Abstract
Background
Few studies have examined the benefits of pulmonary rehabilitation in patients with fibrotic idiopathic pulmonary pneumonia (f-IIP). Here, we report the results of an observational study in routine clinical practice of home-based pulmonary rehabilitation for f-IIP patients.
Methods
A total of 112 consecutive patients (61 with idiopathic pulmonary fibrosis and 51 with fibrotic nonspecific interstitial pneumonitis) were enrolled, of whom 65 had mild-to-moderate disease (forced vital capacity (FVC) ≥50% predicted and diffusing capacity of the lung for carbon monoxide (DLCO) ≥30% predicted) and 47 had severe disease (FVC <50% predicted and/or DLCO <30% predicted). The 2-month pulmonary rehabilitation programme consisted of a once-weekly visit with retraining, therapeutic education and psychosocial support. Patients were provided with an individualised action plan and were followed-up bimonthly for 12 months. Exercise tolerance (6-min stepper test (6MST)), mood (Hospital Anxiety and Depression Scale (HADS)) and quality of life (QoL) (Visual Simplified Respiratory Questionnaire (VSRQ)) were assessed before (T0), immediately after (T2), 6 months after (T8) and 12 months after (T14) the end of the pulmonary rehabilitation programme.
Results
6MST strokes, HADS Anxiety score and VSRQ score were each significantly improved at T2 (n=101), T8 (n=76) and T14 (n=62) compared with T0 values. The improvements in outcomes were not influenced by disease severity or subtype. Patients who completed the study had significantly better baseline FVC and DLCO values than those who did not.
Conclusions
Home-based pulmonary rehabilitation provides long-term benefits in exercise tolerance, anxiety and QoL for patients with f-IIP. Pulmonary rehabilitation should be prescribed systematically as part of the therapeutic arsenal for these patients.
Short abstract
This 12-month follow-up study shows that home-based pulmonary rehabilitation (PR) provides long-term benefits for patients with fibrotic idiopathic interstitial pneumonias. Home-based PR offers an alternative to in-hospital or outpatient PR programmes. http://ow.ly/JVdw30nSOYz
Introduction
Fibrotic idiopathic interstitial pneumonias (f-IIPs) (idiopathic pulmonary fibrosis (IPF) and fibrotic nonspecific interstitial pneumonitis (f-NSIP)) are interstitial lung diseases (ILDs) associated with poor prognosis and impaired daily life activities [1]. f-IIP patients display persistent and severe dyspnoea, exercise intolerance, and poor health-related quality of life (QoL) [2]. Patients with chronic respiratory diseases, including ILDs, have been shown to respond well to pulmonary rehabilitation, a validated and widely used method to improve dyspnoea, exercise tolerance, QoL and mood [3–5]. However, the beneficial effects of pulmonary rehabilitation in patients with ILDs have mainly been established in short-term studies (1–4 weeks) and reports of medium- to long-term effects (6–12 months) have been inconsistent, particularly in patients with progressively worsening fibrosing ILDs [5, 6]. For example, a recent meta-analysis reported no detectable improvements in exercise tolerance (distance in the 6-min walk test (6MWT)) after long-term follow-up (6 months) of f-IIP patients who underwent a 6- to 24-week pulmonary rehabilitation programme [7]. Similarly, a randomised study of 142 patients by Dowman et al. [8] found no benefit on exercise tolerance, QoL or depression scores at 6 months after completion of an 8-week pulmonary rehabilitation programme. However, in their study, Ryerson et al. [9] reported improvements in these same parameters at 6 months after 39 patients completed a 6- to 9-week pulmonary rehabilitation programme. Moreover, while the results were not statistically significant, Vainshelboim et al. [10] showed a trend towards stabilisation of exercise tolerance and QoL in 14 patients at 11 months after a 3-month pulmonary rehabilitation programme, whereas these parameters deteriorated in the control group who did not participate in pulmonary rehabilitation.
In an effort to clarify the benefits of pulmonary rehabilitation in patients with f-IIP, we investigated the long-term effect of a 2-month home-based pulmonary rehabilitation program in a large cohort of patients with IPF and f-NSIP.
Patients and methods
Patients
Between December 2011 and December 2017, 234 patients with a diagnosis of f-IIP were referred to our reference centre for rare pulmonary diseases for a diagnosis and medical decision based on multidisciplinary discussion with radiologists and pathologists. The inclusion criteria were 1) a diagnosis of IPF or f-NSIP according to the international consensus guidelines [11] and 2) no acute exacerbation in the 3 months preceding enrolment. Exclusion criteria were dementia or poorly controlled psychiatric illness, neurological sequelae, or bone and joint disease preventing physical activity. Of the 234 patients, 112 (70 males and 42 females; all Caucasian) gave written informed consent to participate in a home-based pulmonary rehabilitation programme. The study was performed in accordance with the local board on medical ethics. Approval for the use of the data was provided by the Institutional Review Board of the French Learned Society for Pulmonology (CEPRO 2011-039).
