Abstract
Purpose
Immature human oocytes from resected ovaries can be used for research and fertility preservation, though it is unknown whether it is feasible to transport oocytes for these purposes. This study examined in vitro maturation (IVM) outcomes after the transportation of human follicular fluid (HFF) containing oocytes.
Methods
Fourteen patients with endometrial adenocarcinoma were enrolled. Oocytes obtained from the resected ovaries of seven patients were transported with HFF by railway (transportation group). Samples of HFF from the other seven patients were not transported, and IVM was performed promptly (non‐transportation group). The results of oocyte retrieval and IVM were compared.
Results
The average ages in the transportation and non‐transportation groups were 40.1 ± 2.0 and 39.6 ± 1.8 years, respectively, and the average numbers of collected oocytes were 8.1 ± 8.4 and 5.1 ± 5.1, respectively. There was a significant negative correlation between the number of collected oocytes and age. The proportions of oocytes that reached meiosis II (maturation rate) after IVM were 38.6% and 69.2% in the transportation and non‐transportation groups, respectively (P = 0.013).
Conclusion
In this preliminary study, the usefulness of the transportation of HFF was limited. Further studies on maintaining oocyte normality during transportation are necessary for becoming the effective method for research and clinical use.
Keywords: follicular fluid, immature human oocytes, in vitro maturation, oncofertility, transportation
1. INTRODUCTION
A number of studies thus far have investigated the transportation of reproductive tissues from various species for their use in reproductive research and clinical settings. The transportation of immature non‐human oocytes has been reported in species including the cow,1 mouse,2 monkey,3 and pig.4 Most past reports have transported non‐human oocytes at 37°C, with or without a 5% CO2 chamber, for up to 5 hours. The percentages of immature oocytes that mature to meiosis II (MII) and that reach the blastocyst stage have been examined, as have fertilization rates.2, 3, 4 Furthermore, transportation of non‐human ovaries has been reported in the mare,5 pig,6 cat,7 dog,8 and mouse9; these studies have investigated the temperature during ovary transportation as well as several post‐transportation parameters, including the number of collected oocytes, maturation rate, fertilization rate, and rate of reaching the blastocyst stage. Non‐human reproductive tissues have also been transported for the purpose of collecting materials for commercial and research purposes.8 Transported non‐human oocytes are also regarded as valuable translational research materials for human oocyte studies, because it is difficult to obtain sufficiently large numbers of human oocytes.10
There have also been reports on the transportation of human follicular fluid (HFF)11, 12, 13 and ovaries.14, 15, 16, 17 Prior to two decades ago, only a few institutions performed intra‐cytoplasmic sperm injection (ICSI), and therefore, HFF aspirated from stimulated ovaries was transported by car and airplane from satellite clinics to in vitro fertilization centers11, 12, 13; this transportation occurred at distances of 90‐500 km at temperatures of 37°C with no 5% CO2 chamber.11, 12, 13 Transportation of resected human ovaries has also been examined, as it is an important fertility preservation (FP) technique in the field of oncofertility for patients with malignant tumors such as breast and blood cancers.14, 15, 16, 17
Researchers have investigated a variety of outcomes of in vitro maturation (IVM) and artificial reproductive technology (ART) after ovarian transportation for clinical purposes. However, there are few approaches for collecting human oocytes as research materials. Existing methods include obtaining surplus oocytes following fertility treatment18, 19 or collecting oocytes from patients who have undergone surgery for ovarian tumors.20 In 2013, we reported a new method for collecting immature human oocytes for research purposes from the resected ovaries of patients with endometrial adenocarcinoma (EMA).21 In our previous report, the numbers of collected oocytes and IVM outcomes were investigated in eight patients with EMA. There have been several reports of transportation of HFF,11, 12, 13 but none have examined outcomes of IVM of human oocytes collected from transported HFF aspirated from resected ovaries. Currently, HFF transportation is not necessary for ICSI because this technique has become widespread, and is performed even in satellite clinics. In the field of oncofertility, however, it is common at present to transport resected ovarian tissue (ROT) on ice22 or at 4°C.15 In past reports, the maturation rate of immature human oocytes collected from non‐stimulated ROT was about 30%‐40%.17, 23, 24, 25 For clinical and research purposes, it would be beneficial if we could obtain immature human oocytes from transported HFF that was aspirated from resected ovaries at a satellite hospital, and then acquire mature oocytes by IVM. Moreover, collection of oocytes from transported HFF avoids the need to transport whole ovaries and thus may contribute to clinical adaptation when the hospital performing the surgery is in a different location from the ART facility. However, it was reported that rates of maturation and fertilization were low following the transportation of non‐human oocytes.3 One way to collect human oocytes as research material is to transport HFF containing cumulus‐oocyte complexes (COCs) from resected ovaries, though it is still unknown if human oocytes are negatively affected by the transportation process. The IVM process is presumably affected by the transportation of HFF containing COCs, and we think that examining this relationship has scientific significance not only for securing research materials but also for the application to FP. On the other hand, it is also necessary to secure good‐quality oocytes even after transportation of HFF, so as to avoid misinterpretation of study results involving human oocytes.
In the present study, HFF was aspirated from the resected, non‐stimulated ovaries of patients with EMA, and in half the cases the HFF was then transported by train between two university hospitals 250 km apart. We compared the outcomes of IVM of immature human oocytes collected from transported and non‐transported HFF. The purpose of this study was to examine the outcomes of IVM after transportation of HFF containing COCs.
