Summary
Background
The finding of casual sex partners on the internet and methamphetamine use have been described as risk factors for HIV infection in men who have sex with men (MSM). However, the interplay between these factors has not been studied prospectively in one design. This study aims to determine the associations between finding casual sex partners on the internet and incident methamphetamine use and HIV infection.
Methods
In this observational cohort study of Thai MSM, we recruited Bangkok residents aged 18 years or older with a history of penetrative male-to-male sex in the past 6 months. Baseline and follow-up visits were done at a dedicated study clinic in central Bangkok. Men were tested for HIV infection at every study visit and for sexually transmitted infections at baseline. Baseline demographics and HIV risk behaviour information were collected at every visit by audio computer-assisted self-interview. We used a descriptive model using bivariate odds ratios to elucidate the order of risk factors in the causal pathway to HIV incidence and methamphetamine use. We used Cox proportional hazard regression analysis to evaluate covariates for incident methamphetamine use and HIV infection.
Findings
From April 6, 2006, to Dec 31, 2010, 1977 men were screened and 1764 were found eligible. 1744 men were enrolled, of whom 1372 tested negative for HIV and were followed up until March 20, 2012. Per 100 person-years of follow-up, incidence of methamphetamine use was 3·8 (128 events in 3371 person-years) and incidence of HIV infection was 6·0 (212 events in 3554 person-years). In our descriptive model, methamphetamine use, anal sex, and various other behaviours cluster together but their effect on HIV incidence was mediated by the occurrence of ulcerative sexually transmitted infections. Dual risk factors for both incident methamphetamine use and HIV infection were younger age and finding casual sex partners on the internet. Having ever received money for sex was predictive for incident methamphetamine use; living alone or with a housemate, recent anal sex, and ulcerative sexually transmitted infections at baseline were predictive for incident HIV infection.
Interpretation
In MSM in Bangkok, casual sex partner recruitment on the internet, methamphetamine use, and sexually transmitted infections have important roles in sustaining the HIV epidemic. Virtual HIV prevention education, drug use harm reduction, and biomedical HIV prevention methods, such as pre-exposure prophylaxis, could help to reduce or revert the HIV epidemic among MSM in Bangkok.
Funding
US Centers for Disease Control and Prevention and the Johns Hopkins Fogarty AIDS International Training and Research Program.
Introduction
Recent increases in the spread of HIV infection among men who have sex with men (MSM) have coincided with the arrival of the internet (including hand-held technologies and social media) as a convenient way to find casual sex partners.1 During the same time, increases in methamphetamine use to enhance sexual pleasure and a resurgence of unprotected anal intercourse and sexually transmitted infections (STIs) have been reported in MSM.2,3 Several studies have investigated risk characteristics that predict HIV risk behaviour and infection in MSM who are finding casual sex partners on the internet, such as being young, use of methamphetamine and erectile dysfunction drugs, multiple sex partners, engagement in chemsex parties, intentional unprotected anal intercourse, and high prevalence of STIs.4–6 The internet might, therefore, play an important part in recent increases in HIV infection among MSM.5,6
Thai MSM who use drugs for sexual pleasure, such as methamphetamine, are at higher risk for HIV infection.7,8 The prevalence of drug use among Thai MSM has increased substantially during the past decade. In 2003, less than 5% of men included in integrated biological and behavioural HIV surveillance in Bangkok reported drug use during the past 3 months, but by 2014 this had increased to more than 40%.9,10 This increase coincided with an increase in HIV prevalence and incidence in the MSM population: the HIV prevalence increased from 17·3% in 2003 to 30·8% in 2007 and has been stable at around 30% during the ensuing years.8,11 A better understanding of the dynamics between the various epidemiological risk factors in MSM is important for the development of public health interventions.
In April, 2006, we began an observational cohort study among MSM in Bangkok. Here, we analyse and report data collected regarding finding casual sex partners on the internet, other risk behaviours, and incident methamphetamine use and HIV infection.
Methods
Study design and population
In our observational cohort study, we recruited Thai men aged 18 years or older who were Bangkok residents, had a history of penetrative male-to-male sex in the past 6 months, and were available for follow-up visits every 4 months for at least 3 years; follow-up was later extended to 5 years, once 3-year follow-up had been completed. The study was run at the Silom Community Clinic, an HIV testing and research centre in central Bangkok. Men were recruited from HIV testing and counselling services provided at the Silom Community Clinic, at entertainment venues (eg, bars, discos, saunas), through the internet, and by word of mouth. The study design has been described previously8 and only methods relevant to the current analyses are presented here.
The protocol for this study was reviewed and approved by the Ethical Review Committee of the Thailand Ministry of Public Health and by an Institutional Review Board of the US Centers for Disease Control and Prevention. Prior to enrolment, written informed consent was obtained from all participants.
Procedures
Demographic information was recorded at baseline and behavioural data were collected at each study visit using a standardised audio computer-administered self-interview. A clinical examination was performed, medical history taken, and a 25 mL blood sample collected for HIV testing at all visits and for STI evaluation at baseline. Leftover specimens were stored for future testing. Men received pre-test and post-test HIV and risk behaviour counselling during every study visit. Those testing HIV-positive at baseline or during follow-up were referred for antiretroviral treatment as per Thai national guidelines.12 Men with active STIs (laboratory or clinical evidence of Neisseria gonorrhoeae and Chlamydia trachomatis or acute clinical symptoms of herpes simplex virus [HSV] and Treponema pallidum infection) were treated instantly and those without hepatitis B virus immunity were offered vaccination, all free of charge. T pallidum diagnosis and treatment was based on clinical observations and outcomes of sequential testing for the presence of this infection.13
We used the following algorithm to determine if participants found casual sex partners on the internet. First, participants were asked whether they had had any casual sex partners in the past 4 months (yes or no). Men who answered yes were asked how many they had met via the internet. This variable was coded yes if participants reported at least one casual sex partner encountered on the internet; otherwise, it was coded no. Methamphetamine use (defined as use of hydrochloride or crystallised methamphetamine, also known as crystal or ice) to enhance sexual pleasure was coded yes if participants reported use during the past 4 months or ever at baseline. Erectile dysfunction drug use was recorded as yes if use of sildenafil or similar drugs during the same recall period were reported. Otherwise, these variables were coded no. Attendance of so-called chemsex parties (defined as gatherings where people engage in group sex while on drugs) was coded as yes if the participant reported to have ever joined such a party and as no if otherwise. Other risk behaviours were assessed similarly. Because insertive and receptive anal intercourse were collinear among participants reporting attendance of chemsex parties and using methamphetamine, this variable was coded as yes if any anal intercourse was reported and no in the absence of such reports.