Pulmonary function tests and the 6MWT were performed at baseline; all other tests were performed at the patient's home throughout the study (see Assessments section). Forced vital capacity (FVC), forced expiratory volume in 1 s and total lung capacity were measured by spirometry and plethysmography with a Jaeger-Masterlab cabin (Vyaire Medical, Hoechberg, Germany), and single-breath diffusing capacity of the lung for carbon monoxide (DLCO (mL·min−1·mmHg−1)) was measured and corrected for haemoglobin concentration. Values are expressed as percentages of the predicted normal values [12]. Patients with FVC ≥50% predicted and DLCO ≥30% predicted were classified as having mild-to-moderate disease, and patients with FVC <50% predicted and/or DLCO <30% predicted were classified as having severe disease [13]. The 6MWT was performed in accordance with international recommendations [14].
Study design
This was an observational monocentric study with retrospective analysis of structurally captured data. The home-based pulmonary rehabilitation programme has been described in detail elsewhere [15]. Briefly, patients received one supervised in-home 90-min session per week for 2 months. The sessions were tailored based on an initial educational needs assessment, and comprised endurance physical exercise on a cycle ergometer, training in physical activities of daily living, strengthening exercises, therapeutic patient education, psychosocial support, and motivational communication to facilitate health behaviour changes and self-management. Each weekly session was conducted under the direct supervision of a team member, but patients were expected to perform a personalised endurance physical exercise plan, unsupervised, on the other days of the week. Follow-up home visits were performed bimonthly for 12 months after the end of the 2-month pulmonary rehabilitation programme. A detailed accident protocol was included in each patient's pulmonary rehabilitation agreement.
Assessments
Patients were evaluated at home before (±7 days; T0), immediately (±7 days) after (T2), 6 months (±1 month) after (T8) and 12 months (±1 month) after (T14) the end of the 2-month pulmonary rehabilitation programme. Exercise tolerance was evaluated using the 6-min stepper test (6MST), as previously described [16]. This test was selected because it is feasible for home use in patients with pulmonary disease and it has been verified to detect improved functional capacity after pulmonary rehabilitation in patients with chronic obstructive pulmonary disease (COPD) [17]. A change of 40 strokes (1 stroke=a half-cycle up or down) has been proposed as the minimal clinically important difference (MCID) for COPD patients [18]. Psychological state was determined using the Hospital Anxiety and Depression Scale (HADS) [19]. This questionnaire comprises 14 items (seven each for anxiety and depression), with responses being scored on a scale of 0–3 to indicate symptom frequency (minimum and maximum subscores of 0 and 21; lower is better). For both subscores, a change of 1.5 points is considered the MCID for COPD patients [20]. The Visual Simplified Respiratory Questionnaire (VSRQ) was initially developed for COPD patients, and is a validated and reliable questionnaire to assess health-related QoL [21]. It comprises eight visual analogue scales ranging from 0 to 10 (minimum and maximum scores of 0 and 80; higher is better). The MCID for COPD patients is considered 3.4 points.
Statistical analysis
Categorical variables are expressed as frequencies and percentages, and continuous variables are expressed as means with standard deviation. Normality of distribution was checked graphically and using the Shapiro–Wilk test. Comparisons of groups according to disease severity (mild-to-moderate versus severe) and disease type (IPF versus f-NSIP) were performed using the t-test for baseline continuous variables and the Chi-squared test for sex ratio.
Changes in study outcomes over time (6MST strokes, HADS scores and VSRQ score) were assessed using a linear mixed model with time as a fixed effect and patient as a random effect. When the time effect was significant, post hoc tests were performed to compare the baseline value and each follow-up assessment. Normality of the model residuals was checked for each outcome. Changes in study outcomes according to disease severity or type were assessed by including a time interaction term in a linear mixed model. All analyses were repeated for the cohort who completed the 12-month follow-up (n=62; sensitivity analysis). Results are expressed as means and 95% confidence intervals. All analyses were performed using a two-tailed test with an α-level of 0.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
The clinical and functional baseline characteristics of the patients included in the study are summarised by disease severity and type in table 1. Of the total cohort of 112 patients, 21% were treated with anxiolytics and/or antidepressants, 34% were on long-term oxygen therapy, and 53% were on ambulatory oxygen. Disease severity was considered mild-to-moderate in 65 patients and severe in 47 patients. All of the baseline characteristics except age and sex were significantly different between these two groups.
TABLE 1.
