Abstract
The majority of giant hepatic cavernous hemangiomas are asymptomatic and can safely be observed. However, when a lesion becomes symptomatic, affecting quality of life or cannot be distinguished from a malignancy, then operative therapy should be considered. We herein present a case of a symptomatic 12cm × 14cm × 17cm “mega” hemangioma (>10cm) of the left hepatic lobe. This lesion was initially refractory to transarterial embolization of the left hepatic artery, but was subsequently treated successfully with a left lateral extended hepatic segmentectomy (resection). We thus advocate a rational treatment algorithm for management of hepatic “mega” hemangiomas.
Keywords: hepatic cavernous hemangioma, transarterial embolization, liver resection
Introduction
Cavernous hemangiomas are the most prevalent (73%) benign tumors affecting the liver, with incidence up to 7.3% on autopsy series.1 They are also a common incidental finding on routine imaging, and tend to be small (< 1 cm), stable, and asymptomatic. Lesions >4 to 5 cm are considered giant hemangiomas; despite large intra-abdominal growth, giant hemangiomas generally remain asymptomatic in most cases.1–2 Hepatic hemangiomas are more prevalent in middle-aged women, can progress in size during pregnancy and can be diagnosed via multiple imaging modalities including ultrasound, MRI, and CT, but the gold standard for diagnosis remains IV contrast-enhanced abdominal CT.1–2
Management of giant hemangiomas is controversial, and prophylactic treatment via surgical resection or other means has historically been the standard of care to avert potential grave complications, such as rupture/bleeding, thrombosis, or disseminated intravascular coagulation / consumptive thrombocytopenia (Kasabach-Merritt Syndrome).2 However, prevention of rupture should not be considered a lone indication for surgical extirpation of an asymptomatic lesion.3 Conversely, treatment should be offered to patients with symptomatic lesions affecting quality of life, and the literature describes various approaches that can safely and effectively be employed.4–6
It is well established that the preponderance of giant hepatic hemangiomas are asymptomatic, in spite of their foreboding designation.1–10 Therefore, we feel it would be useful to introduce a new distinct terminology, “mega,” for hepatic hemangiomas measuring >10cm, as these lesions are far more likely to provoke symptoms with possible complications. Herein, we report a case of a symptomatic hepatic mega-hemangioma, and advocate a rational treatment algorithm for managing patients with this condition. 1–12
Case Report
A 34-year-old otherwise healthy male was referred to our general surgery clinic at TAMC (Tripler Army Medical Center) for evaluation of a firm, large, non-tender abdominal mass, hepatic cavernous hemangioma, easily visible and palpable on physical exam which protruded and extended across the midline of the abdominal wall from the right upper quadrant, and also extended caudally midway between the xiphoid and umbilicus. The patient first noticed the mass approximately two months prior to evaluation, and complained of moderate abdominal discomfort and intermittent early satiety. However, his primary concerns were the rapid growth, and increasing visibility of this hepatic mass on his abdominal wall.
Of note, he was found to be near thrombocytopenic with a platelet count in the low 100,000 per microlitre (range 150,000–450,000) and anemic with a hemoglobin and hematocrit that nadired at 11.6g/dL (12–16) and 34.7% (40–50), respectively. His coagulation profile was also noted to be abnormal, with a prothrombin time of 15.5sec (10–13), a fibrinogen of 102mg/dL (200–500), and an elevated D-dimer, though he did not experience any bleeding stigmata or manifest signs of thrombocytopenia. A subsequent CT examination of his abdomen and pelvis with IV and oral contrast were performed, which revealed a 12cm x 14cm x 17cm lesion located in the left hepatic lobe, consistent with a mega-hemangioma. (Figure 1)
Figure 1.
