Abstract
The interactive effects of ocean warming and invasive species are complex and remain a source of uncertainty for projecting future ecological change. Climate-mediated change to trophic interactions can have pervasive ecological consequences, but the role of invasion in mediating trophic effects is largely unstudied. Using manipulative experiments in replicated outdoor mesocosms, we reveal how near-future ocean warming and macrophyte invasion scenarios interactively impact gastropod grazing intensity and preference for consumption of foundation macroalgae (Ecklonia radiata and Sargassum vestitum). Elevated water temperature increased the consumption of both macroalgae through greater grazing intensity. Given the documented decline of kelp (E. radiata) growth at higher water temperatures, enhanced grazing could contribute to the shift from kelp-dominated to Sargassum-dominated reefs that is occurring at the low-latitude margins of kelp distribution. However, the presence of a native invader (Caulerpa filiformis) was related to low consumption by the herbivores on dominant kelp at warmer temperatures. Thus, antagonistic effects between climate change and a range expanding species can favour kelp persistence in a warmer future. Introduction of species should, therefore, not automatically be considered unfavourable under climate change scenarios. Climatic changes are increasing the need for effective management actions to address the interactive effects of multiple stressors and their ecological consequences, rather than single threats in isolation.
Keywords: global climate change, ocean warming, herbivory, invasive species, Caulerpa filiformis, Ecklonia radiata
1. Introduction
Climate change is a global environmental threat with consequences for ecological community structure and ecosystem function [1–3]. Community-level effects associated with climate change stem from combinations of direct effects (i.e. phenology, biology, physiology, distribution) [4–6] and indirect effects such as changes in biological interactions (e.g. trophic structures, competition) [7–9]. The predictability of such community-level responses to climate change is challenged by shifts in species' range and expansions of introduced species or ‘invasions’, which can facilitate alternations in species interactions and ecosystem properties [10–13]. Both introduced (i.e. non-native) and native species can shift distribution in response to climate change, but the interactive effects of such changes for community structure and function are still not well understood [14–18].
Marine invasions are predicted to increase with climate change [19,20], but only a limited body of literature has empirically evaluated how invasive species will alter ecosystem dynamics under such scenarios [21–23]. While the effects of invasion and different aspects of climate change have often been evaluated separately, these stressors can interact in complex ways [24–27]. Theoretical models forecast that the interactive effects between global warming and invasive species will most often be additive or synergistic [24,25]. In aquatic systems, for example, warming temperatures may reduce survival of native cold-adapted species and facilitate the establishment and reproductive success of non-native warm-adapted species [21]. This could lead to invasive species dominating over native species (i.e. owing to efficiency to compete or avoid predation), altering the structure and function of communities and intensifying impacts of climate change on ecosystem properties.
The influence of ocean warming on ecosystem dynamics often depends on the extent that such environmental change alters species interactions [8]. For example, plant–herbivore interactions may be altered at elevated ocean temperatures, as a result of a mismatch between production and herbivore consumption [28–30]. Increasing ocean temperatures enhance respiration, metabolism and grazing activity of herbivores up to their thermal maxima. However, photosynthetic production in many primary producers does not vary as temperature increases [31–34].
Climate-mediated change to trophic interactions can have substantial consequences for marine systems [35–36]. In particular, ocean warming is predicted to increase the metabolism of herbivores [8,34,37], intensifying the pressure on key native macrophytes in temperate rocky reef systems, as tropical herbivores expand into temperate latitudes [2,36]. Concurrently, native tropical algae are expanding their distribution into temperate habitats prompting novel shifts in competitive and trophic interactions [38–40]. While we have an emerging understanding of climate-mediated impacts of range expanding consumers on native algae [2,36], the impacts and consequences of range expanding algae on competitive and trophic interactions are poorly known.