Baseline characteristics of patients
| f-IIP | p-value | IPF | f-NSIP | p-value | |||
| All | Mild-to-moderate | Severe | |||||
| Patients | 112 | 65 | 47 | 61 | 51 | ||
| Age years | 66.8±10.2 | 67.9±9.3 | 65.1±11.3 | 0.15 | 66.8±10.0 | 66.7±10.6 | 0.97 |
| Male/female | 70/42 | 41/24 | 29/18 | 0.88 | 46/15 | 24/27 | 0.002 |
| BMI kg·m−2 | 27.4±4.8 | 28.8±5.0 | 25.4±3.6 | <0.001 | 27.8±4.3 | 27.0±5.3 | 0.36 |
| FVC % pred | 69.7±19.7 | 78.6±17.1 | 57.4±16.2 | <0.001 | 68.6±18.0 | 71.1±21.6 | 0.52 |
| FEV1 % pred | 70.1±19.4 | 78.3±17.2 | 59.3±16.8 | <0.001 | 69.6±18.3 | 70.8±20.9 | 0.74 |
| TLC % pred | 67.6±15.2 | 73.8±15.0 | 58.7±10.4 | <0.001 | 66.7±14.4 | 68.7±16.2 | 0.55 |
| DLCO % pred | 35.9±12.7 | 42.4±10.3 | 23.9±6.5 | <0.001 | 33.4±9.8 | 39.1±15.1 | 0.033 |
| 6MWT distance m | 357±116 | 391±109 | 303±109 | <0.001 | 370±113 | 338±120 | 0.20 |
| 6MWT nadir SpO2 % | 83±7 | 86±6 | 80±7 | <0.001 | 82±7 | 86±7 | 0.011 |
| 6MST strokes | 364±150 | 413±151 | 292±116 | <0.001 | 371±144 | 356±157 | 0.63 |
| HADS score | 15.2±7.0 | 14.1±6.7 | 16.6±7.4 | 0.067 | 15.0±6.7 | 15.5±7.5 | 0.70 |
| VSRQ score | 37.3±16.4 | 40.8±15.0 | 32.3±17.1 | 0.007 | 37.6±16.4 | 36.9±16.5 | 0.82 |
Data are presented as n or mean±sd, unless otherwise stated. f-IIP: fibrosing idiopathic pulmonary pneumonia; IPF: idiopathic pulmonary fibrosis; f-NSIP: fibrotic nonspecific interstitial pneumonitis; BMI: body mass index; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; TLC: total lung capacity; DLCO: diffusing lung capacity of the lung for carbon monoxide; 6MWT: 6-min walk test; SpO2: arterial oxygen saturation measured by pulse oximetry; 6MST: 6-min stepper test; HADS: Hospital Anxiety and Depression Scale; VSRQ: Visual Simplified Respiratory Questionnaire.
61 of the patients were diagnosed with IPF and 51 with f-NSIP. The proportion of males in the IPF group (male/female 46/15) was significantly greater than that in the f-NSIP group (male/female 24/27) (table 1). Gas exchange parameters (DLCO and nadir arterial oxygen saturation measured by pulse oximetry during the 6MWT) were significantly lower in the IPF group than in the f-NSIP group, but there were no other significant differences in baseline parameters between the two disease groups.
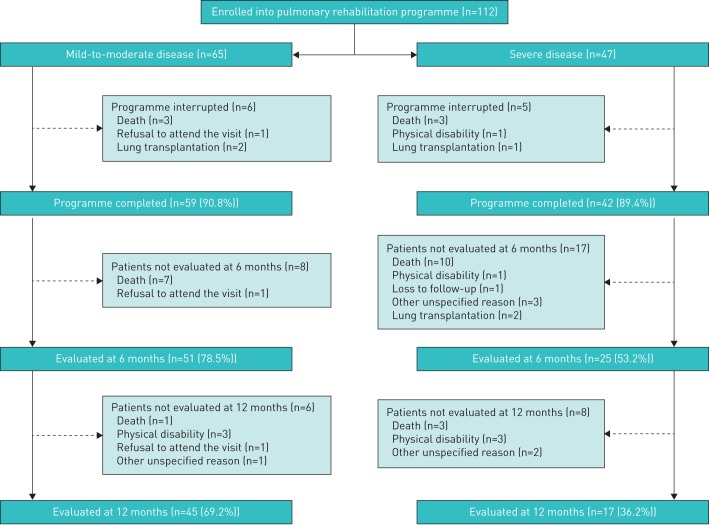
The disposition of the 112 patients is summarised in figure 1. 27 patients died and five underwent lung transplantation during the 14-month study. No adverse events or deaths attributable to the pulmonary rehabilitation or exercise retraining were recorded during or after the programme. In total, 101 patients (90.2%) completed the pulmonary rehabilitation programme and 62 (55.4%) completed all follow-up visits. Of the 50 patients who did not attend the 12-month follow-up visit, 20 had mild-to-moderate disease and 30 had severe disease. Patients who dropped out had more severe disease at baseline than those who completed the study, according to their functional characteristics (supplementary table S1).
FIGURE 1.
Flow diagram of patient disposition.
As shown in table 2, exercise tolerance (6MST strokes), QoL (VSRQ score), anxiety (HADS Anxiety score), and overall anxiety and depression (HADS Total score) were all significantly improved at T2 (the end of the 2-month programme) and at both T8 and T14 (6- and 12-month follow-up, respectively) compared with T0. After 12 months, the mean changes were 41.6 (95% CI 15.2–68.0) strokes for the 6MST, −1.5 (95% CI −2.9–0.1) for the HADS Anxiety score and 4.2 (95% CI 0.6–7.7) for the VSRQ score. In sensitivity analyses of the 62 patients who completed the T14 visit, the 6MST strokes and HADS Anxiety score were significantly improved, whereas the HADS Depression score and VSRQ score did not change (table 3).