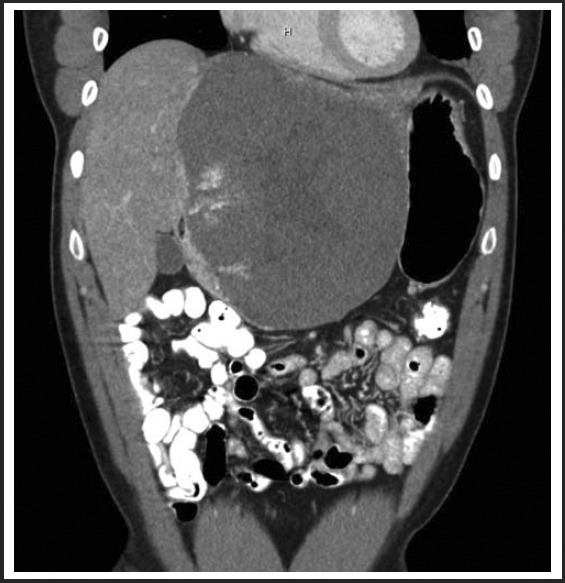
12cm × 14cm low density heterogeneous mass within the left hepatic lobe on CT performed in the Fall of 2010. The mass demonstrated peripheral pooling of contrast initially with centripetal filling on delayed images using triphase IV contrast abdominal CT, consistent with a cavernous hemangioma.
Due to the patient's initial moderate symptoms of abdominal pain and early satiety after meals, we opted for conservative management and consulted our Interventional Radiology Service to perform a transarterial embolization procedure, with the goals of decreasing the size of the mass and relieving his gastrointestinal symptoms.
While undergoing radiologic interventions, pre-embolization angiography revealed a normal course of the celiac arterial axis. A selective angiography was next performed by means of a VS1 catheter, demonstrating two vessels from the left hepatic artery that were supplying the hemangioma. (Figure 2) Further subselection of the left hepatic artery demonstrated contrast pooling with a characteristic blush to the known hemangioma. Bead Block® (Terumo Europe/ Biocompatibles) microspheres were used to embolize the left hepatic artery with an immediate result; there was decreased blood flow and no significant blush was seen in the post-embolization angiogram. (Figure 3) The patient tolerated the procedure well and there were no complications.
Figure 2.
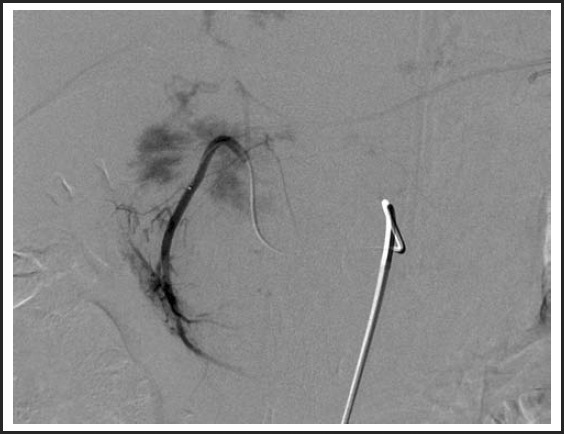
Pre-embolization arteriogram.
Figure 3.
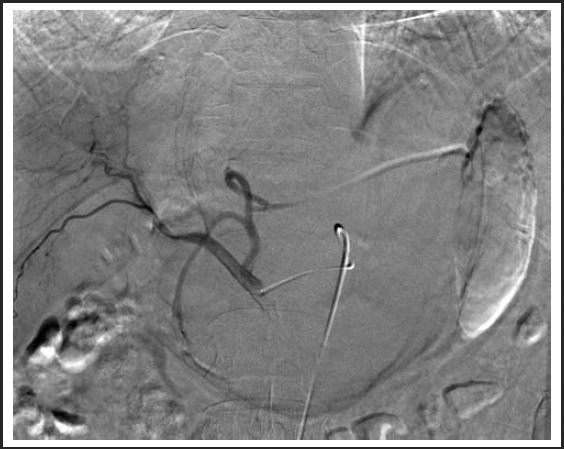
Post-embolization of the left hepatic arterial trunk with 6ml of Bead Block 500–700 microspheres.
Post-procedure imaging at 3 months revealed that the lesion had been refractory to the transarterial embolization procedure of the left hepatic artery, as it effectively did not downsize, but it did help alleviate some of his abdominal symptoms. (Figure 4) This symptomatic relief was short lived however since after a few months, the patient continued to have abnormal serum laboratory tests and worsening of other baseline symptoms which include: increasing abdominal girth and new-onset shortness of breath with moderate physical exertion. The lesion had also become tender to palpation on physical examination.