2. MATERIALS AND METHODS
2.1. Patients
In our previous report, we investigated eight patients under 45 years old who had a preoperative diagnosis of stage I EMA.21 In this study, we recruited another 14 patients with the same cancer stage from January 2014 to December 2016. All patients required hysterectomy and bilateral oophorectomy, and FP was not an option. In this study, therefore, all collected oocytes were used only for research purposes. Patient characteristics, including age, are summarized in Tables 1 and 2. In seven of the 14 newly recruited patients (case numbers 2, 4, 5, 6, 8, 9, and 10), we transported the COCs obtained from the follicles of resected ovaries in each patient's HFF, and defined these patients as the “transportation group.” The HFF of the remaining seven patients (case numbers 1, 3, 7, 11, 12, 13, and 14) was not transported, and these patients were defined as the “non‐transportation group.” The case numbers correspond to the order in which the patients underwent surgery.
Table 1.
Summary of patient data
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | 41 | 40 | 38 | 41 | 38 | 43 | 43 | 41 | 37 | 41 | 38 | 39 | 39 | 39 |
| Transport or non‐transport | non‐transport | Transport | non‐transport | Transport | Transport | Transport | non‐transport | Transport | Transport | Transport | non‐transport | non‐transport | non‐transport | Non |
| Transport Method | ‐ | Bottle | ‐ | Bottle | Box | Box | ‐ | Box | Box | Box | ‐ | ‐ | ‐ | ‐ |
| Collected oocytes (n) | 5 | 6 | 16 | 5 | 21 | 4 | 1 | 2 | 19 | 0 | 1 | 4 | 5 | 4 |
| Number of oocytes for IVM (n) | 4 | 1 | 8 | 4 | 14 | 4 | 1 | 2 | 19 | 0 | 1 | 3 | 5 | 4 |
| Meiosis II Stage (n) | 4 | 0 | 8 | 1 | 6 | 2 | 0 | 2 | 6 | 0 | 1 | 2 | 2 | 1 |
| BMI (kg/m2) | 23.6 | 37.6 | 25.2 | 20.4 | 31.4 | 30.7 | 37.5 | 34.8 | 37.1 | 19.0 | 19.4 | 22.3 | 19.7 | 19.3 |
BMI, body mass index; IVM, in vitro maturation.
Table 2.
Comparison of current and past cases, and transport and non‐transport groups
| Past research17 | Current cases | P | Transport group | Non‐transport group | P | ||
|---|---|---|---|---|---|---|---|
| Number of cases | 8 | 14 | Number of cases | 7 | 7 | ||
| Age (years old) | 39.8 ± 3.4 | 39.9 ± 1.9 | 0.924 | Age (years old) | 40.1 ± 2.0 | 39.6 ± 1.8 | 0.589 |
| BMI (kg/m2) | N.D | 27.0 ± 7.5 | ‐ | BMI (kg/m2) | 30.1 ± 7.6 | 23.9 ± 6.4 | 0.121 |
| Collected oocytes (number) | 10.9 ± 10.1 | 6.6 ± 6.8 | 0.254 | Collected oocytes (number) | 8.1 ± 8.4 | 5.1 ± 5.1 | 0.433 |
| Maturation rate (%) | 12.6 | 50.0 | <0.001 | Maturation rate (%) | 38.6 | 69.2 | 0.013 |
BMI, body mass index; N.D, no data available.
Of note, in case 10 the patient had previously undergone resection of the right ovary for torsion, as well as ovarian cystectomy twice on the left side. In case 11, the patient had complications of systemic lupus erythematosus and anti‐phospholipid antibody syndrome, so she had been receiving azathioprine for an extended period prior to our study.
Because oocytes were not collected for clinical use, none of the patients underwent ovarian stimulation prior to surgery, for instance with follicular stimulation hormone (FSH) or human chorionic gonadotropin (hCG). Because patients with EMA frequently have atypical genital bleeding or irregular menstrual cycles, the day of the menstrual cycle at the time of surgery was not recorded.
2.2. Aspiration of HFF
We previously reported our method of obtaining HFF from resected ovaries.21 In brief, the surgeon removed one or both ovaries as soon as possible after the start of the operation. In case 10, the right ovary had previously been resected, and therefore, only the left ovary was resected. In the non‐transportation group, resected ovaries were immediately placed in warm saline and moved to the laboratory. Using a single 19‐gauge needle containing 1‐2 mL of flushing medium from the IVM Kit (Cooper Surgical, Trumbull, CT, USA), the surface of each ovary was punctured about 15 times in the location where follicle‐like structures were visible macroscopically. In the transportation group, aspiration of HFF was performed in the same way, immediately after resection of the ovaries in the operating room. To ensure a uniform technique, all HFF aspiration procedures in both groups were performed by the same senior physician (H.S).
