deficient mouse eggs is reversed by mouse CD9, human CD9, or mouse CD81; polyadenylated mRNA injection developed for molecular analysis of sperm‐egg fusion. Dev Biol. 2002;247:327–334. [DOI] [PubMed] [Google Scholar]
- 79. Cohen J, Scott R, Alikani M et al. Ooplasmic transfer in mature human oocytes. Mol Hum Reprod. 1998;4:269–280. [DOI] [PubMed] [Google Scholar]
- 80. Dale B, Wilding M, Botta G et al. Pregnancy after cytoplasmic transfer in a couple suffering from idiopathic infertility: case report. Hum Reprod. 2001;16:1469–1472. [DOI] [PubMed] [Google Scholar]
- 81. Levy R, Elder K, Menezo Y. Cytoplasmic transfer in oocytes: biochemical aspects. Hum Reprod Update. 2004;10:241–250. [DOI] [PubMed] [Google Scholar]
- 82. Barritt J, Willadsen S, Brenner C, Cohen J. Cytoplasmic transfer in assisted reproduction. Hum Reprod Update. 2001;7:428–435. [DOI] [PubMed] [Google Scholar]
- 83. Perez GI, Trbovich AM, Gosden RG, Tilly JL. Mitochondria and the death of oocytes. Nature. 2000;403:500–501. [DOI] [PubMed] [Google Scholar]
- 84. Van Blerkom J, Sinclair J, Davis P. Mitochondrial transfer between oocytes: potential applications of mitochondrial donation and the issue of heteroplasmy. Hum Reprod. 1998;13:2857–2868. [DOI] [PubMed] [Google Scholar]
- 85. Barritt JA, Brenner CA, Malter HE, Cohen J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum. Reprod. 2001;16:513–516. [DOI] [PubMed] [Google Scholar]
- 86. Tachibana M, Sparman M, Sritanaudomchai H et al. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zoon KC. Letter to Sponsors / Researchers ‐ Human Cells Used in Therapy Involving the Transfer of Genetic Material By Means Other Than the Union of Gamete Nuclei. 2001.
- 88. Liang CG, Han Z, Cheng Y, Zhong Z, Latham KE. Effects of ooplasm transfer on paternal genome function in mice. Hum. Reprod. 2009;24:2718–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang J, Wang CW, Krey L et al. In vitro maturation of human preovulatory oocytes reconstructed by germinal vesicle transfer. Fertil. Steril. 1999;71:726–731. [DOI] [PubMed] [Google Scholar]
- 90. Liu H, Wang CW, Grifo JA, Krey LC, Zhang J. Reconstruction of mouse oocytes by germinal vesicle transfer: maturity of host oocyte cytoplasm determines meiosis. Hum. Reprod. 1999;14:2357–2361. [DOI] [PubMed] [Google Scholar]
- 91. Takeuchi T, Ergun B, Huang TH, Rosenwaks Z, Palermo GD. A reliable technique of nuclear transplantation for immature mammalian oocytes. Hum. Reprod. 1999;14:1312–1317. [DOI] [PubMed] [Google Scholar]
- 92. Eichenlaub‐Ritter U, Boll I. Age‐related nondisjunction, spindle formation and progression through maturation of mammalian oocytes. Prog Clin Biol Res. 1989;318:259–269. [PubMed] [Google Scholar]
- 93. Dailey T, Dale B, Cohen J, Munne S. Association between nondisjunction and maternal age in meiosis II human oocytes. Am J Hum Genet. 1996;59:176–184. [PMC free article] [PubMed] [Google Scholar]
- 94. Volarcik K, Sheean L, Goldfarb J, Woods L, Abdul‐Karim FW, Hunt P. The meiotic competence of in‐vitro matured human oocytes is influenced by donor age: evidence that folliculogenesis is compromised in the reproductively aged ovary. Hum Reprod. 1998;13:154–160. [DOI] [PubMed] [Google Scholar]
- 95. Henderson SA, Edwards RG. Chiasma frequency and maternal age in mammals. Nature. 1968;217:22–28. [DOI] [PubMed] [Google Scholar]
- 96. Crowley PH, Gulati DK, Hayden TL, Lopez P, Dyer R. A chiasmahormonal hypothesis relating Down syndrome and maternal age. Nature. 1979;280:417–418. [DOI] [PubMed] [Google Scholar]
- 97. Takeuchi T, Neri QV, Katagiri Y, Rosenwaks Z, Palermo GD. Effect of treating induced mitochondrial damage on embryonic development and epigenesis. Biol Reprod. 2005;72:584–592. [DOI] [PubMed] [Google Scholar]
- 98. Palermo GD, Takeuchi T, Rosenwaks Z. Technical approaches to correction of oocyte aneuploidy. Hum Reprod. 2002;17:2165–2173. [DOI] [PubMed] [Google Scholar]
- 99. Meirelles F, Smith L. Mitochondrial genotype segregation in a mouse heteroplasmic lineage produced by embryonic karyoplast transplantation. Genetics. 1997;145:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. He Z, Liu HC, Rosenwaks Z. Cryopreservation of nuclear material as a potential method of fertility preservation. Fertil Steril. 2003;79:347–354. [DOI] [PubMed] [Google Scholar]