Figure 4.
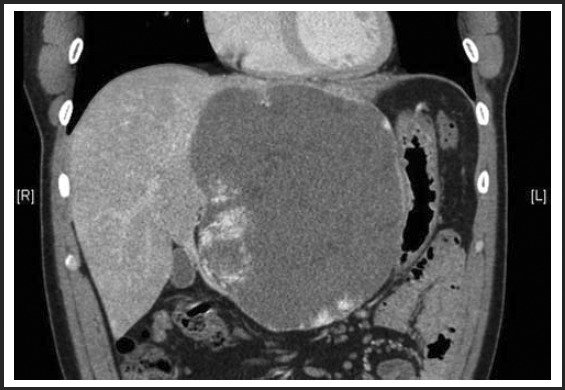
No significant change in size or appearance of mega hemangioma three months status post-embolization, measuring 12.3cm × 13.7cm × 17.2cm.
The patient was offered a repeat embolization by our interventional radiologist, but he declined and opted instead for an elective surgical resection or enucleation of his hemangioma. This was completed successfully 9 months following his embolization procedure. Upon entry into the abdomen, a large liver hemangioma was readily apparent replacing the left lobe of liver. There was no blood noted or evidence of other pathology within the abdomen. The extent of the lesion was assessed and it was determined that it occupied and greatly expanded the entire left lateral segment without extension beyond the falciform ligament. The decision was made intraoperatively to perform a left lateral extended segmentectomy to include the involved segments 2 and 3 and to ensure complete resection of the mass. Hemostasis was good following the liver parenchymal dissection with a moderate amount of blood loss (500mL, estimated blood loss). The patient remained hemodynamically stable throughout the procedure and was transferred to the ICU for close postoperative monitoring.
His postoperative course was relatively uncomplicated. He required transfusion with one unit of packed red blood cells for symptomatic anemia on postoperative day #2 and one unit of cryoprecipitate due to low fibrinogen and low grade DIC (disseminated intravascular coagulation). He maintained a stable hemoglobin/hematocrit and coagulation profile following his transfusions. He was discharged home on postoperative day #6 ambulating and tolerating a regular diet with well-controlled incisional pain while on oral analgesics.
At six-month clinic follow-up, the patient was completely asymptomatic, and he had no complaints related to his surgery. A single post-procedure CT scan of the abdomen demonstrated normal post-surgical changes, along with expected hypertrophy of his right hepatic lobe. (Figure 5) There was no recurrent hemangioma evident. The patient was again seen at 18 months after surgery in our clinic and he remained asymptomatic without complications.
Figure 5.
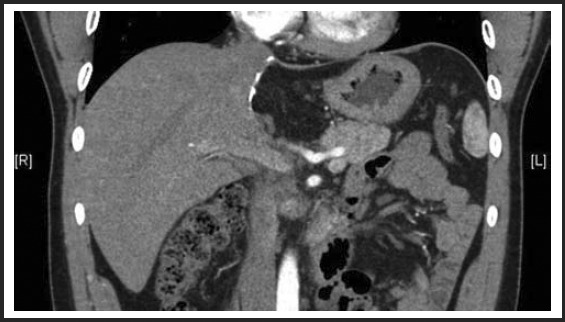
Six month status-post extended left lateral segmentectomy.