The Caulerpa genus is one of the most successful groups of invasive algae around the world. Caulerpa filiformis is a green alga found in South Africa, Mozambique, Peru and Australia [38–41]. On the east coast of Australia, C. filiformis has become locally abundant on temperate shallow rocky reefs well outside its historic distribution [40–42]. These range expansions have negative effects on co-occurring macrophytes [43] representing a similar invasive behaviour as Caulerpa taxifolia and Caulerpa racemosa in southeastern Australia, the Mediterranean and United States [44,45]. Although C. filiformis has been considered ‘native’ in southeastern Australia by some authors [40,46], and ‘invasive’ by others [47,48], some impacts have been described [43] and their origin is still unsolved [47,48]. Hence, this species has been carefully termed here as a ‘native invader’ [43,49,50], based on biological invasion definitions by [51]. Caulerpa filiformis is structurally simpler than common co-occurring macroalgal species (e.g. Sargassum spp. and Ecklonia radiata) and, once established, can form large and persistent mono-specific stands that can spread via vegetative reproduction. The cover of C. filiformis is greater at subtidal than intertidal sites and mean percentage cover varies between 1 and 62% [40]. Caulerpa filiformis is heavily chemically defended with active secondary metabolites (e.g. caulerpenyne) which putatively leach into the surrounding water, impacting nearby macrophytes and potentially altering diversity of epibiotic assemblages [46]. Caulerpa filiformis is unpalatable to several key herbivores [48], which contributes to the dominance of this taxon once established.
Turbinid gastropods are common and abundant generalist herbivores in shallow subtidal reefs of eastern Australia [52,53]. Density of turbinid gastropods, such as Turbo militaris varies from 1 to 30 individuals per 4 m2 depending on protected or non-protected areas [54]. This species co-occurs and preferentially consumes brown algae E. radiata and Sargassum spp. rather than the chemically defended C. filiformis [47,55]. What is unknown, however, is how future ocean conditions, particularly warming, might directly alter the condition and palatability of these macrophytes, as well as the preferences and grazing rates of herbivores. Understanding interactions among these key ecological processes is necessary to predict likely future ecological consequences of climate-mediated invasions.
Here, we investigated the interactive effects of ocean warming and invasion (using the ‘native invader’ C. filiformis) on herbivory of native macroalgae. Using a series of manipulative experiments, we evaluate how gastropod grazing intensity and preference impacted consumption of native algae under combinations of near-future ocean warming and invasion scenarios. We hypothesized that temperature will increase gastropod grazing activity and intensify consumption of preferred native macrophytes, but this impact will be ameliorated in the presence of the chemically defended invasive macrophyte, C. filiformis.
2. Material and methods
(a). Experimental system
To test hypotheses about the influence of ocean warming and biological invasions on trophic interactions, three experiments were conducted in an outdoor mesocosm system at the National Marine Science Centre (NMSC) in Coffs Harbour, Australia (30.3022° S, 153.1189° E). The system was composed of 20, 230 l round, fibreglass, outdoor mesocosms (80 cm diameter × 45 cm high), 20 aquariums (30 cm length × 19.5 cm width × 20.5 cm high) and 20 trays (81 cm length × 61 cm width × 9 cm high). Each tray contained one aquarium (30 cm length × 19.5 cm width × 20.5 cm high) that received water from the respective mesocosm connected by a pipe at a rate of 2 l min−1. Mesocosms were set up in orthogonal combinations of ocean warming (temperature level: current (23°C), and future (26°C)) and invasion (C. filiformis present and absent, hereafter: invaded and non-invaded, respectively). An increase in ocean temperature of 3°C approximates near-future changes predicted by the Representative Concentration Pathway (RCP) 8.5 climate model for 2081–2100 [56,57]. Each mesocosm and aquarium was supplied with 50 µm filtered seawater (at a flow rate of 2 l min−1) continuously sourced from an adjacent beach (30.2670° S, 153.1407° E). Water temperature was controlled using heater chiller units (Aquahort Ltd, Omana Beach, New Zealand), and oxygen levels and water movement were maintained by bubbling ambient air into each mesocosm. The outdoor mesocosms were situated under shade cloth and exhibited diurnal cycles where water temperatures varied by less than 1°C. Water temperature and salinity were measured daily with a Hach HQ40d multiprobe calibrated with high precision buffers. The average (s.e.) measured and calculated seawater conditions for each treatment are presented in the electronic supplementary material, table S1.