2.3. Transportation of HFF
Aspirated HFF containing COCs was transported from Tohoku University (Sendai, Japan) to Akita University (Akita, Japan) by high‐speed rail. In this study, no ovarian stimulation was performed prior to surgery, so the amount of HFF was only a few milliliters per patient and evaporation was a concern. Therefore, when HFF was transported in a vacuum‐insulated bottle (as described below), it was mixed with Sage IVM washing medium (Cooper Surgical) containing 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid (HEPES). When HFF was transported under 5% CO2 (as described below), it was mixed HFF with Sage IVM maturation medium (Cooper Surgical, Trumbull, CT, USA), which does not contain HEPES. The total volume of transported HFF and either washing medium or maturation medium was about 7 mL, depending on the volumes of the tubes and dishes. In the first two patients (cases 2 and 4), HFF mixed with washing medium was transported in a 300‐ml vacuum‐insulated bottle (Tiger Co., Ltd., Tokyo, Japan). The HFF mixture was first put in a 15‐ml Falcon conical tube (BD Biosciences, Franklin Lakes, NJ, USA), and this tube was placed in warm water (37°C) within the vacuum‐insulated bottle. This method is shown in Figure 1A,B. We defined this group as the “bottle group.” In the next five patients (cases 5, 6, 8, 9, and 10), HFF mixed with maturation medium was transported in a CellBox® plastic container (Corefront, Tokyo, Japan) at 36°C under 5% CO2. The HFF mixture was placed into 6‐cm laboratory dishes that were then covered by gas‐permeable film (Corefront). The 5% CO2 and 36°C conditions within the CellBox® plastic container were maintained with a CulturePal® CO2 generator (Corefront) and a Thermopack® (Corefront), respectively. This system is shown in Figure 1C‐F. We defined this group as the “box group.” The HFF transportation procedures in both groups were performed by the same senior physician (H.S).
Figure 1.
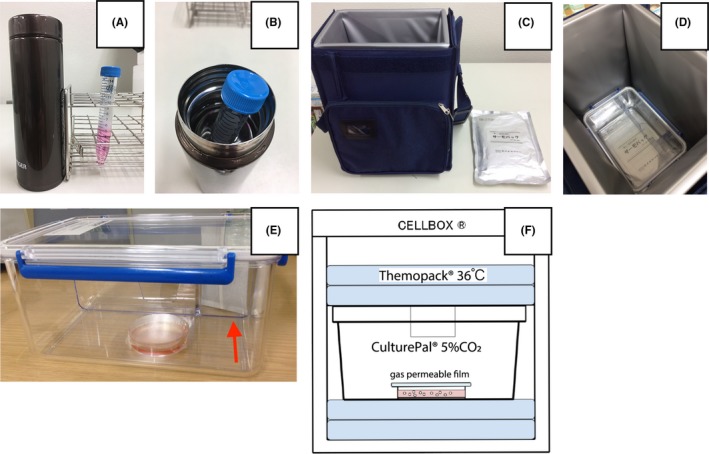
Techniques for transporting human follicular fluid. A, Appearance of the 300‐mL vacuum‐insulated bottle and the Falcon conical tube. B, Falcon conical tube in a 300‐mL vacuum‐insulated bottle filled with warm water (37°C). C, CellBox® and Thermopack®. D, Appearance of CulturePal® inside CellBox®. E, Dish with HFF and Sage IVM maturation medium is contained in CulturePal®. White bag shown by red arrow is the CulturePal® CO2 generator. F, Schema of Thermopack® and CulturePal® in CellBox®
2.4. Collection of immature oocytes and in vitro maturation
After the HFF was delivered to the laboratory at Akita University, we quickly collected oocytes (Figure 2A) using a light microscope. We washed the collected oocytes in Sage IVM washing medium (Cooper Surgical), transferred them to Sage IVM maturation medium (Cooper Surgical) with 75 IU of FSH and 75 IU of luteinizing hormone (HMG Injection Teizo; Asuka Pharmaceutical, Tokyo, Japan), and kept them in an incubator (5% CO2, 5% O2; 37°C) for 24 hours. We previously reported the clinical method we used for IVM.26 In patients 2, 3, and 5, some oocytes were subjected to Sage IVM maturation medium with MG‐132 (Merck Millipore, Billerica, MA, USA) for 24 hours to assess the ability of proteasome inhibitor to inhibit maturation of oocytes. For this protocol, we used five oocytes from patient 2, eight from patient 3, and seven from patient 5. This study does not report the data from the MG‐132 experiments. Also, oocytes with strong deformation were excluded from IVM. The results of the experiments are summarized in detail in Table 1.
Figure 2.
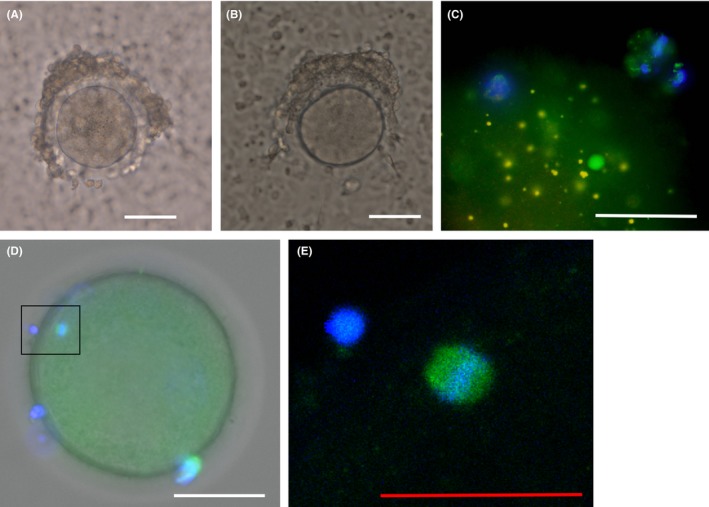
Collected oocytes from resected ovaries and immunofluorescent staining. A, Collected immature oocytes before in vitro maturation in patient 8. B, An oocyte in meiosis II showing the first polar body after in vitro maturation in patient 8. C, Immunofluorescent staining of a mature oocyte in patient 5. D, Immunofluorescent staining of a mature oocyte in patient 13. The spindle is enclosed in a square box. E, Enlarged view of the spindle corresponding to the boxed area in (D). White scale bar indicates 50 µm. Red scale bar indicates 25 µm. Green, microtubules; blue, DAPI
After IVM, we checked the meiosis stage of each oocyte to determine whether the first polar body was extruded, as shown in Figure 2B. Mature, metaphase II oocytes with extruded polar bodies were defined in accordance with previous reports.27, 28 Oocytes without polar bodies were incubated for an additional 24 hours, after which a final check of meiosis stage was performed. We defined the “maturation rate” as the percentage of oocytes that reached mature metaphase II as a result of IVM, as described in previous reports.24, 28
2.5. Immunofluorescent staining of oocytes
After IVM, the maturation status of oocytes was assessed using immunofluorescence staining with a previous technique.21, 29 In brief, the oocytes were fixed with 3.7% formaldehyde and incubated for 15 minutes in 0.2% Triton X‐100. To detect microtubules, the primary antibody was mouse monoclonal anti‐β‐tubulin antibody (Sigma‐Aldrich, St. Louis, MO, USA), and the secondary antibody was Alexa Fluor® 488‐conjugated goat anti‐mouse IgG (2.0 mg/mL; Life Technologies, Carlsbad, CA, USA). Oocytes were mounted in VECTASHIELD® Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA, USA) for chromosome observation.