TABLE 2.
Evaluation of exercise tolerance, anxiety, depression and quality of life before (T0), immediately after (T2), 6 months after (T8) and 12 months after (T14) the end of the pulmonary rehabilitation programme for all patients
| T0 | T2 | T8 | T14 | Global p-value | |
| Patients | 112 | 101 | 76 | 62 | |
| 6MST strokes | 364±150 | 417±169*** | 452±173** | 453±186** | <0.001 |
| HADS Total score | 15.2±7.0 | 13.5±6.4** | 12.4±6.7** | 12.3±6.4* | 0.008 |
| HADS Anxiety score | 8.6±4.5 | 7.5±3.9** | 7.0±3.8*** | 7.0±4.2** | 0.001 |
| HADS Depression score | 6.6±3.8 | 5.9±3.5 | 5.4±3.8 | 5.3±3.3 | 0.23 |
| VSRQ score | 37.3±16.4 | 42.7±15.3** | 42.1±16.5 | 45.0±16.7* | 0.013 |
Data are presented as n or mean±sd, unless otherwise stated. 6MST: 6-min stepper test; HADS: Hospital Anxiety and Depression Scale; VSRQ: Visual Simplified Respiratory Questionnaire. *: p<0.05; **: p<0.01; ***: p<0.001, significant difference from T0.
TABLE 3.
Evaluation of exercise tolerance, anxiety, depression, and quality of life before (T0), immediately after (T2), 6 months after (T8) and 12 months after (T14) the end of the pulmonary rehabilitation programme for the 62 patients who completed the study
| Variable | T0 | T2 | T8 | T14 | Global p-value |
| Patients | 62 | 62 | 62 | 62 | |
| 6MST strokes | 408±145 | 472±150*** | 462±167*** | 453±186** | <0.001 |
| HADS Total score | 13.6±6.9 | 12.3±6.2 | 11.2±5.9*** | 12.3±6.4 | 0.009 |
| HADS Anxiety score | 8.0±4.5 | 7.2±4.0* | 6.5±3.6*** | 7.0±4.2* | 0.002 |
| HADS Depression score | 5.6±3.3 | 5.1±3.0 | 4.7±3.3 | 5.3±3.3 | 0.24 |
| VSRQ score | 42.0±16.6 | 45.9±14.4 | 45.0±15.8 | 45.0±16.7 | 0.21 |
Data are presented as n or mean±sd, unless otherwise stated. 6MST: 6-min stepper test; HADS: Hospital Anxiety and Depression Scale; VSRQ: Visual Simplified Respiratory Questionnaire. *: p<0.05; **: p<0.01; ***: p<0.001, significant difference from T0.
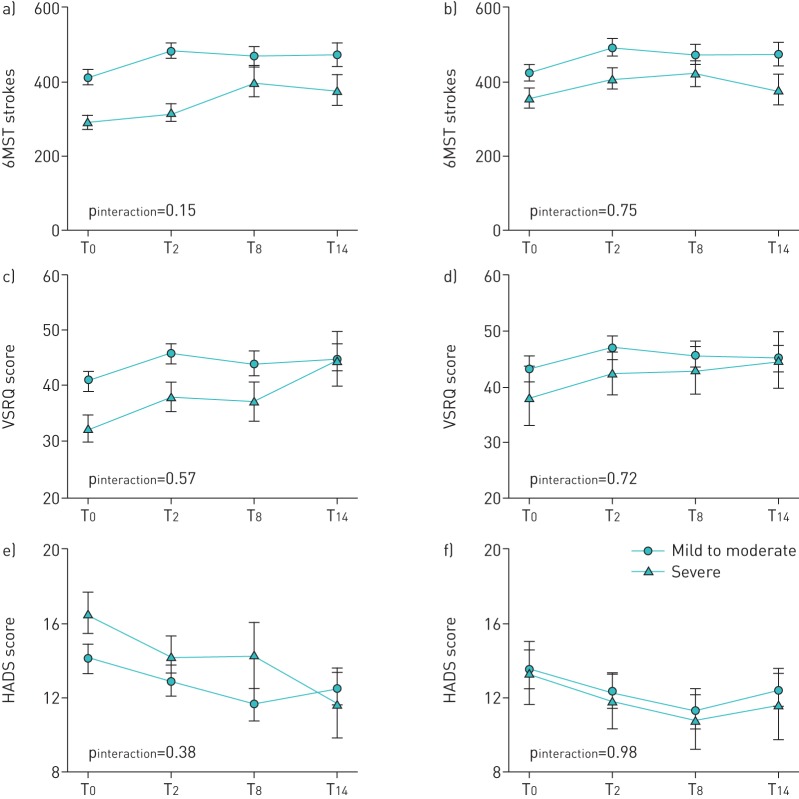
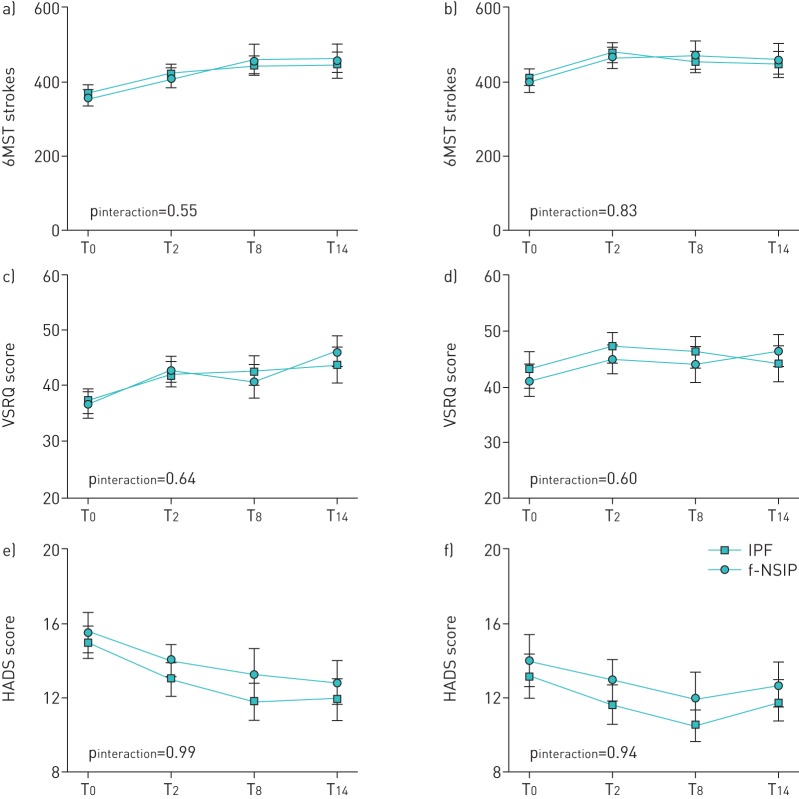
Disease severity and subtype had no effects on the 6MST strokes, HADS scores or VSRQ score (figures 2 and 3), and this was also true for the sensitivity analyses.