The first experiment tested the interactive effects of ocean warming and invasion (presence of C. filiformis, hereafter Caulerpa) on the consumption rate of native macrophytes (E. radiata and Sargassum vestitum, hereafter Ecklonia and Sargassum, respectively) by a large turbinid gastropod (T. militaris). Ecklonia and Sargassum are two of the most common and ubiquitous taxa characterizing Australia's temperate reefs and play a key role in underpinning temperate biodiversity and ecosystem services [58,59]. This experiment ran in the tanks for 15 days for Ecklonia and 35 days for Sargassum from December 2017 to January 2018. The duration of the experiments was determined by the time required for the gastropod to eat close to 100% of any macrophyte individual in at least one mesocosm. Each tank contained four cages (enclosed plastic mesh baskets 26.5 cm length × 18.5 cm width × 10.5 cm high) with a mesh on top. Inside each of two cages, we placed native macrophytes (n = 3) without gastropods (autogenic control, one cage for each algal species); whereas the other two cages contained macrophyte plants (n = 3; one cage for each algal species) exposed to gastropod grazing (one T. militaris for each cage). These individual numbers were used to simulate natural densities considering the dimensions of the cages. Ten Caulerpa plants were placed outside of the cages in each mesocosm, to simulate (i) dominance condition in the tanks that correspond approximately 30% (mean percentage cover) of the substrate considering similar abundances found in nature (between 1 and 62%, see [43]), and (ii) to simulate chemical effect in the mesocosm water, but avoiding physical influence of gastropod grazing on the invasive alga. Individual Caulerpa plants were replaced if their fronds began to bleach or necrose throughout the experiment.
Ecklonia (17.8 ± 0.3 cm length and 4.3 ± 0.1 g weight, mean ± s.e.), Sargassum (20.9 ± 0.3 cm length and 17.7 ± 0.2 g weight) and gastropods (T. militaris 7.9 ± 0.1 cm length and 141.8 ± 3.9 g weight) were collected from nearby rocky reefs and Caulerpa plants (28.3 ± 0.6 cm length and 33.4 ± 0.8 g weight) were collected from the closest accessible location at Anna Bay (32.7898° S, 152.1157° E). Collected Ecklonia plants were in their second stage of growth, which is in between juveniles and adults [60], Sargassum were small adults and Caulerpa adult plants. Algae were attached to a plastic grid weighted with two rocks and enclosed in permeable cloth to facilitate their natural erect position in the tanks. The gastropods were acclimatized in aquarium conditions with the same mesocosm temperature for three weeks prior being included in any experiment. The wet weight of Ecklonia, Sargassum and Caulerpa was determined prior being placed into the mesocosms, and again when removed at the end of the experiment, by patting dry with paper towel and weighing without the mesh or weights. The weight and length of the gastropods were also measured at the start and end of the experiment.
The photosynthetic health of macrophytes was measured as effective quantum yield (ΔF/F′m) on day 1, 7 and 15 for Ecklonia, or on day 1 and 35 of the experiment for Sargassum, respectively. Effective quantum yield was determined using a pulse amplitude modulation (PAM) fluorometer (Diving-PAM, Walz, Effeltrich, Germany); where ΔF = F′m − Ft, with F′m being the maximal fluorescence, and Ft the steady-state fluorescence under illumination at time t [61,62]. Plants were dark acclimated for at least 15 min prior to measurements using leaf clips. Fluorescence was measured by holding the fibreoptic of the PAM fluorometer 1 mm from the algae frond using a clip in situ in the mesocosm. The algae surface was then exposed to a pulsed measuring beam of weak red light (0.15 µmol m−2 s−1, 650 nm) to measure Ft. Once the signal was stabilized (5 s), a pulse of saturating light (6000 µmol m−2 s−1) was applied and F′m was recorded. Measurements commenced in the morning, at the same time each day around 08.00, after plants had been exposed to approximately 2 h of natural daylight and were completed in less than 2 h. One reading per individual plant was recorded on haphazardly selected areas of the algal frond. To ensure independence in analyses, individual readings were averaged to provide a mean value of ΔF/F′m for each mesocosm.