2.6. Statistical analysis
Data were analyzed by the two‐sided Fisher's exact test or Pearson's correlation using SPSS software, version 21.0 (SPSS, Chicago, IL, USA). P values <0.05 were considered statistically significant.
3. RESULTS
The summarized data of patients in this study are shown in Tables 1 and 2. The average number of oocytes collected from each of the 14 patients was 6.6 ± 6.8, with no significant differences between the transportation and non‐transportation groups (P = 0.433), as shown in Table 2. The number of oocytes collected per patient ranged from 0 to 21. Patient age and body mass index (BMI) did not differ significantly between the transportation and non‐transportation groups.
As illustrated in Figure 3A, there was a significant negative correlation between the number of collected oocytes and patient age (R 2 = 0.404, P = 0.015). The addition of data from eight patients in our previous study20 further strengthened the negative correlation between the number of collected oocytes and patient age (R 2 = 0.610, P < 0.001), as shown in Figure 3B.
Figure 3.
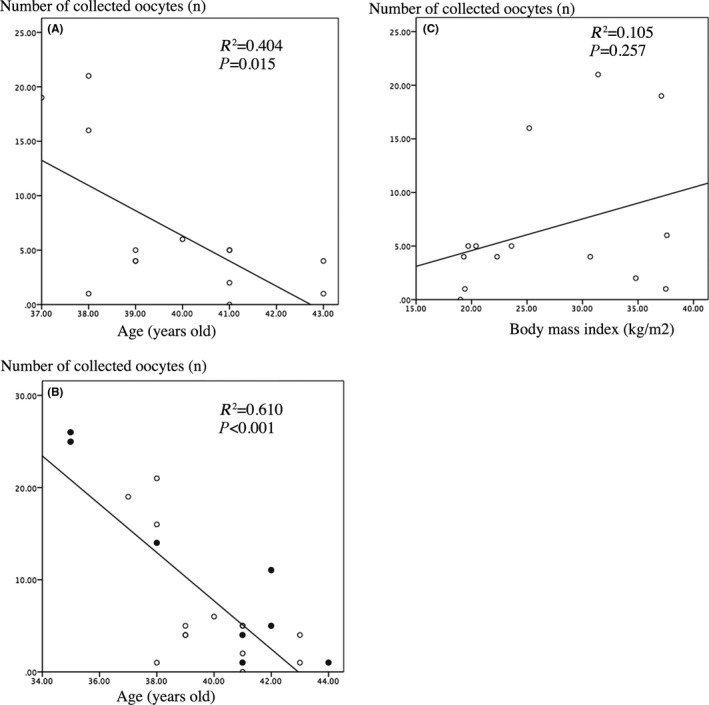
Correlations between number of collected oocytes and both age and body mass index. A, Correlation between the number of collected oocytes and age in the 14 patients in this study. B, Correlation between the number of collected oocytes and age in 22 patients: 14 from this study and 6 from our previous research.17 Black circles indicate the patients in our previous report. C, Correlation between the number of collected oocytes and body mass index in the 14 patients in this study
Data on the BMI of the 14 patients in this study are shown in Tables 1 and 2. The average BMI was 27.0 ± 7.5 kg/m2, which is categorized as overweight.29 There was no significant correlation between the number of collected oocytes and BMI, as shown in Figure 3C (R 2 = 0.105, P = 0.257).
The average duration of train transportation from Tohoku University to Akita University, defined as the time from ovary resection to the beginning of IVM in the incubator at Akita University, was 261 ± 14 min. The maturation rate in the non‐transportation group was significantly higher than that in the transportation group (P = 0.013). As shown in Figure 4, there was a significant difference in the maturation rate in the non‐transportation group, the bottle group (cases 2 and 4), and the box group (cases 5, 6, 8, 9, and 10; two‐sided Fisher's exact test, P = 0.032). The maturation rates in the bottle group and box group were 20.0% and 41.0%, respectively, indicating no significant difference (P = 0.634). The maturation rate of the non‐transportation group was significantly higher than that in the box group (P = 0.042), but did not differ significantly from that of the bottle group (P = 0.060).
Figure 4.