- 101. Sato A, Kono T, Nakada K et al. Gene therapy for progeny of mito‐mice carrying pathogenic mtDNA by nuclear transplantation. Proc Natl Acad Sci USA. 2005;102:16765–16770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Craven L, Tuppen HA, Greggains GD et al. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature. 2010;465:82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhang J, Zhuang G, Zeng Y, Acosta C, Shu Y, Grifo J. Pregnancy derived from human nuclear transfer. Fertil Steril. 2003;80:S56. [Google Scholar]
- 104. Liu L, VanderElst J, Dhont M. In vitro parthenogenetic development of mouse oocytes following reciprocal transfer of chromosome spindle between in vivo‐matured oocytes and in vitro‐matured oocytes. Biol Reprod. 2003;68:186–189. [DOI] [PubMed] [Google Scholar]
- 105. Bai ZD, Liu K, Wang XY. Developmental potential of aged oocyte rescued by nuclear transfer following parthenogenetic activation and in vitro fertilization. Mol Reprod Dev. 2006;73:1448–1453. [DOI] [PubMed] [Google Scholar]
- 106. Mitsui A, Yoshizawa M, Matsumoto H, Fukui E. Improvement of embryonic development and production of offspring by transferring meiosis‐II chromosomes of senescent mouse oocytes into cytoplasts of young mouse oocytes. J Assist Reprod Genet. 2009;26:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kasahara A, Ishikawa K, Yamaoka M et al. Generation of trans‐mitochondrial mice carrying homoplasmic mtDNAs with a missense mutation in a structural gene using ES cells. Hum Mol Genet. 2006;15:871–878. [DOI] [PubMed] [Google Scholar]
- 108. Tanaka A, Nagayoshi M, Awata S et al. Metaphase II karyoplast transfer from human in‐vitro matured oocytes to enuclueated mature oocytes. Reprod Biomed Online. 2009;19:514–520. [DOI] [PubMed] [Google Scholar]
- 109. Nishino T, Kamiguchi Y, Tateno H, Sengoku K, Ishikawa M. A cytogenetic study of human oocytes unfertilized in in‐vitro fertilization (IVF). Acta Obstetrica et Gynaecologica Japonica. 1994;46:95–101. [PubMed] [Google Scholar]
- 110. Oldenbourg R. A new view on polarization microscopy. Nature. 1996;381:811–812. [DOI] [PubMed] [Google Scholar]
- 111. Tanaka A, Nagayoshi M, Tanaka I, Kusunoki H. Direct Visualization of Mataphase‐II Chromosomes in Human Oocytes Under an Inverted Microscope. Recent Patents Med Imag. 2011;1:84–88. [Google Scholar]
- 112. Tachibana M, Amato P, Sparman M et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Paull D, Emmanuele V, Weiss KA et al. Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants. Nature. 2013;493:632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhang J, Liu H, Luo S et alFirst live birth using human oocytes reconstituted by spindle nuclear transfer for mitochondrial DNA mutation causing Leigh syndrome. Fertil Steril. 2016;106:e375–e376. [Google Scholar]
- 115. Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta. 2002;1576:128–14. [DOI] [PubMed] [Google Scholar]
- 116. Stojanovski D, Johnston AJ, Streimann I, Hoogenraad NJ, Ryan MT. Import of nuclear‐encoded proteins into mitochondria. Exp Physiol. 2003;88:57–64. [DOI] [PubMed] [Google Scholar]
- 117. Falkenberg M, Gaspari M, Rantanen A, Trifunovic A, Larsson NG, Gustafsson CM. Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nat Genet. 2002;31:289–294. [DOI] [PubMed] [Google Scholar]
- 118. Yamaoka M, Isobe K, Shitara H, Yonekawa H, Miyabayashi S, Hayashi JI. Complete repopulation of mouse mitochondiral DNA‐less cells with rat mitochondrial DNA restores translation but not mitochondrial respiratory function. Genetics. 2000;155:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kenyon L, Moraes C. Expanding the functional human mitochondrial DNA database by the establishment of primate xenomitochondrial cybrids. Proc Natl Acad Sci USA. 1997;94:9131–9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tachibana M, Sparman M, Mitalipov S. Chromosome transfer in mature oocytes. Nat. Protoc. 2010;5:1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]