Prevalent and incident HIV infection was determined using OraQuick HIV-1/2 Rapid Test (OraSure Technologies, Bethlehem, PA, USA), and if reactive, confirmed according to the Thai national algorithm with three rapid tests on blood: Determine HIV-1/2 (Abbott Laboratories, Tokyo, Japan), DoubleCheck II HIV-1&2 (Organics, Yavne, Israel) or SDBioline HIV-1/2 3.0 (Standard Diagnostics, Kyonggi-do, South Korea), and Capillus HIV-1/HIV-2 (Trinity Biotech, Jamestown, NY, USA) or Core HIV-1/2 (CORE Diagnostics, Birmingham, UK). Baseline blood specimens were tested for antibodies against ulcerative STIs (ie, HSV-1 and HSV-2, with HerpeSelect 1 and 2 Elisa, Focus Diagnostics, Cypress, CA, USA), and T pallidum by rapid plasma reagin (RPR) assay (Macro-VueTM RPR 18 mm Circle Card Test, Becton Dickinson Microbiology Systems, Sparks, MD, USA) confirmed by immunochromatography (Determine Syphilis-TP, Abbott Laboratories, Tokyo, Japan). Participants with RPR of at least 1 : 8 and positive immunochromatography were considered positive for T pallidum (current or past infection).13 Outcomes of this testing were subsequently used in the analysis as presence or absence of ulcerative STIs.
Statistical analysis
The initial sample size of the HIV uninfected portion of cohort (n=1114) was based on an estimated HIV prevalence of 17% at baseline and HIV incidence of three per 100 person-years (95% CI 2–4) of follow-up. Because of the open character of the cohort we replaced HIV seroconverted men and those lost-to-follow-up.
To evaluate bivariate associations among predictive factors and conceptualise their position in the causal pathway to incident HIV infection, a descriptive model was created on the basis of odds ratios (ORs) between the factors. Directions among variables in the model are based on theoretical assumptions about the sequence of events, not on empirical evidence.
Multivariate analyses considered two outcome variables, studied sequentially. Our first analysis aimed to predict incident methamphetamine use from available demographic and risk characteristics (excluding HIV infection), followed by a second analysis to predict HIV incidence. The latter analysis also included methamphetamine use as a predictor of HIV risk.
Methamphetamine use incidence density was calculated as the number of men newly reporting this behaviour divided by the total number of person-years of follow-up among HIV-negative men never having used meth amphetamine at baseline. Person-time was calculated with the midpoint between the date of last report of no use and date of first use, or, in men consistently reporting no use, the date of the last visit.
HIV incidence density was calculated as the number of seroconversions divided by the number of person-years of follow-up among those testing HIV-negative at baseline. The date of seroconversion was defined as the midpoint between the date of the last HIV-negative and first HIV-positive visit. The person-time contributed was from baseline to the midpoint date of the seroconversion interval, or to the date of the last visit for men who were persistently HIV-negative.
Covariates of time to first report of methamphetamine use or to HIV seroconversion were analysed using Cox proportional hazards regression. For use in models, we selected variables significant in bivariate analysis and those of theoretical importance on the basis of previous research. Models included baseline demographic and behavioural information as fixed and the past 4-month risks as time-dependent covariates.
Participants who missed a follow-up visit resulting in missing data or records were excluded from the analysis. Missing values for specific variables were rare for most risk factors. If data were missing despite visits, these data were substituted using the first available response obtained during subsequent visits; if no data were available, we used an indicator variable for the missing response category so the variable could be included in multivariate modelling.
The proportional hazards assumption for Cox regression analysis was assessed by scaled Schoenfeld residuals. Hazard ratios and 95% CIs were calculated for these associations and p values of 0·05 or less were considered statistically significant. Correlation between Kaplan-Meier methamphetamine and HIV incidence curves was evaluated using Pearson’s R coefficient (two-sided test). All analyses were done with STATA, version SE14.
Role of the funding source
The funders of this study had no role in study design; data gathering, analysis, or interpretation; or writing of the report. The corresponding author had full access to all the data and carries the final responsibility for the decision to submit for publication.
Results
From April 6, 2006 to Dec 31, 2010, 1977 men were screened and 1764 were found eligible. 1744 were enrolled, of whom 1372 tested HIV negative and were followed-up until March 20, 2012. The overall retention rate during the study period was 81·7%. At baseline, the median age was 26 years (IQR 22–30), nearly half of participants had at least a university-level education, most were employed, and nearly half were living alone or with a housemate (table 1).