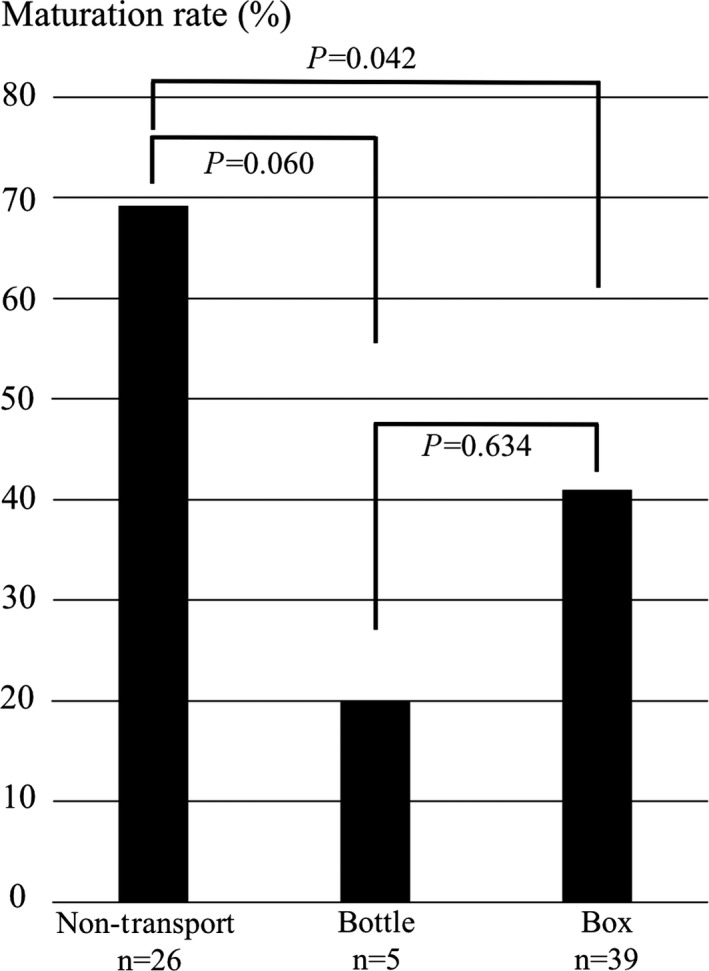
Comparison of maturation rates in the transportation and non‐transportation groups. Non‐Transport, non‐transportation group; Bottle, bottle group; Box, box group. The bars indicate the percentages of oocytes that reached mature metaphase II as a result of in vitro maturation. Two‐sided Fisher's exact test was used for statistical analysis
As shown in Figure 2, meiotic spindles with normal chromosome alignment during meiosis II were observed in both the transportation group and non‐transportation group.
4. DISCUSSION
Portable incubators set at 37°C without 5% CO2 were used in a past report on HFF transportation.12 In our investigation, we transported HFF in a tube placed in warm water (37°C) within a vacuum bottle or in a CellPorter® container at 36°C under 5% CO2 maintained by a CO2 generator. In non‐human species, two studies reported the usefulness of 5% CO2 during transportation of mouse oocytes.3, 4 By contrast, another report concluded that there was no significant difference in the maturation rate of immature mouse oocytes transported with or without a CO2 chamber.2 No previous studies examined whether a CO2 chamber was useful for maintaining the quality of immature oocytes during HFF transportation; therefore, we included this method in this study and compared the results of IVM with those following transportation using a vacuum‐insulated bottle. The maturation rate was higher when a CO2 chamber was used than when it was not, but this difference was not significant, as shown in Figure 4. One limitation of this study was that the number of oocytes was too low to conclusively assess the utility of the CO2 chamber. There was no significant difference in the maturation rate in the non‐transportation group and the bottle group, but the maturation rate in the bottle group was 20.0%. The maturation rate is not yet high enough to transport HFF satisfactory, even in a box with a 40.0% maturation rate; however, it may be possible to minimize the impact of transportation by adjusting the HFF transport environment.
Since the non‐transportation group was by definition not adversely affected by HFF transportation, it is natural that it achieved a higher maturation rate than the transportation group. Due to the small number of cases in this study, further research is necessary to determine the optimal gas environment for HFF transportation.
In two past reports on HFF transportation, the transport tube contained only HFF, with no additional medium.11, 12 Porcine follicular fluid that was kept at a low temperature for a long time was found to result in reduced oocyte quality.6 Also, it was reported that high pH in the transportation environment lowered embryo quality.30 In this study, we mixed HFF with maturation medium in order to prevent evaporation and to improve the HFF environment during transportation. Additional research is required to determine whether HFF should be transported alone or mixed with maturation medium.
Most studies of IVM of human oocytes collected from resected ovaries have been conducted in the field of oncofertility,16, 24, 31, 32, 33 with reported maturation rates ranging from 3% to 100%. In recent years, the maturation rate in FP has been reported to range from 30% to 60%.34 When oocytes were collected from non‐stimulated transported ovaries, the maturation rates showed wide discrepancies, ranging from 3.1% to 66.7%.16, 28 By contrast, the maturation rate without HCG stimulation in the common population of patients with polycystic ovary syndrome was approximately 50%‐80%.34 In our study, the maturation rate of human oocytes after transportation of HFF was 38.6%. While this rate was not markedly lower than in past studies, it still must be further improved by optimizing HFF transportation methods and further analyzing the relationship between IVM results and transportation distances. In the field of FP, it has been reported that given the age of some patients, at least 8‐10 MII oocytes are necessary for a live birth.34, 35 In this study, the average number of oocytes collected from the patients in the transportation group was 8.1 ± 8.4. In previous reports on FP, the average number of oocytes collected from ROT ranged from 2.3 to 14.7 per patients.24, 34, 36 Transportation may be beneficial not only for the purpose of collecting research material, particularly since in this study there were no significant differences between the transportation and non‐transportation groups in the number of oocytes collected, but also for increasing the number of MII oocytes collected for FP by improving maturation rate. It is important to consider that when transported oocytes are used in research, their quality needs to be maintained to prevent misleading outcomes. In this regard, this study evaluated maturation rate and immunofluorescent staining of transported oocytes, but further investigation of oocyte quality is needed in the future.