FIGURE 2.
Evaluation of exercise tolerance (6-min stepper test (6MST)), quality of life (Visual Simplified Respiratory Questionnaire (VSRQ)) and mood (Hospital Anxiety and Depression Scale (HADS)) in the fibrosing idiopathic pulmonary pneumonia patient population and the subset who completed the study according to disease severity. a, b) Number of strokes performed in the 6MST, c, d) VSRQ score and e, f) HADS score were assessed for all patients (a, c and e) and the 62 patients who completed the study (b, d and f) before (T0), immediately after (T2), 6 months after (T8) and 12 months after (T14) the end of the pulmonary rehabilitation programme. Data are presented as mean±se. The p-value for the interaction between time and disease severity (i.e. change in the variable with time compared between the mild-to-moderate and severe disease groups) is indicated.
FIGURE 3.
Evaluation of exercise tolerance (6-min stepper test (6MST)), quality of life (Visual Simplified Respiratory Questionnaire (VSRQ)) and mood (Hospital Anxiety and Depression Scale (HADS)) in the patients with idiopathic pulmonary fibrosis (IPF) and fibrotic nonspecific interstitial pneumonitis (f-NSIP). a, b) Number of strokes performed in the 6MST, c, d) VSRQ score and e, f) HADS score were assessed for the subsets of patients with IPF (n=61) (a, c and e) and NSIP (n=51) (b, d and f) before (T0), immediately after (T2), 6 months after (T8) and 12 months after (T14) the end of the pulmonary rehabilitation programme. Data are presented as mean±se. The p-value for the interaction between time and disease aetiology (i.e. change in the variable with time compared between the disease groups) is indicated.
Discussion
Our study shows significant long-term (12 months) benefits of a 2-month home-based pulmonary rehabilitation programme for f-IIP patients with bimonthly visits during follow-up. The main result of our pragmatic study is that the improvements in exercise tolerance and anxiety observed at the end of the pulmonary rehabilitation programme were maintained for 12 months. Interestingly, these benefits were experienced by all patients, even those with severe f-IIP.