Table 1:
Demographic characteristics
| Participants (n=1372) | |
|---|---|
| Age (years) | |
| 18–21 | 264 (19%) |
| 22–29 | 724 (53%) |
| ≥30 | 384 (28%) |
| Education | |
| Primary | 37 (3%) |
| Secondary or vocational | 699 (51%) |
| University and above | 636 (46%) |
| Study or work status | |
| Work | 797 (58%) |
| Work and study | 523 (38%) |
| Neither | 52 (4%) |
| Current living situation | |
| With family | 546 (40%) |
| With partner | 193 (14%) |
| Alone or with housemate | 633 (46%) |
| Used erectile dysfunction drugs | |
| Yes | 147 (11%) |
| No | 1225 (89%) |
| Anal sex | |
| Yes | 1279 (93%) |
| No | 93 (7%) |
| Total number of male partners | |
| None | 64 (5%) |
| 1–5 | 786 (57%) |
| ≥6 | 522 (38%) |
| Condom use with male partner | |
| Always | 551 (40%) |
| Not always | 728 (53%) |
| Not applicable | 93 (7%) |
| Group sex | |
| Yes | 461 (34%) |
| No | 910 (66%) |
| Coerced into sex | |
| Yes | 212 (15%) |
| No | 1159 (84%) |
| Received money for sex | |
| Yes | 239 (17%) |
| No | 1133 (83%) |
| Internet recruitment of casual sex partners | |
| Yes | 541 (39%) |
| No | 831 (61%) |
| Attended chemsex party | |
| Yes | 59 (4%) |
| No | 1000 (73%) |
| Unknown or missing | 313 (23%) |
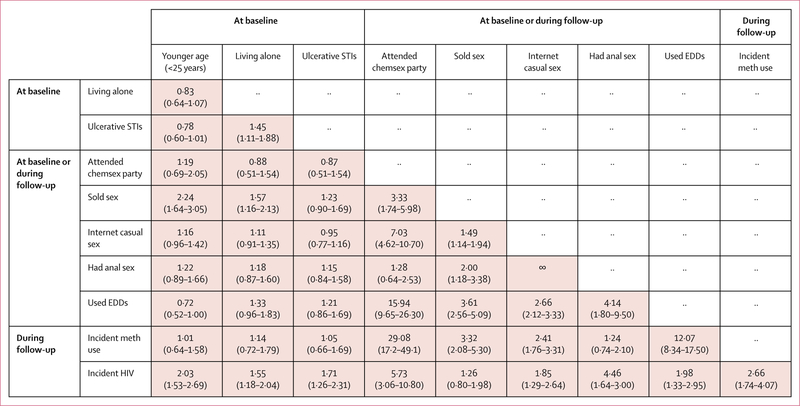
Finding casual sex partners on the internet was strongly associated with attending chemsex parties, which in turn was highly associated with reported use of erectile dysfunction drugs, incident methamphetamine use, and HIV seroconversion (figure 1). Finding casual sex partners on the internet was also strongly associated with practising anal sex, and anal sex was associated with reports of erectile dysfunction drug use and HIV seroconversion (figure 1). Incident methamphetamine use was also highly associated with erectile dysfunction drug use.
Figure 1: Matrix of bivariate odds ratios (95% CI) between risk characteristics among men who have sex with men.
EDD=erectile dysfunction drugs. Internet casual sex=finding casual sex partners on the internet. Meth=methamphetamine. STI=sexually transmitted infection.
The conceptual model (figure 2) uses bivariate associations between variables to reflect their position in the causal pathway to incident HIV infection. Bivariate associations between finding casual sex partners on the internet, attendance of chemsex parties, erectile dysfunction drug use, anal sex history, and incident methamphetamine use form a cluster of behaviours, but their association with incident HIV infection is moderate and mediated by the presence or absence of ulcerative STIs (figure 2).
Figure 2: Conceptual model of incident HIV infection.
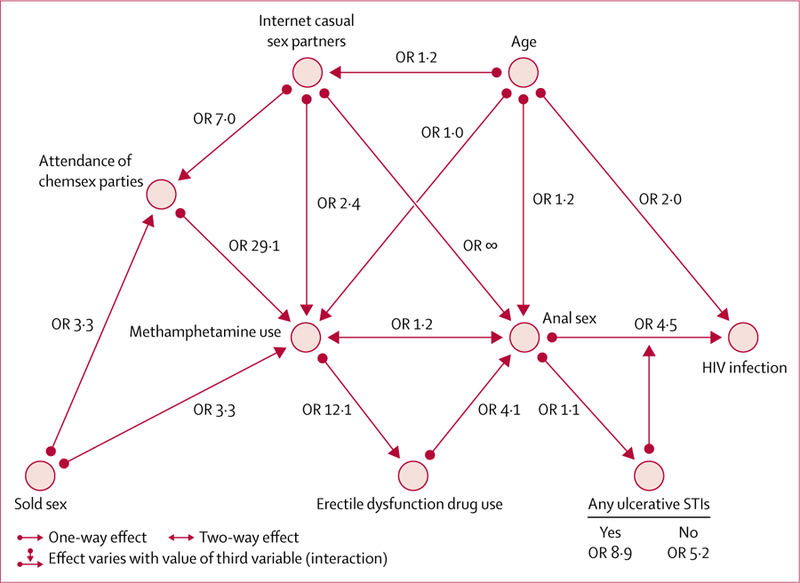
OR=odds ratio. STIs=sexually transmitted infections.
138 (10·1%, 95% CI 8·5–11·8) HIV-uninfected men had history of methamphetamine use before baseline. The 1234 men without such a history contributed follow-up of 3371 person-years. The median midpoint between the date of last report of no use and date of first use was 124 days (IQR 120–136). Incident methamphetamine use was reported by 128 men, yielding a crude incidence of 3·80 per 100 person-years (95% CI 3·17–4·51; table 2). Methamphetamine use incidence decreased with increasing age (table 2). The Kaplan-Meier cumulative incidence of methamphetamine use was 14·9% (95% CI 12·5–17·6; figure 3).