Discussion
With the advent of minimally invasive interventional radiology techniques, transarterial embolization has emerged as a reasonable and often effective treatment option for this benign condition given its low rate of morbidity.5,7–8 In our current case, while the embolization procedure did not visibly downsize the lesion, it also did not complicate or negatively impact the subsequent operative intervention of definitive hepatic resection. Additionally, it has been suggested that embolization therapy can have a positive effect on the technical aspect of the surgical procedure because it facilitates mobilization of the liver by reducing the overall volume of the hemangioma via occlusion of the main feeding vessels from the hepatic arteries. This is especially relevant for patients at high risk for bleeding during and after surgery, such as those with centrally located hemangiomas and those with hemangiomas in close proximity to vascular structures such as portal or hepatic veins.8 Embolization therapy has been successfully utilized to alleviate symptoms of mass-effect abdominal pain and discomfort in the time period prior to surgical intervention 5,7–8 Some institutions even advocate same day embolization followed by liver resection.1 Therefore, it is our recommendation that initial treatment with transarterial embolization for life-altering symptomatic mega hepatic hemangiomas (> 10 cm) be considered as either the sole therapy or as a staging (bridge) procedure prior to definitive surgery.1,5,7–8,12 In summary, we advocate a stepwise assessment of size, location, growth rate with IV contrast CT or MR imaging plus adding clinical symptomatology as the initial work-up of mega hepatic hemangiomas (>10 cm) leading to upfront embolization if indicated, then to definitive surgical therapy if embolization fails (Figure 6). In a similar vein the American College of Gastroenterology in their practice guideline recommends that for life-style altering symptomatic giant hemangiomas greater than 10 cm that these patients be referred for definitive surgical or non-surgical therapy by an experienced healthcare team of providers.13
Figure 6.
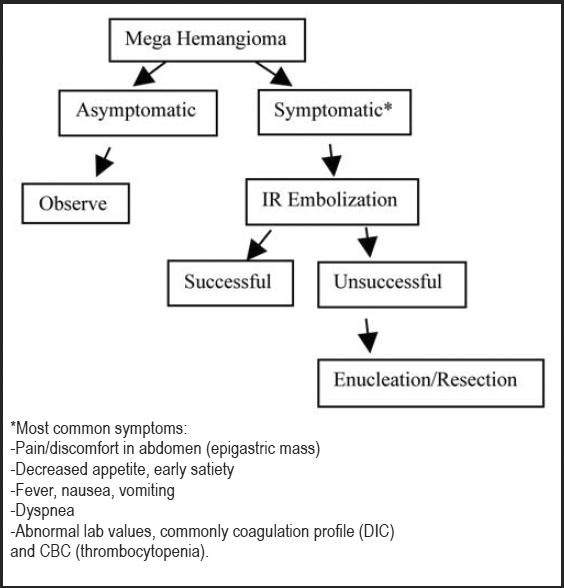
Proposed treatment algorithm for management of mega hepatic hemangioma (>10 cm).
This case report may be criticized for its exclusion of other treatment modalities, most notably radiofrequency (RFA) and microwave ablations (MWA). However, in general these modalities are reserved for moderate size hepatic lesions (5–10cm) with the caveat that MWA tends to work better than RFA for relatively larger hemangiomas, and MWA's effectiveness compared to RFA is not hampered much by the “current/heat-sink” phenomenon for hemangiomas that are located adjacent to portal or major hepatic veins, nor by the local impedence of ablated (dessicated) tissue, hence these methods have been shown to be promising for definitive hepatic hemangioma treatment in some cases.14–15 The effectiveness of these ablative modalities however are limited by the “heat sink” phenomenon for hemangiomas that are located adjacent to portal or major hepatic veins. Comparison of these ablative modalities to embolization is a potential area of further study that can help determine how ablation fits into our current proposed treatment algorithm outlined above.15 Radiation and liver transplantation are alternative treatment options; however the first is for unresectable cases and is not considered definitive and the second is generally reserved for multiple simultaneous mega hemangiomas (>10cm) in which resection of a large volume of liver parenchyma would risk post-op liver failure.6,16
Conclusion
Hepatic “mega” hemangiomas (>10cm) can be safely observed when asymptomatic given their low risk for spontaneous or traumatic rupture and other complications.3 Treatment should be reserved for symptomatic patients after completing a thorough history and physical evaluation to exclude other etiologies for their complaints.2,4 When treatment is indicated based on life-quality altering symptoms, it is prudent to begin with a minimally-invasive interventional radiology procedure (embolization or ablation) given its inherently low risk of morbidity such as bleeding compared to surgery.8,14–15 If however embolization or ablative therapies are ineffective, hepatic surgical resection or enucleation remains the definitive treatment for these refractory cases.2,4,6
Footnotes
The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
Conflict of Interest
None of the authors identify a conflict of interest.