Since 2008, several studies have established that pulmonary rehabilitation is safe, and has immediate beneficial effects on exercise tolerance, QoL and dyspnoea in patients with ILDs [5, 8, 22–25]. However, relatively few studies have examined the medium- and long-term benefits of pulmonary rehabilitation in f-IIP patients, and the results have varied considerably. For example, the randomised study of Dowman et al. [8] found that benefits in exercise tolerance, QoL and depression were not sustained at 6 months after exercise training, whereas the cohort studied by Ryerson et al. [9] showed sustained benefits in the same parameters at the same time-point after pulmonary rehabilitation. Additionally, the f-IIP patients studied by Vainshelboim et al. [10] showed a tendency towards stabilisation of 6MWT and QoL benefits at 11 months post-pulmonary rehabilitation compared with baseline, whereas these parameters deteriorated in the control group. The conflicts between our study and those of Vainshelboim et al. [10] and Dowman et al. [8] may be due to differences in patient cohorts, even though the latter study included patients with better prognosis than our f-IIP patients (e.g. asbestosis, sarcoidosis, hypersensitivity pneumonitis) [8]. Our results are consistent with those of a recent study by Perez-Bogerd et al. [26], who reported that pulmonary rehabilitation-induced improvements in exercise tolerance, health status and muscle force in patients with ILDs were maintained at 1-year follow-up. However, it should be noted that the study of Perez-Bogerd et al. [26] differed from ours in several respects: it included fewer patients with IIP (10 out of 30), the pulmonary rehabilitation programme was of 6 months duration (which is unusually long) and the “12-month” assessment was actually performed 6 months after the end of the 6-month pulmonary rehabilitation programme. In addition, the study by Perez-Bogerd et al. [26] used the 6MWT to assess exercise endurance, whereas we employed the 6MST. The latter test is more practical than the 6MWT, easy to perform at home [17], reproducible and sensitive. Moreover, the number of strokes in the 6MST correlates well with the distance travelled in the 6MWT in ILD patients [16].
Anxiety and depression are common in patients with ILDs. In a study of 50 patients with f-IIP (29 IPF and 21 NSIP), 54% had anxiety and 28% had depression (HADS subscores ≥8 for both) [27]. In another study, 59 out of 118 (49%) IPF patients had a score greater than the cut-off value of 15 on the Wakefield Self-assessment of Depression Inventory questionnaire [28]. Home-based pulmonary rehabilitation may improve anxiety, depression and psychological health by breaking the vicious circle of negative emotions, unpleasant breathing sensations and poor exercise performance [29].
We assessed QoL in our f-IIP patients using the VSRQ, a short questionnaire that has been validated in COPD patients [21], but not in ILD patients. We have used the VSRQ since the beginning of our home-based pulmonary rehabilitation programmes, which initially included predominantly COPD patients [15], and we continue to use it with other patients because it is informative and easy to use. We found a significant improvement above the MCID of 3.4 points that persisted for at least 12 months. Several additional studies have identified pulmonary rehabilitation-derived improvements in QoL, including one of 440 ILD patients (202 IPF, 21 NSIP and 217 other ILDs), but those studies used the 36-item Short-Form Health Survey, which includes physical and psychological components [5, 10, 30].
Our finding that pulmonary rehabilitation benefits are sustained for at least 12 months has not previously been demonstrated in f-IIP patients. We speculate that the home-based aspect was a crucial factor in this success. First, on unsupervised days, the patients could select whichever physical activities they found most enjoyable, which is undoubtedly easier at home. A patient is more likely to integrate physical activities into his/her daily life and to pursue them long term if they are involved in developing their own individualised action plan that takes into account their preferences and available facilities, is based on activities of daily living, and is combined with a home-based exercise retraining programme [31]. Second, the home setting allowed the patient's partner and/or caregivers to become involved. Finally, the bimonthly visit to the patient's home allowed the specialised pulmonary rehabilitation team to address difficulties, demonstrate solutions and provide motivational reinforcement, not only to the patient but also their partner and/or caregivers. Pulmonary rehabilitation gains can only be sustained if the patient also receives therapeutic education and behavioural/motivational support. Several studies have confirmed the interest of ILD patients in therapeutic education [32]. The motivational and behavioural aspects of a pulmonary rehabilitation programme [33] also give the patient confidence, and establish a faster therapeutic alliance between the patient and the rehabilitation team [34].
Although f-IIPs are progressive and fatal diseases with a median survival time of <4 years after diagnosis, treatment advances show encouraging signs of slowing disease progression and declining pulmonary function [35]. Only 36 patients (32%) had dropped out of our study after 6 months, comparable to the findings of Ryerson et al. [9] (30% at 6 months), suggesting that pulmonary rehabilitation could be a meaningful addition to the available treatment options. Pulmonary rehabilitation is currently the safest and most effective nondrug treatment known to improve dyspnoea, depression and anxiety, and should be offered to all f-IIP patients as part of a broader supportive care plan [3, 11, 29]. Particularly when prescribed soon after diagnosis, pulmonary rehabilitation could not only improve exercise tolerance and QoL but could also assist the patient, partner and/or caregiver in initiating the difficult, and often protracted, work of acceptance and end-of-life planning. This is particularly important, since palliative care is requested but rarely implemented in patients with IPF before the end of life [36].
There are several limitations to our study. Most patients received immunosuppressive agents, corticosteroids (for f-NSIP) and/or antifibrotic agents (for IPF), which is a potential confounding factor. We could not standardise the treatments or take them into account during data analysis because the treatments were implemented after the beginning of the pulmonary rehabilitation programme and the doses were adjusted according to individual patient needs. Although the VSRQ has not been validated in ILD patients, it is an easy method for patients to assess their respiratory-related QoL and it has been validated in studies assessing pulmonary rehabilitation programmes in patients with a variety of respiratory disorders, including COPD and severe asthma. We do not know how many more days the participants exercised by themselves, within and after the 2-month period. However, an important aspect of our patient's overall care is to trust that they are motivated to make long-term changes in their health behaviour (with the help of their entourage). This is routinely accomplished by motivating them, valuing the progress made during follow-up appointments, helping them to make adjustments where necessary and giving them confidence in their ability to act (empowerment and self-management). Lastly, the study was not randomised, and patients selected the home-based design according to their preference and/or the distance to the nearest pulmonary rehabilitation centre. This could represent a recruitment bias. However, home-based pulmonary rehabilitation has proven long-lasting benefits [15, 37–39] and caters well to patients who have limited mobility, lack motivation to participate at a pulmonary rehabilitation centre, do not wish to be hospitalised, have transportation problems, live a long distance from the nearest centre or do not wish to leave their home [3, 40].