Table 2:
Bivariate and multivariate analyses of risk factors for incident methamphetamine use
| Incident methamphetamine use (cases/person-years) |
Crude methamphetamine incidence (95% CI) |
Bivariate analysis hazard ratio (95% CI) |
Multivariate analysis hazard ratio (95% CI) |
|
|---|---|---|---|---|
| Total | 128/3371 | 3·8 (3·2–4·5) | ·· | ·· |
| Age (years) | ||||
| 18–21 | 31/492 | 6·3 (4·3–8·9) | 3·0 (1·7–5·1) | 2·0 (1·2–3·6) |
| 22–29 | 75/1811 | 4·1 (3·3–5·2) | 2·0 (1·2–3·2) | 1·6 (1·0–2·6) |
| ≥30 | 22/1069 | 2·1 (1·3–3·1) | 1 (ref) | 1 (ref) |
| Education | ||||
| Primary | 3/44 | 6·8 (1·4–19·9) | 2·8 (0·9–8·9) | ·· |
| Secondary or vocational | 80/1499 | 5·3 (4·2–6·6) | 2·2 (1·5–3·1) | ·· |
| University and above | 45/1828 | 2·8 (1·8–3·3) | 1 (ref) | ·· |
| Study or work status | ||||
| Work | 61/2031 | 3·0 (2·3–3·9) | 1 (ref) | ·· |
| Work and study | 56/1218 | 4·6 (3·5–6·0) | 1·5 (1·1–2·2) | ·· |
| Neither | 11/122 | 9·0 (4·5–16·1) | 3·1 (1·6–5·9) | ·· |
| Current living situation | ||||
| With family | 44/1416 | 3·1 (2·3–4·2) | 1 (ref) | ·· |
| With partner | 13/471 | 2·8 (1·5–4·7) | 0·9 (0·5–1·6) | ·· |
| Alone or with housemate | 71/1486 | 4·8 (3·7–6·0) | 1·5 (1·0–2·2) | ·· |
| Used erectile dysfunction drugs in past 4 months | ||||
| Yes | 61/194 | 31·5 (24·1–40·4) | 16·5 (11·6–23·4) | ·· |
| No | 67/3177 | 2·1 (1·6–2·7) | 1 (ref) | ·· |
| Anal sex in past 4 months | ||||
| Yes | 126/3099 | 4·1 (3·4–4·8) | 5·2 (1·3–20·9) | ·· |
| No | 2/272 | 0·8 (0·1–26·6) | 1 (ref) | ·· |
| Total number of male partners | ||||
| None | 2/242 | 0·8 (0·1–3·0) | 1 (ref) | ·· |
| 1–5 | 54/2307 | 2·3 (1·8–3·0) | 2·7 (0·6–11·0) | ·· |
| ≥6 | 72/823 | 8·7 (6·9–11·0) | 9·7 (2·4–39·7) | ·· |
| Condom use with male partner in past 4 months* | ||||
| Always | 60/1955 | 3·1 (2·3–4·0) | 1 (ref) | ·· |
| Not always | 66/1144 | 5·8 (4·5–7·3) | 1·8 (1·3–2·6) | ·· |
| Ever had group sex | ||||
| Yes | 56/465 | 12·0 (9·1–15·6) | 4·8 (3·4–6·9) | ·· |
| No | 72/2906 | 2·5 (1·9–3·1) | 1 (ref) | ·· |
| Ever coerced into sex | ||||
| Yes | 27/529 | 5·1 (3·4–7·4) | 1·47 (1·0–2·2) | ·· |
| No | 101/2839 | 3·6 (2·9–4·3) | 1 (ref) | ·· |
| Ever received money for sex | ||||
| Yes | 27/226 | 12·0 (7·9–17·4) | 3·4 (2·2–5·3) | 2·3 (1·4–3·6) |
| No | 101/3146 | 3·2 (2·6–3·9) | 1 (ref) | 1 (ref) |
| Finding casual sex partners on the internet in past 4 months | ||||
| Yes | 69/988 | 7·0 (5·4–8·8) | 2·8 (2·0–4·0) | 2·1 (1·4–3·0) |
| No | 59/2383 | 2·5 (1·9–3·2) | 1 (ref) | 1 (ref) |
| Attended chemsex party in past 4 months* | ||||
| Yes | 22/53 | 41·7 (26·1–63·2) | 14·9 (9·3–24·0) | 10·0 (6·2–16·4) |
| No | 85/3060 | 2·78 (2·2–3·4) | 1 (ref) | 1 (ref) |
Only significant variables are reported for the multivariate analysis.
Missing values for this factor were handled by an indicator variable.
Figure 3:
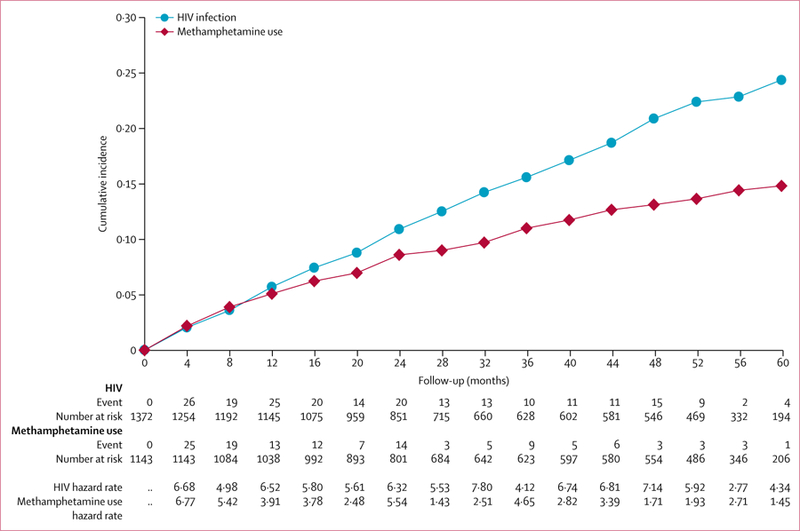
Kaplan-Meier cumulative incidence of newly reported methamphetamine use and HIV seroconversion
In bivariate and multivariate Cox proportional hazard regression analyses, factors independently associated with incident methamphetamine use were younger age at baseline, reported having ever received money for sex, finding casual sex partners on the internet within the past 4 months, and attendance of chemsex parties within the past 4 months (table 2).
372 (21%, 95% CI 19–23) men tested positive for HIV at baseline. The remaining 1372 men contributed 3554 person-years of follow-up. The median midpoint between the date of participants’ last HIV-negative and first HIV-positive visit was 122 days (IQR 117–127). Of the 1372 HIV-negative men, 212 seroconverted, yielding an HIV incidence of 6·0 per 100 person-years (95% CI 5·2–6·8). HIV incidence declined with increasing age (table 3). The Kaplan-Meier cumulative incidence of HIV infection was 24·4% (95% CI 21·4–27·8; figure 3).