We carried out a second experiment in the 20 aquariums to compare gastropod grazing activity (independent of macrophytes) among treatments by investigating grazing scars on wax surfaces [63,64], over a 4 day period. One dental wax square (4.5 × 4.5 cm) glued onto a ceramic tile (15 × 15 cm) was placed in a separate aquarium with an individual gastropod (T. militaris 8.1 ± 0.1 cm length and 145.3 ± 4.7 g weight) acclimated as above. The duration of this experiment was determined by the time required for a gastropod to make notable radula scrapings on wax surfaces from grazing on fast-growing epilithic algae in at least one mesocosm.
A third experiment ran for 4 days in the tanks that were cleaned immediately following the first experiment. It was a multiple choice assay to evaluate whether ocean warming influences the grazing preferences of gastropods with respect to Ecklonia, Sargassum and Caulerpa. Elucidating preference hierarchies of gastropods is important to understand the magnitude of herbivory on preferred algal species relative to less preferred species [65,66]. The duration of this experiment was determined by the time required for a gastropod to eat close to 100% of any macrophyte individual in at least one mesocosm. For this experiment, 40 cages were cleaned of epiphytes and biofilms by placing them in freshwater for 2 days and then carefully scrubing them with a brush and rinsing them with a jet of freshwater. Two of these cages were placed in each of the 20 mesocosms (n = 10 per temperature level: 23°C and 26°C). In each mesocosm, one cage had one plant of Ecklonia, Sargassum and Caulerpa as autogenic controls without gastropods. The other cage also had one plant of each species with one individual gastropod. The Ecklonia, Sargassum and Caulerpa plants ranged in size from 25.4 ± 0.7 cm length (6.4 ± 0.3 g weight), 13.7 ± 0.5 cm length (13.9 ± 0.3 g weight) and 25 ± 0.7 cm length (12.9 ± 0.3 g weight), respectively.
(b). Data analysis
Experiments 1 and 2 had two orthogonal fixed factors: warming (two levels, current 23°C and future 26°C) and invasion (two levels, invaded and non-invaded). Consumed biomass of native algae, yield and per cent cover of gastropod bites were analysed with general linear models (GLM) using a Gaussian distribution. The effects of treatments on the health of algae of autogenic control (weight and photosynthetic yield) were tested separately (see the electronic supplementary material, table S2). Experiment 3 had two orthogonal fixed factors: warming (two levels, current 23°C and future 26°C) and species (three levels, Caulerpa, Ecklonia and Sargassum). The amount of biomass consumed was calculated with the equation: Consumption (Si × Cf/Ci) − Sf, where Si and Sf were the mass of the plants exposed to gastropods before (initial or i) and after (final or f) the assay, respectively; and Ci and Cf were the biomass of the paired autogenic control plants before and after the assay, respectively [67]. The consumption data were normally distributed and thus analysed using the Hotelling's T2 test [68]. The Caulerpa weight loss differences between autogenic control and herbivory treatment were tested using Kruskal–Wallis. A post hoc Tukey test was used when significant differences were found. The GLMs and Kruskal–Wallis tests were performed using the R software [69] using the packages lme4 and MASS. The Hotelling's T2 test was performed using SPSS software.
3. Results
There were no direct effects of warming or invasion on the weight of Ecklonia and photosynthetic yield of Sargassum (autogenic controls) (electronic supplementary material, table S2a,d). All native algae in the autogenic controls remained in visibly good health during the experiment. There was an effect of warming on the weight of Sargassum with higher values in 26°C (electronic supplementary material, table S2c). Additionally, there was a significant effect of warming on the photosynthetic yield of Ecklonia with higher values in 23°C and the interaction with invasion was also significant with higher values without Caulerpa presence at 23°C (electronic supplementary material, table S2b).
(a). Macrophyte biomass consumed and photosynthetic yield
A significant effect of ocean warming on grazing of the kelp Ecklonia was observed, with greater biomass being consumed at 26°C than at 23°C (figure 1a; electronic supplementary material, table S3a). The interaction between ocean warming and invasion was also significant with less consumption of kelp under an invasion scenario (Caulerpa present) at 26°C (electronic supplementary material, table S3a). Temperature also had significant effects on grazing on Sargassum, with greater biomass consumed under warmer conditions (figure 1b; electronic supplementary material, table S3b). However, the interaction between warming and invasion had no significant effect on grazing on Sargassum (electronic supplementary material, table S3b). There were no direct effects of warming or invasion on the photosynthetic yield of native macrophytes (electronic supplementary material, table S4).