On the other hand, the current results indicate that HFF transportation may not yet be clinically feasible in FP because the maturation rate in the transportation group was low. Of note, the maturation rate in this study was significantly higher than that in our past report,21 presumably due to the different medium used. We previously performed IVM using handmade culture medium with M199 (Gibco Labs, Grand Island, NY, USA), whereas in this study we used commercial maturation medium.
Transportation of ROT is advantageous in oncofertility because unlike with HFF, the transportation techniques are already established. In the future, however, transportation of HFF may be beneficial for oncofertility applications in cases where the hospital performing the pathological diagnosis of resected ovaries is geographically distant from the hospital involved in FP. Such hospitals are relatively rare in Japan; for instance, while in 2017 there were more than 400 cancer hospitals nationwide, there were only 30 hospitals with the ability to cryopreserve both oocytes and ovarian tissue.37 It may also allow human oocytes to be stored as research materials during transportation from operating hospitals to research institutions. In this study, mature oocytes in meiosis II were obtained in both the transportation and non‐transportation groups, and we anticipate that it will be feasible to use these oocytes for the study of blastocysts, for instances using parthenogenetic activation techniques.
Thus far, there have been few reports on the number of oocytes collected from resected, non‐stimulated ovaries.16, 21, 27 One study found a negative correlation between age and the number of oocytes collected from the resected ovaries of 23 patients with gynecologic benign disease.27 On the other hand, a surprisingly positive correlation was reported between age and the number of collected oocytes in patients younger than their mid‐30s.16 For the first time, we examined the correlation between age and the number of oocytes collected from the resected ovaries of patients with endometrial adenocarcinoma, many of whom were overweight. In this study, the mean BMI was 27.6 ± 7.5 kg/m2, which is considered overweight. As shown in Figure 3C, there was no significant correlation between BMI and the number of collected oocytes, even in overweight individuals. This is consistent with a previous study that found no correlation between BMI and the number of collected oocytes in patients with breast cancer and a mean BMI of 21.4.33
In this study, we performed IVM after transporting HFF for more than 4 hours. One report showed that the amount of non‐esterified fatty acids in the follicular fluid was significantly positively correlated with BMI; therefore, it is possible that the follicular fluid composition may affect oocyte quality.27, 38 The maturation rate after HFF transportation was relatively low in our study, at 38.6%, and further research is necessary to determine how IVM after transportation is affected by the composition of the follicular fluid. We have not yet attempted fertilization of mature oocytes after IVM. An examination of fertility rates after transportation of HFF will be necessary to better assess the quality of oocytes after transportation. If fertilization experiments are difficult from an ethical point of view, the use of parthenogenetic activation may be beneficial in the future.
Finally, we performed time‐lapse imaging of IVM incubation in patient 3, and as shown in the supplemental movie, this confirmed the extrusion of the first polar body. It will be helpful to use time‐lapse imaging in the future to compare differences in oocyte maturation between the transportation and non‐transportation groups.
The major limitation of this study was that we did not examine patients’ anti‐Müllerian hormone (AMH) levels and menstruation cycles. It was previously reported that the number of collected oocytes was positively correlated with AMH level, but not menstruation cycle.33
Another limitation is that the study design did not involve assigning half of each patient's oocytes to the transportation group and the other half to the non‐transportation group, thus providing an internal control. An average of 6.6 oocytes, ranging from 0 to 21, was collected per patient. Since oocytes were collected from non‐stimulated resected ovaries, fewer than 2 oocytes were obtained from 4 of 14 patients, as shown in Table 1. Although it is difficult to secure collected oocytes, in order to accurately evaluate the usefulness of HFF transportation, it will be necessary in future studies to evaluate IVM results by dividing the oocytes from each patient into two groups, only one of which undergoes the intervention. Considering this limitation and the fact that transportation significantly reduces the maturation rate, we concluded that the usefulness of the present HFF transportation technique was limited. A study in pigs reported that follicular fluid acidosis influenced DNA fragmentation of the oocyte nucleus.39 If HFF transport causes a similar adverse effect, studies using post‐transportation oocytes may result in inaccurate conclusion. In this study, an average of 8.1 ± 8.4 oocytes per patient and a maturation rate of 38.6% were obtained following HFF transportation. In FP, even one mature oocyte is valuable,34, 35 and in research setting, human oocytes are needed in many situations, for example, when creating parthenogenetic embryonic stem cells.40 In order to reduce the adverse effects on oocytes, research on improving HFF transportation method is needed in the future.
In conclusion, we transported by railway the HFF of patients with EMA and examined for the first time the results of IVM after HFF transportation. Improvements in transportation methods should yield better IVM results. Further studies on maintaining oocyte normality during HFF transportation are necessary before this technique can be effectively used to transfer oocytes for research purposes and FP.
In the future, these data may also be applied to the field of oncofertility after studies on fertility rates have been performed.