Table 3:
Bivariate and multivariate analysis of risk factors for incident HIV infection
| Incident HIV (cases/person-years) |
Crude HIV incidence (95% CI) |
Bivariate analysis hazard ratio (95% CI) |
Multivariate analysis hazard ratio (95% CI) |
|||
|---|---|---|---|---|---|---|
| Total | 212/3554 | 6·0 (5·2–6·8) | ·· | |||
| Age group (years) | ||||||
| 18–21 | 49/537 | 9·1 (6·8–12·1) | 2·5 (1·6–3·7) | 2·5 (1·7–3·9) | ||
| 22–29 | 122/1917 | 6·4 (5·3–7·6) | 1·7 (1·2–2·5) | 1·68 (1·2–2·4) | ||
| ≥30 | 41/1100 | 3·7 (2·7–5·0) | 1 (ref) | 1 (ref) | ||
| Education | ||||||
| Primary | 3/46 | 6·5 (1·3–19·1) | 1·4 (0·4–4·4) | ·· | ||
| Secondary or vocational | 122/1668 | 7·3 (6·1–8·7) | 1·5 (1·2–2·0) | ·· | ||
| University and above | 87/1841 | 4·7 (3·8–5·8) | 1 (ref) | ·· | ||
| Study or work status | ||||||
| Work | 101/2120 | 4·7 (3·9–5·8) | 1 (ref) | ·· | ||
| Work and study | 100/1304 | 7·7 (6·2–9·3) | 1·6 (1·2–2·1) | ·· | ||
| Neither | 11/130 | 8·4 (4·2–15·1) | 1·8 (1·0–3·3) | ·· | ||
| Current living situation | ||||||
| With family | 69/1504 | 4·6 (3·6–5·8) | 1 (ref) | 1 (ref) | ||
| With partner | 28/502 | 5·6 (3·7–8·1) | 1·2 (0·8–1·9) | 1·2 (0·8–1·9) | ||
| Alone or with housemate | 115/1547 | 7·4 (6·1–8·9) | 1·6 (1·2–2·2) | 1·5 (1·1–2·1) | ||
| Ever used methamphetamine to increase sexual pleasure | ||||||
| Yes | 28/197 | 14·2 (9·4–20·5) | 2·7 (1·8–4·0) | 2·0 (1·2–3·1) | ||
| No | 184/3357 | 5·5 (4·7–6·3) | 1 (ref) | 1 (ref) | ||
| Use of erectile dysfunction drugs in past 4 months | ||||||
| Yes | 29/267 | 10·9 (7·3–15·6) | 1·9 (1·3–2·9) | ·· | ||
| No | 183/3288 | 5·6 (4·8–6·4) | 1 (ref) | ·· | ||
| Anal sex in past 4 months | ||||||
| Yes | 208/3278 | 6·3 (5·5–7·3) | 4·4 (1·6–11·8) | 3·6 (1·3–9·9) | ||
| No | 4/276 | 1·5 (0·4–3·7) | 1 (ref) | 1 (ref) | ||
| Total number of male partners in past 4 months | ||||||
| 0 | 3/245 | 1·2 (0·2–3·6) | 1 (ref) | ·· | ||
| 1–5 | 135/2370 | 5·7 (4·8–6·7) | 4·7 (1·5–14·7) | ·· | ||
| ≥6 | 74/939 | 7·9 (6·2–9·9) | 6·5 (2·0–20·6) | ·· | ||
| Condom use with male partner in past 4 months* | ||||||
| Always | 96/1966 | 4·9 (4·0–5·0) | 1 (ref) | ·· | ||
| Not always | 112/1312 | 8·5 (7·0–10·3) | 1·8 (1·3–2·3) | ·· | ||
| Presence of circumcision at baseline | ||||||
| Yes | 18/411 | 4·4 (2·6–6·9) | 1 (ref) | ·· | ||
| No | 194/3143 | 6·2 (5·3–6·9) | 1·4 (0·9–2·3) | ·· | ||
| Ever had group sex | ||||||
| Yes | 53/536 | 9·9 (7·4–12·9) | 1·9 (1·4–2·6) | ·· | ||
| No | 159/3019 | 5·3 (4·5–6·2) | 1 (ref) | ·· | ||
| Ever coerced into sex | ||||||
| Yes | 46/581 | 7·9 (5·8–10·6) | 1·4 (1·0–2·0) | ·· | ||
| No | 166/2971 | 5·6 (4·8–6·5) | 1 (ref) | ·· | ||
| Paid money for sex in past 4 months | ||||||
| Yes | 18/386 | 4·7 (2·8–7·4) | 1 (ref) | ·· | ||
| No | 194/3169 | 6·1 (5·3–7·0) | 1·3 (0·8–2·1) | ·· | ||
| Received money for sex in past 4 months | ||||||
| Yes | 22/301 | 7·3 (4·6–11·1) | 1·2 (0·8–1·9) | ·· | ||
| No | 190/3169 | 5·8 (5·0–6·6) | 1 (ref) | ·· | ||
| Finding casual sex partners on the internet in past 4 months | ||||||
| Yes | 38/381 | 10·0 (7·0–13·7) | 2·0 (1·4–2·9) | 1·6 (1·0–2·6) | ||
| No | 174/3173 | 5·5 (4·7–6·7) | 1 (ref) | 1 (ref) | ||
| Ever attended chemsex party* | ||||||
| Yes | 13/49 | 26·8 (14·3–45·8) | 5·3 (2·9–9·7) | 2·9 (1·4–5·7) | ||
| No | 67/1317 | 5·1 (3·9–6·5) | 1 (ref) | 1 (ref) | ||
| Any ulcerative STIs at baseline | ||||||
| Yes | 154/2171 | 7·1 (6·0–8·3) | 1·7 (1·3–2·3) | 1·7 (1·3–2·3) | ||
| No | 58/1384 | 4·2 (3·2–5·4) | 1 (ref) | 1 (ref) | ||
Only significant variables are reported for the multivariate analysis. STI=sexually transmitted infection.
Missing values for this factor were handled by an indicator variable.