In conclusion, home-based pulmonary rehabilitation provided long-term benefits in exercise tolerance, QoL and mood for patients with f-IIP. Pulmonary rehabilitation at home offers an alternative to centre-based pulmonary rehabilitation programmes (in-hospital or outpatient), greatly expanding the number of patients with access to care, supervised by a transdisciplinary team qualified in this field.
Acknowledgements
We thank the rehabilitation team from FormAction Santé (Pérenchies, France): Gaelle Tywoniuk, Sophie Duriez, Matthieu Grosbois, Florence Urbain, Virginie Wauquier and Marjorie Lambinet. The authors also wish to thank Anne M. O'Rourke (Scientific and Medical Writing, San Diego, CA, USA) for editing of the manuscript.
Footnotes
This article has supplementary material available from openres.ersjournals.com.
Author contributions: B. Wallaert, L. Duthoit and J-M. Grosbois had full access to all data, and take responsibility for the integrity of the data and the accuracy of data analysis, including and especially any adverse effects. E. Drumez, H. Behal, L. Wemeau and C. Chenivesse contributed substantially to the study design, data analysis and interpretation, and writing of the manuscript.
Conflict of interest: B. Wallaert reports personal fees from Roche and Boehringer Ingelheim, outside the submitted work.
Conflict of interest: L. Duthoit has nothing to disclose.
Conflict of interest: E. Drumez has nothing to disclose.
Conflict of interest: H. Behal has nothing to disclose.
Conflict of interest: L. Wemeau reports personal fees from Roche and Boehringer Ingelheim, outside the submitted work.
Conflict of interest: C. Chenivesse has nothing to disclose.
Conflict of interest: J-M. Grosbois reports that FormAction Santé received financial support from Adair, France Oxygène, Homeperf, LVL Medical, Orkyn, Santélys, Santeo, SOS Oxygène, Sysmed, VitalAire and ARS Hauts de France, during the conduct of the study.
References
- 1.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Vries J, Kessels BL, Drent M. Quality of life of idiopathic pulmonary fibrosis patients. Eur Respir J 2001; 17: 954–961. [DOI] [PubMed] [Google Scholar]
- 3.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188: e13–e64. [DOI] [PubMed] [Google Scholar]
- 4.Gomes-Neto M, Silva CM, Ezequiel D, et al. Impact of pulmonary rehabilitation on exercise tolerance and quality of life in patients with idiopathic pulmonary fibrosis: a systematic review and meta-analysis. J Cardiopulm Rehabil Prev 2018; 38: 273–278. [DOI] [PubMed] [Google Scholar]
- 5.Dowman L, Hill CJ, Holland AE. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev 2014; 10: CD006322. [DOI] [PubMed] [Google Scholar]
- 6.Alison JA, McKeough ZJ, Johnston K, et al. Australian and New Zealand pulmonary rehabilitation guidelines. Respirology 2017; 22: 800–819. [DOI] [PubMed] [Google Scholar]
- 7.Cheng L, Tan B, Yin Y, et al. Short- and long-term effects of pulmonary rehabilitation for idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Clin Rehabil 2018; 32: 1299–1307. [DOI] [PubMed] [Google Scholar]
- 8.Dowman LM, McDonald CF, Hill CJ, et al. The evidence of benefits of exercise training in interstitial lung disease: a randomised controlled trial. Thorax 2017; 72: 610–619. [DOI] [PubMed] [Google Scholar]
- 9.Ryerson CJ, Cayou C, Topp F, et al. Pulmonary rehabilitation improves long-term outcomes in interstitial lung disease: a prospective cohort study. Respir Med 2014; 108: 203–210. [DOI] [PubMed] [Google Scholar]
- 10.Vainshelboim B, Oliveira J, Fox BD, et al. Long-term effects of a 12-week exercise training program on clinical outcomes in idiopathic pulmonary fibrosis. Lung 2015; 193: 345–354. [DOI] [PubMed] [Google Scholar]
- 11.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Respiratory Society Standardized Lung Function Testing. Official statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16: 1–100. [PubMed] [Google Scholar]
- 13.King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. [DOI] [PubMed] [Google Scholar]
- 14.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 15.Grosbois JM, Gicquello A, Langlois C, et al. Long-term evaluation of home-based pulmonary rehabilitation in patients with COPD. Int J Chron Obstruct Pulmon Dis 2015; 10: 2037–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delourme J, Stervinou-Wemeau L, Salleron J, et al. Six-minute stepper test to assess effort intolerance in interstitial lung diseases. Sarcoidosis Vasc Diffuse Lung Dis 2012; 29: 107–112. [PubMed] [Google Scholar]