DISCLOSURES
Conflicts of interest: Hiromitsu Shirasawa, Natsuki Ono, Yukiyo Kumazawa, Wataru Sato, Naoki Sato, Motomasa Ihara, Nobuo Yaegashi, and Yukihiro Terada declare that they have no conflicts of interest. Human rights statements and informed consent: This protocol for research including human subjects was approved by a suitably constituted Ethics Committee. This study was approved by the Ethical Review Board of Akita University (No. 787, June. 2011, revised in 2016) and Tohoku University (No. 316, Jan. 2013, revised in 2016). We thoroughly explained to the patients that all obtained oocytes would be used only for IVM research and not for research on fertilization or for parthenogenetic activation. All procedures were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Animal studies: None of the authors performed any research on animal subjects in this study.
Supporting information
ACKNOWLEDGEMENTS
Financial support was provided by JSPS KAKENHI Grant Number JP16K20170 (H.S.). We thank for Yohei Onodera, Mibuki Anzai, and Kazumasa Takahashi of the Department of Obstetrics and Gynecology, Akita University Graduate School of Medicine, who collaborated in the process of IVM. We appreciate the English proofreading provided by ZENIS Co., Ltd. No author has any potential conflict of interest.
Shirasawa H, Ono N, Kumazawa Y, et al. Oocyte collection and in vitro maturation after train transportation of human follicular fluid aspirated from resected non‐stimulated ovaries of patients with endometrial adenocarcinoma. Reprod Med Biol. 2019;18:180–189. 10.1002/rmb2.12265
Hiromitsu Shirasawa and Natsuki Ono contributed equally to this work.
REFERENCES
- 1. Powell AM, Talbot NC, Wells KD, Kerr DE, Pursel VG, Wall RJ. Cell donor influences success of producing cattle by somatic cell nuclear transfer. Biol Reprod. 2004;71:210–216. [DOI] [PubMed] [Google Scholar]
- 2. Chen N, Liow SL, Yip WY, Tan LG, Ng SC. Influence of cysteamine supplementation and culture in portable dry‐incubator on the in vitro maturation, fertilization and subsequent development of mouse oocytes. Theriogenology. 2005;63:2300–2310. [DOI] [PubMed] [Google Scholar]
- 3. Chen N, Liow SL, Abdullah RB, et al. Developmental competence of transported in‐vitro matured macaque oocytes. Reprod Biomed Online. 2006;12:50–59. [DOI] [PubMed] [Google Scholar]
- 4. Fujii A, Kaedei Y, Tanihara F, et al. In vitro maturation and development of porcine oocytes cultured in a straw or dish using a portable incubator with a CO2 chamber. Reprod Domest Anim. 2010;45:619–624. [DOI] [PubMed] [Google Scholar]
- 5. Guignot F, Bezard J, Palmer E. Effect of time during transport of excised mare ovaries on oocyte recovery rate and quality after in vitro maturation. Theriogenology. 1999;52:757–766. [DOI] [PubMed] [Google Scholar]
- 6. Kim HJ, Choi SH, Son DS, et al. Effect of exposure duration of ovaries and oocytes at ambient temperature on parthenogenetic development of porcine follicular oocytes. J Reprod Dev. 2006;52:633–638. [DOI] [PubMed] [Google Scholar]
- 7. Naoi H, Otoi T, Shimamura T, et al. Developmental competence of cat oocytes from ovaries stored at various temperature for 24 h. J Reprod Dev. 2007;53:271–277. [DOI] [PubMed] [Google Scholar]
- 8. Hanna C, Long C, Hinrichs K, Westhusin M, Kraemer D. Assessment of canine oocyte viability after transportation and storage under different conditions. Anim Reprod Sci. 2008;105:451–456. [DOI] [PubMed] [Google Scholar]
- 9. Kamoshita K, Okamoto N, Nakajima M, et al. Investigation of in vitro parameters and fertility of mouse ovary after storage at an optimal temperature and duration for transportation. Hum Reprod. 2016;31:774–781. [DOI] [PubMed] [Google Scholar]
- 10. Nichols S, Harvey A, Gierbolini L, Gonzalez‐Martinez J, Brenner C, Bavister B. Long‐distance transportation of primate embryos developing in culture: a preliminary study. Reprod Biomed Online. 2010;20:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coetsier T, Verhoeff A, De Sutter P, Roest J, Dhont M. Transport‐in‐vitro fertilization/intracellular sperm injection: a prospective randomized study. Hum Reprod. 1997;12:1654–1656. [DOI] [PubMed] [Google Scholar]
- 12. Buckett WM, Fisch P, Dean NL, Biljan MM, Tan SL. In vitro fertilization and intracytoplasmic sperm injection pregnancies after successful transport of oocytes by airplane. Fertil Steril. 1999;71:753–755. [DOI] [PubMed] [Google Scholar]
- 13. Takanashi Y, Abe Y, Shibui Y, et al. Effect of oocyte transportation time on the clinical results of transport in vitro fertilization/intracytoplasmic sperm injection‐embryo transfer. Reprod Med Biol. 2004;3:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donnez J, Martinez‐Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12:519–535. [DOI] [PubMed] [Google Scholar]
- 15. Dittrich R, Lotz L, Keck G, et al. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril. 2012;97:387–390. [DOI] [PubMed] [Google Scholar]
- 16. Wilken‐Jensen HN, Kristensen SG, Jeppesen JV, Yding AC. Developmental competence of oocytes isolated from surplus medulla tissue in connection with cryopreservation of ovarian tissue for fertility preservation. Acta Obstet Gynecol Scand. 2014;93:32–37. [DOI] [PubMed] [Google Scholar]