In bivariate and multivariate analyses, factors independently associated with HIV seroconversion were younger age at baseline, living alone or with a housemate, ever having used methamphetamine to increase sexual pleasure, practising anal sex, attendance of chemsex parties within the past 4 months, finding casual sex partners on the internet within the past 4 months, and baseline presence of ulcerative STIs (antibodies against HSV-1 or 2 or T pallidum infection; table 2). HIV incidence was highly correlated with the incidence of methamphetamine use (Pearson’s R 0·99, p<0·0001).
Discussion
Our study shows ongoing and mutually associated twin epidemics of methamphetamine use and HIV infection among MSM in Bangkok. After adjusting for other risk factors, HIV incidence was high among MSM who reported methamphetamine use for sexual pleasure. Risk factors for both methamphetamine use and HIV incidence were younger age, finding casual sex partners on the internet, and having attended a chemsex party. Additionally, ever having received money for sex was predictive for incident methamphetamine use, whereas living alone or with a housemate, practice of anal sex, and presence of ulcerative STIs at baseline were predictive of incident HIV infection. Because most risk factors identified in our analysis might or might not directly affect incident HIV infection, we created a conceptual HIV transmission model to better elucidate their order in the causal pathway to infection, on the basis of bivariate ORs between them. The model shows the existence of a cluster of risk behaviours, such as finding casual sex partners online, attendance of chemsex parties, erectile dysfunction drug use, and anal sex, associated with incident HIV infection and accelerated in the presence of ulcerative STIs.
The increasing use of methamphetamine reported in our study population is part of a troubling global trend of increased stimulant and other drug use, including erectile dysfunction drugs, in MSM.2,14,15 Methamphetamine use might promote risk-taking behaviour by lowering protective tolerance and perception of risk while increasing sexual energy, libido, and stamina. These, in turn, have been found to be associated with more frequent and more vigorous sexual intercourse, often with multiple consecutive partners or during group sex. These behaviours could result in recurrent and longer exposures, possibly in the presence of anogenital trauma.16–18 Moreover, methamphetamine use has been implicated in the emergence of temporal clusters and chains of acute HIV infection in MSM, as shown by phylogenetic analyses,19–21 which suggests that acute HIV infection might account for up to 80% of new infections in MSM. Factors found associated with phylogenetic clusters included younger age, methamphetamine use, prevalent STIs, frequent and rapid partner turnover, phenotypic representations of HIV with a history of successful previous transmissions, or with X4 or C5 tropism.19–22 Methamphetamine use in MSM was initially identified as a public health problem in Australia and the USA some 20 years ago, and HIV epidemics associated with its use have subsequently been observed among urban MSM throughout the industrialised world and Asia Pacific,6,17,23 and now in Bangkok, Thailand.
As the methamphetamine use epidemic progresses among MSM in Bangkok, so will associated epidemics of mental health and social problems, including drug dependence. In an earlier analysis24 from our cohort, the presence of co-occurring epidemics, or syndemics, seemed to be associated with increased HIV prevalence and incidence. Another factor likely to increase over time is parenteral methamphetamine use. Whereas injection drug use was rarely reported in our cohort, anecdotal information collected from social media among MSM increasingly refers to the benefits of injection over other ways of methamphetamine use.
The association between finding casual sexual partners on the internet, attendance of chemsex parties, methamphetamine use, anal intercourse, and HIV seroconversion in MSM has been described previously.5,25–28 However, to our knowledge, these associations had not been confirmed simultaneously in a single prospective design, as was done in our study. Finding casual sex partners on the internet, attendance of chemsex parties, and methamphetamine use remained significant in the Cox regression model, after adjusting for additional risk factors such as anal sex and ulcerative STI. This suggests that other unmeasured risk behaviours might have, at least in part, increased HIV incidence in MSM: for example, by facilitating assortative mixing among risktaking men or by expanding sexual networks into higherrisk subpopulations, such as those in whom acute HIV infection is a more frequent occurrence.
The internet has become a worldwide venue for communication, education, and finding new acquaintances, including new sex partners. It is being used more frequently for these purposes by MSM in Bangkok and elsewhere. Several recent changes in sociocultural behaviours and norms, as well as technological advances, might have influenced the increasing incidence of HIV infection observed in our study. The internet is especially popular in young same-sex oriented or interested men, who might want to explore or assess their sexuality without adopting a gay identity or revealing their sexual preferences or behaviours to others. Many websites and mobile applications primarily featuring issues of interest to MSM have appeared throughout Thailand, facilitating finding of casual sex partners independent of sexual orientation or identity. Sex with a casual partner is inherently riskier, partly because HIV serostatus is often unknown and sex might more often be accomplished by use of methamphetamine and erectile dysfunction drugs. As was shown in our study, finding sexual partners on the internet and use of stimulant and sexual enhancement drugs prior to and during sex is associated with increased risk of HIV infection. However, the association between these factors has been difficult to document for several reasons. To ascertain the additive risk of internet and metha mphetamine use on HIV incidence with an acceptable degree of assurance, a large cohort of high-risk MSM must be followed prospectively with frequent assessment of sexual behaviour and HIV status. Such studies are costly, logistically challenging, and require clinical and laboratory infrastructure and an enabling environment. The latter is particularly important because MSM can remain a hidden, stigmatised population in many societies and might be difficult to locate, recruit, and retain in a longitudinal study
HIV incidence estimates among MSM in Bangkok have remained high for more than a decade. Previous studies have assessed HIV incidence in this population as being within 4·1–7·7 per 100 person-years between 2003 and 2013.9,11 These data provide solid evidence for a continuing epidemic of HIV infection. This ongoing high HIV incidence, 10 years after recognition of the epidemic among MSM, is in complete contrast with the heterosexual HIV epidemic in the late 1980s and early 1990s in Thailand. During that time, high rates of infection were brought rapidly under control by widespread preventive interventions, particularly consistent condom use.29 The HIV incidence in Bangkok MSM is also higher than reported among similar populations in other countries. Studies from western Europe, North America, Australia, and elsewhere in Asia report annual HIV incidence rates between 1% and 3% of the population.3
The results of our analysis are subject to several limitations. Our study population might not be representative of the general MSM population in Bangkok, nor of MSM in other geographical areas. Self-selected men volunteered to take part or were recruited from clients voluntarily coming for HIV testing and counselling at the study clinic. Behavioural assessment measures relied on self-reports, which might have been subject to social desirability and recall bias. However, data were collected by audio computerassisted self-interviewing, covering a short interval of 4 months between study visits. This type of interviewing has been shown to reduce under-reporting of sensitive behaviours,30 while shorter recall periods allow participants to remember their activities more precisely and minimise recall bias. Also, frequent study visits improved our ability to link reported behavior temporally with HIV incidence. However, we could not determine whether a certain risk preceded or followed an HIV seroconversion during a study interval. Finally, our descriptive model of HIV incidence is a conceptual framework based on theoretical assumptions and bivariate empirical associations found in our study. It was created to visualise, understand, and communicate the complex associations, mutual reinforcements, and interactions between the variables of which it consists. Despite the insights gained from the model, background epidemiological factors, such as the prevalence of acute HIV infection in the sexual network, could not be included. Hence, it does not represent causality and its external validity in other situations remains to be seen.