- 17.Grosbois JM, Riquier C, Chehere B, et al. Six-minute stepper test: a valid clinical exercise tolerance test for COPD patients. Int J Chron Obstruct Pulmon Dis 2016; 11: 657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pichon R, Couturaud F, Mialon P, et al. Responsiveness and minimally important difference of the 6-minute stepper test in patients with chronic obstructive pulmonary disease. Respiration 2016; 91: 367–373. [DOI] [PubMed] [Google Scholar]
- 19.Lepine JP, Godchau M, Brun P. Anxiety and depression in inpatients. Lancet 1985; 2: 1425–1426. [DOI] [PubMed] [Google Scholar]
- 20.Puhan MA, Frey M, Büchi S, et al. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes 2008; 6: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez T, Arnould B, Grosbois J-M, et al. Validity, reliability, and responsiveness of a new short Visual Simplified Respiratory Questionnaire (VSRQ) for health-related quality of life assessment in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2009; 4: 9–18. [PMC free article] [PubMed] [Google Scholar]
- 22.Holland A, Hill C. Physical training for interstitial lung disease. Cochrane Database Syst Rev 2008; 4: CD006322. [DOI] [PubMed] [Google Scholar]
- 23.Spruit MA, Janssen DJA, Franssen FME, et al. Rehabilitation and palliative care in lung fibrosis. Respirology 2009; 14: 781–787. [DOI] [PubMed] [Google Scholar]
- 24.Rammaert B, Leroy S, Cavestri B, et al. Home-based pulmonary rehabilitation in idiopathic pulmonary fibrosis. Rev Mal Respir 2011; 28: e52–e57. [DOI] [PubMed] [Google Scholar]
- 25.Wallaert B, Masson N, Le Rouzic O, et al. Effects of pulmonary rehabilitation on daily life physical activity of fibrotic idiopathic interstitial pneumonia patients. ERJ Open Res 2018; 4: 00167-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez-Bogerd S, Wuyts W, Barbier V, et al. Short and long-term effects of pulmonary rehabilitation in interstitial lung diseases: a randomised controlled trial. Respir Res 2018; 19: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallaert B, Monge E, Le Rouzic O, et al. Physical activity in daily life of patients with fibrotic idiopathic interstitial pneumonia. Chest 2013; 144: 1652–1658. [DOI] [PubMed] [Google Scholar]
- 28.Akhtar AA, Ali MA, Smith RP. Depression in patients with idiopathic pulmonary fibrosis. Chron Respir Dis 2013; 10: 127–133. [DOI] [PubMed] [Google Scholar]
- 29.Ferrara G, Luppi F, Birring SS, et al. Best supportive care for idiopathic pulmonary fibrosis: current gaps and future directions. Eur Respir Rev 2018; 27: 170076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huppmann P, Sczepanski B, Boensch M, et al. Effects of inpatient pulmonary rehabilitation in patients with interstitial lung disease. Eur Respir J 2013; 42: 444–453. [DOI] [PubMed] [Google Scholar]
- 31.Alison JA, McKeough ZJ. Pulmonary rehabilitation for COPD: are programs with minimal exercise equipment effective? J Thorac Dis 2014; 6: 1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland AE, Wadell K, Spruit MA. How to adapt the pulmonary rehabilitation programme to patients with chronic respiratory disease other than COPD. Eur Respir Rev 2013; 22: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol 2009; 65: 1232–1245. [DOI] [PubMed] [Google Scholar]
- 34.Bourbeau J, Lavoie KL, Sedeno M. Comprehensive self-management strategies. Semin Respir Crit Care Med 2015; 36: 630–638. [DOI] [PubMed] [Google Scholar]
- 35.Raghu G, Chen S-Y, Yeh W-S, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med 2014; 2: 566–572. [DOI] [PubMed] [Google Scholar]
- 36.Kreuter M, Bendstrup E, Russell A-M, et al. Palliative care in interstitial lung disease: living well. Lancet Respir Med 2017; 5: 968–980. [DOI] [PubMed] [Google Scholar]
- 37.Maltais F, Bourbeau J, Shapiro S, et al. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2008; 149: 869–878. [DOI] [PubMed] [Google Scholar]
- 38.Holland AE, Mahal A, Hill CJ, et al. Home-based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial. Thorax 2017; 72: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horton EJ, Mitchell KE, Johnson-Warrington V, et al. Comparison of a structured home-based rehabilitation programme with conventional supervised pulmonary rehabilitation: a randomised non-inferiority trial. Thorax 2018; 73: 29–36. [DOI] [PubMed] [Google Scholar]
- 40.Hayton C, Clark A, Olive S, et al. Barriers to pulmonary rehabilitation: characteristics that predict patient attendance and adherence. Respir Med 2013; 107: 401–407. [DOI] [PubMed] [Google Scholar]