- 17. Yin H, Jiang H, Kristensen SG, Andersen CY. Vitrification of in vitro matured oocytes collected from surplus ovarian medulla tissue resulting from fertility preservation of ovarian cortex tissue. J Assist Reprod Genet. 2016;33:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holubcova Z, Blayney M, Elder K, Schuh M. Human oocytes. Error‐prone chromosome‐mediated spindle assembly favors chromosome segregation defects in human oocytes. Science. 2015;348:1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sakakibara Y, Hashimoto S, Nakaoka Y, Kouznetsova A, Hoog C, Kitajima TS. Bivalent separation into univalents precedes age‐related meiosis I errors in oocytes. Nat Commun. 2015;6:7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsutsumi M, Fujiwara R, Nishizawa H, et al. Age‐related decrease of meiotic cohesins in human oocytes. PLoS ONE. 2014;9:e96710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shirasawa H, Kumagai J, Sato W, Kumazawa Y, Sato N, Terada Y. Retrieval and in vitro maturation of human oocytes from ovaries removed during surgery for endometrial carcinoma: a novel strategy for human oocyte research. J Assist Reprod Genet. 2013;30:1227–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosendahl M, Schmidt KT, Ernst E, et al. Cryopreservation of ovarian tissue for a decade in Denmark: a view of the technique. Reprod Biomed Online. 2011;22:162–171. [DOI] [PubMed] [Google Scholar]
- 23. Abir R, Ben‐Aharon I, Garor R, et al. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum Reprod. 2016;31:750–762. [DOI] [PubMed] [Google Scholar]
- 24. Segers I, Mateizel I, Van Moer E et al. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising "ex vivo" method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet. 2015;32:1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Imesch P, Scheiner D, Xie M, et al. Developmental potential of human oocytes matured in vitro followed by vitrification and activation. J Ovarian Res. 2013;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shirasawa H, Kumazawa Y, Sato W, Ono N, Terada Y. In vitro maturation and cryopreservation of oocytes retrieved from intra‐operative aspiration during second enucleation for ovarian tumor: a case report. Gynecol Oncol Rep. 2017;19:180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cha KY, Koo JJ, Ko JJ, Choi DH, Han SY, Yoon TK. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. Fertil Steril. 1991;55:109–113. [DOI] [PubMed] [Google Scholar]
- 28. Grynberg M, Poulain M, le Parco S, Sifer C, Fanchin R, Frydman N. Similar in vitro maturation rates of oocytes retrieved during the follicular or luteal phase offer flexible options for urgent fertility preservation in breast cancer patients. Hum Reprod. 2016;31:623–629. [DOI] [PubMed] [Google Scholar]
- 29. Shirasawa H, Kumagai J, Sato E, et al. Novel method for immunofluorescence staining of mammalian eggs using non‐contact alternating‐current electric‐field mixing of microdroplets. Sci Rep. 2015;5:15371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Itoi F, Tokoro M, Terashita Y, et al. Offspring from mouse embryos developed using a simple incubator‐free culture system with a deoxidizing agent. PLoS ONE. 2012;7:e47512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Son WY, Park SJ, Hyun CS, Lee WD, Yoon SH, Lim JH. Successful birth after transfer of blastocysts derived from oocytes of unstimulated woman with regular menstrual cycle after IVM approach. J Assist Reprod Genet. 2002;19:541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prasath EB, Chan ML, Wong WH, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29:276–278. [DOI] [PubMed] [Google Scholar]
- 33. Takae S, Sugishita Y, Yoshioka N, et al. The role of menstrual cycle phase and AMH levels in breast cancer patients whose ovarian tissue was cryopreserved for oncofertility treatment. J Assist Reprod Genet. 2015;32:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shirasawa H, Terada Y. In vitro maturation of human immature oocytes for fertility preservation and research material. Reprod Med Biol. 2017;16:258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cobo A, Garcia‐Velasco JA, Coello A, Domingo J, Pellicer A, Remohi J. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil Steril. 2016;105:755–764.e8. [DOI] [PubMed] [Google Scholar]
- 36. Safian F, Khalili MA, Karimi‐Zarchi M, Mohsenzadeh M, Ashourzadeh S, Omidi M. Developmental competence of immature oocytes aspirated from antral follicles in patients with gynecological diseases. Iran J Reprod Med. 2015;13:507–512. [PMC free article] [PubMed] [Google Scholar]
- 37. Takai Y. Recent advances in oncofertility care worldwide and in Japan. Reprod Med Biol. 2018;17:356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Valckx SD, Arias‐Alvarez M, De Pauw I et al. Fatty acid composition of the follicular fluid of normal weight, overweight and obese women undergoing assisted reproductive treatment: a descriptive cross‐sectional study. Reprod Biol Endocrinol. 2014;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wongsrikeao P, Otoi T, Karja NW, Agung B, Nii M, Nagai T. Effects of ovary storage time and temperature on DNA fragmentation and development of porcine oocytes. J Reprod Dev. 2005;51:87–97. [DOI] [PubMed] [Google Scholar]
- 40. Yu Y, Gao Q, Zhao HC, et al. Ascorbic acid improves pluripotency of human parthenogenetic embryonic stem cells through modifying imprinted gene expression in the Dlk1‐Dio3 region. Stem Cell Res Ther. 2015;6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