With an HIV prevalence of around 30% and a history of drug use in the past 6 months of more than 40% in MSM enrolled in integrated biological and behavioural HIV surveillance in Bangkok,10 the results from our study reinforce the view of no alteration in the recent trajectory of the HIV epidemic and its determinants in Thailand. Widespread use of the internet among Thai MSM provides ample opportunities for HIV prevention education, especially for those at the highest risk for infection. Decriminalisation of recreational drug use, such as methamphetamine use for sexual pleasure; increased recognition and openness about subcultural drug-use patterns and sexual habits among MSM; harm-reduction approaches; drug-use education; and dependency treatment are urgently needed. Such changes would demystify methamphetamine use and widen options for prevention and treatment over penalising measures. Greater HIV prevention effforts that also address social structural determinants of HIV risk not modifiable at the clinic, voluntary counselling and testing, or behavioural level, such as equal rights, legal recognition, and prevention of discrimination and stigma of MSM in health-care settings,3 are essential to halt the spread of HIV infection among MSM in Thailand and elsewhere.
Research in context.
Evidence before this study
We searched PubMed for publications about internet and methamphetamine use and HIV infection in men who have sex with men (MSM). We used combinations of the search terms “internet”, “methamphetamine”, “men who have sex with men”, “HIV infection”, and “Thailand”, and restricted our search to studies published between Jan 1, 2005, and Dec 31, 2016. We used 2005 as the start date of our search because the first report of HIV prevalence among MSM in Thailand was published in that year. The 2005 study identified a large, previously undescribed epidemic of HIV infection among MSM in Bangkok, which has remained uncontrolled until the present date. Our search returned more than 500 publications, mostly from the industrialised world; only few originated from southeast Asia or Thailand. The association between internet and methamphetamine use, sexual risk behaviour, and HIV prevalence and incidence among MSM in high-income countries appeared to be well established and subject of a variety of preventive interventions. No such reports could be found for MSM in Thailand.
Added value of this study
To our knowledge, our study is the first to prospectively investigate the interplay between finding casual sex partners online and incident methamphetamine use and HIV seroconversion in MSM. Our results show expanding epidemics of stimulant drug use and HIV infection in MSM in Bangkok, facilitated by internet use and accelerated in the presence of ulcerative sexually transmitted infections (STIs).
Implications of all available evidence
Our findings add to the documentation of the continuing and ever-growing HIV epidemic in relation to internet and methamphetamine use in MSM, globally. They also point to the inadequacy of past HIV prevention efforts, insufficient investment in the internet as a preventive venue, little political will to decriminalise stimulant drug use, inability to implement effective STI control, and delayed implementation of HIV pre-exposure prophylaxis. If the HIV epidemic in MSM in Bangkok is to be controlled, virtual HIV prevention education, drug use harm reduction and treatment, biomedical HIV prevention methods, and effective STI control will all need to be implemented concurrently. Only such a holistic prevention approach could possibly mitigate the risk of HIV infection in this population, in whom the HIV incidence is among the highest documented worldwide.
Acknowledgments
We gratefully acknowledge the staff of the HIV/STD Research Program and the Laboratory and IT Sections of the Thailand Ministry of Public Health–US Centers for Disease Control and Prevention Collaboration for their assistance in conducting this study. The personnel of the Silom Community Clinic and all study participants are thanked for their valuable contribution: without their help this research would not have been possible. PP received support from the Thailand Ministry of Science and Technology, the Phramongkutklao College of Medicine, and the Johns Hopkins University Fogarty AIDS International Training and Research Program. Results and conclusions reported in this paper are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention.
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Phunlerd Piyaraj, Department of Parasitology, Phramongkutklao College of, Medicine, Bangkok, Thailand; Thailand Ministry of Public Health-US Centers for Disease Control and Prevention Collaboration, Nonthaburi, Thailand; Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA.
Prof Frits van Griensven, Department of Epidemiology and Biostatistics, University of California, San Francisco, CA, USA; Thai Red Cross AIDS Research Center, Bangkok, Thailand.
Timothy H Holtz, Thailand Ministry of Public Health-US Centers for Disease Control and Prevention Collaboration, Nonthaburi, Thailand; Division of HIV/AIDS Prevention, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Prof Kenrad E Nelson, Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA.