References
- 1.Bailey J, Di Carlo S, Blackwell J, Gomez D. Same day arterial embolization followed by hepatic resection for treatment of giant haemangioma. BMJ Case Rep. 2016 Feb 25; doi: 10.1136/bcr-2015-213259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoekstra LT, Bieze M, Erdogan D, Roelofs JJTH, Beuers UHW, van Gulik TM. Management of giant liver hemangiomas: an update. Expert Review of Gastroenterology & Hepatology. 2013;7(3):263+. doi: 10.1586/egh.13.10. [DOI] [PubMed] [Google Scholar]
- 3.Plackett TP, Lin-Hurtubise KM. Hepatic hemangiomas and parachuting. Aviat Space Environ Med. 2008 Oct;79(10):986–988. doi: 10.3357/asem.2358.2008. [DOI] [PubMed] [Google Scholar]
- 4.Bajenaru N, Balaban V, Savulescu F, Campeanu I, Patrascu T. Hepatic hemangioma-review. Journal of Medicine and Life. 2015;8(Spec Issue):4–11. [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma A, Kaspar M, Siddiqui M, Kim J. Enucleation after Embolization of Liver Failure-Causing Giant Liver Hemangioma. Am J Case Rep. 2015 Aug 24;16:563–567. doi: 10.12659/AJCR.893298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, Huang Z-Y, Ke C-S, et al. Surgical Treatment of Giant Liver Hemangioma Larger than 10cm: A Single Center's Experience with 86 Patients. Medicine. 2015 Aug;94(34):e1420. doi: 10.1097/MD.0000000000001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun JH, Nie CH, Zhang YL, et al. Transcatheter Arterial Embolization Alone for Giant Hepatic Hemangioma. PLoS One. 2015 Aug 19;10(8) doi: 10.1371/journal.pone.0135158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topaloğlu S, Oğuz S, Kalayc O, et al. Preoperative arterial embolization of large liver hemangiomas. Diagn Interv Radiol. 2015 May-Jun;21(3):222–228. doi: 10.5152/dir.2014.14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H, Chen Z, Prasoon P, et al. Surgical management for giant liver hemangiomas greater than 20cm in size. Gut and Liver. 2011;5(2):228–233. doi: 10.5009/gnl.2011.5.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho HY, Wu TH, Yu MC, et al. Surgical management of giant hepatic hemangiomas: complications and review of the literature. Chang Gung Med J. 2012;35:70–77. doi: 10.4103/2319-4170.106164. [DOI] [PubMed] [Google Scholar]
- 11.Uetama T, Yoshida H, Hirakata A, et al. A symptomatic giant hepatic hemangioma treated with hepatectomy. J Nippon Med Sch. 2011;78:34–39. doi: 10.1272/jnms.78.34. [DOI] [PubMed] [Google Scholar]
- 12.Lupinacci RM, Szejnfeld D, Farah JF. Spontaneous rupture of a giant hepatic hemangioma: sequential treatment with preoperative transcatheter arterial embolization and conservative hepatectomy. G Chir. 2011;32:469–472. [PubMed] [Google Scholar]
- 13.Marrero J, Ahn J, Reddy K, et al. ACG clinical Guidelines: The Diagnosis and Management of Focal Liver Lesions. Am J Gastroenterology (advance online publication) 2014 Aug 19; doi: 10.1038/ajg.2014.213. [DOI] [PubMed] [Google Scholar]
- 14.Gao J, Ke S, Ding X-M, et al. Radiofrequency ablation for large hepatic hemangiomas: Initial experience and lessons. Surgery. 2013;153:78–85. doi: 10.1016/j.surg.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Ziemlewicz TJ, Wells SA, Lubner MA, et al. Microwave Ablation of Giant Hepatic Cavernous Hemangiomas. CardioVascular and Interventional Radiology. 2014;37:1299–1305. doi: 10.1007/s00270-014-0934-x. [DOI] [PubMed] [Google Scholar]
- 16.Gaspar L, Mascarenhas F, Sada Costa M, et al. Radiation therapy in the unresectable cavernous hemangioma of the liver. Radiotherapy and Oncology. 1993 Oct;29(1):45–50. doi: 10.1016/0167-8140(93)90172-5. [DOI] [PubMed] [Google Scholar]