References
- 1.Roth Y Zero feet away: the digital geography of gay social media. J Homosex 2016; 63: 437–42. [DOI] [PubMed] [Google Scholar]
- 2.Fisher DG, Reynolds GL, Napper LE. Use of crystal meth, viagra and sexual behaviour. Curr Opin Infect Dis 2010; 23: 53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyrer C, Baral SD, Van Griensven F, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet 2012; 380: 367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry M, Raymond HF, Kellogg T, McFarland W. The internet, HIV serosorting and transmission risk among men who have sex with men, San Francisco. AIDS 2008; 22: 787–89. [DOI] [PubMed] [Google Scholar]
- 5.Rosser BR, Miner MH, Bockting WO, et al. HIV risk and the internet: results of the Men’s INTernet Sex (MINTS) study. AIDS Behav 2009; 13: 746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei C, Guadamuz TE, Lim SH, Huang Y, Koe S. Patterns and levels of illicit drug use among men who have sex with men in Asia. Drug Alc Dep 2012; 120: 246–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Griensven F, Thanprasertsuk S, Jommaroeng R, et al. Evidence of a previously undocumented epidemic of HIV infection among men who have sex with men in Bangkok, Thailand. AIDS 2005; 19: 521–26. [DOI] [PubMed] [Google Scholar]
- 8.van Griensven F, Thienkrua W, McNicholl J, et al. Evidence of an explosive epidemic of HIV infection in a cohort of men who have sex with men in Thailand. AIDS 2013; 27: 825–32. [DOI] [PubMed] [Google Scholar]
- 9.van Griensven F, Varangrat A, Wimonsate W, et al. Trends in HIV prevalence, estimated HIV incidence, and risk behavior among men who have sex with men in Bangkok, Thailand, 2003–2007. J Acquir Immune Defic Syndr 2010; 53: 234–39. [DOI] [PubMed] [Google Scholar]
- 10.Health Department. Integrated behavioral and biological HIV surveillance, 2003–2014 Bangkok: Division of AIDS, TB and STI, Health Department, Bangkok Metropolitan Administration, 2015. [Google Scholar]
- 11.van Griensven F, Holtz TH, Thienkrua W, et al. Temporal trends in HIV-1 incidence and risk behaviours in men who have sex with men in Bangkok, Thailand, 2006–13: an observational study. Lancet HIV 2015; 2: e64–70. [DOI] [PubMed] [Google Scholar]
- 12.Manosuthi W, Ongwandee S, Bhakeecheep S, et al. Guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents 2014, Thailand. AIDS Res Ther 2015; 12: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtz T, Thienkrua W, McNicholl J, et al. Prevalence of treponema pallidum seropositivity and herpes simplex virus type 2 infection in a cohort of men who have sex with men, Bangkok, Thailand, 2006–2010. Int J STD AIDS 2012; 23: 424–28. [DOI] [PubMed] [Google Scholar]
- 14.Halkitis PN, Parsons JT, Stirratt MJ. A double epidemic: crystal methamphetamine drug use in relation to HIV transmission among gay men. J Homosex 2001; 41: 17–35. [DOI] [PubMed] [Google Scholar]
- 15.Worth H, Rawstorne P. Crystallizing the HIV epidemic: methamphetamine, unsafe sex, and gay diseases of the will. Arch Sex Behav 2005; 34: 483–86. [DOI] [PubMed] [Google Scholar]
- 16.Mansergh G, Shouse R, Marks G, et al. Methamphetamine and sildenafil (Viagra) use are linked to unprotected receptive and insertive anal sex, respectively, in a sample of men who have sex with men. Sex Transm Infect 2006; 82: 131–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mimiaga MJ, Fair AD, Mayer KH, et al. Experiences and sexual behaviors of HIV-infected MSM who acquired HIV in the context of crystal methamphetamine use. AIDS Educ Prevent 2008; 20: 30–41. [DOI] [PubMed] [Google Scholar]
- 18.Prestage G, Grierson J, Bradley J, Hurley M, Hudson J. The role of drugs during group sex among gay men in Australia. Sex Health 2009; 6: 310–17. [DOI] [PubMed] [Google Scholar]
- 19.Brenner BG, Roger M, Routy J-P, et al. High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis 2007; 195: 951–59. [DOI] [PubMed] [Google Scholar]
- 20.Lewis F, Hughes GJ, Rambaut A, Pozniak A, Brown AJL. Episodic sexual transmission of HIV revealed by molecular phylodynamics. PLoS Med 2008; 5: e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenner BG, Roger M, Stephens D, et al. Transmission clustering drives the onward spread of the HIV epidemic among men who have sex with men in Quebec. J Infect Dis 2011; 204: 1115–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berlier W, Bourlet T, Lawrence P, et al. Selective sequestration of X4 isolates by human genital epithelial cells: implication for virus tropism selection process during sexual transmission of HIV. J Med Virol 2005; 77: 465–74. [DOI] [PubMed] [Google Scholar]
- 23.Vu NTT, Holt M, Phan HTT, et al. The relationship between methamphetamine use, sexual sensation seeking and condomless anal intercourse among men who have sex with men in Vietnam: results of a community-based, cross-sectional study. AIDS Behav 2017; 21: 1105–16. [DOI] [PubMed] [Google Scholar]
- 24.Guadamuz T, McCarthy K, Wimonsate W, et al. Psychosocial health conditions and HIV prevalence and incidence in a cohort of men who have sex with men in Bangkok, Thailand: evidence of a syndemic effect. AIDS Behav 2014; 18: 2089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garofalo R, Herrick A, Mustanski BS, Donenberg GR. Tip of the iceberg: young men who have sex with men, the internet, and HIV risk. Am J Public Health 2007; 97: 1113–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berg RC. Barebacking among MSM internet users. AIDS Behav 2008; 12: 822–33. [DOI] [PubMed] [Google Scholar]
- 27.Ostrow DG, Plankey MW, Cox C, et al. Specific sex drug combinations contribute to the majority of recent HIV seroconversions among MSM in the MACS. J Acquir Immune Defic Syndr 2009; 51: 349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko N-Y, Koe S, Lee H-C, Yen C-F, Ko W-C, Hsu S-T. Online sex-seeking, substance use, and risky behaviors in Taiwan: results from the 2010 Asia internet MSM sex survey. Arch Sex Behav 2012; 41: 1273–82. [DOI] [PubMed] [Google Scholar]
- 29.van Griensven F, Phanuphak N, Srithanaviboonchai K. Biomedical HIV prevention research and epidemic control in Thailand: two sides of the same coin. Sex Health 2014; 11: 180–99. [DOI] [PubMed] [Google Scholar]
- 30.van Griensven F, Naorat S, Kilmarx PH, et al. Palmtop-assisted self-interviewing for the collection of sensitive behavioral data: randomized trial with drug use urine testing. Am J Epidemiol 2006; 163: 271–78. [DOI] [PubMed] [Google Scholar]