Figure 1.
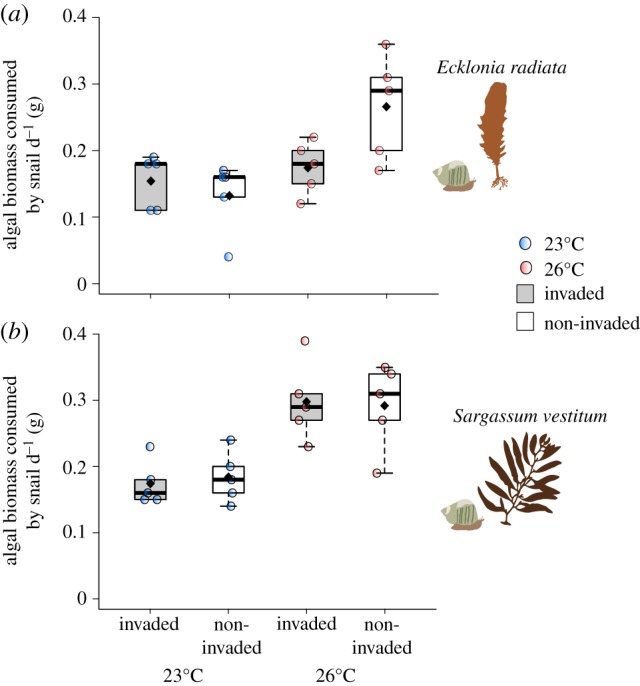
Algal biomass consumed by Turbo militaris under invaded and non-invaded conditions at 23°C and 26°C for Ecklonia radiata (a) and Sargassum vestitum (b). Box plot represents the median, Q1, Q3, minimum and maximum values, and outliers, black diamonds represent mean values; each circle represents a mesocosm.
(b). Patterns of gastropod grazing intensity
The intensity of grazing on dental wax was significantly higher at 26°C in the absence of invasion (no Caulerpa presence) (figure 2; electronic supplementary material, table S5). Both main effects of warming and invasion increased grazing rates, but the interaction between these factors was not significant (electronic supplementary material, table S5). Grazing rates were higher at 26°C but lower under invasion conditions (figure 2; electronic supplementary material, table S5).
Figure 2.
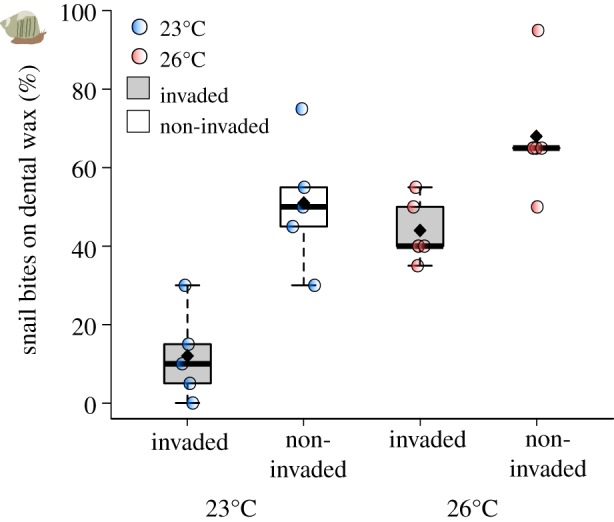
Grazing rates of Turbo militaris on experimental dental wax in invaded and non-invaded conditions at 23°C and 26°C. Box plot represents the median, Q1, Q3, minimum and maximum values, and outliers, black diamonds represent mean values; each circle represents a mesocosm.
(c). Feeding preferences
Turbo militaris consumed significantly more Ecklonia and Sargassum than Caulerpa (post hoc tests following Hotelling's T2, p < 0.05; electronic supplementary material, table S6; figure 3). These clear preferences of T. militaris did not change with water temperature (Hotelling's T2, p > 0.05; electronic supplementary material, table S6; figure 3). The consumption of Caulerpa was very low and weight loss in this species did not differ significantly from that in autogenic controls (electronic supplementary material, table S7).
Figure 3.
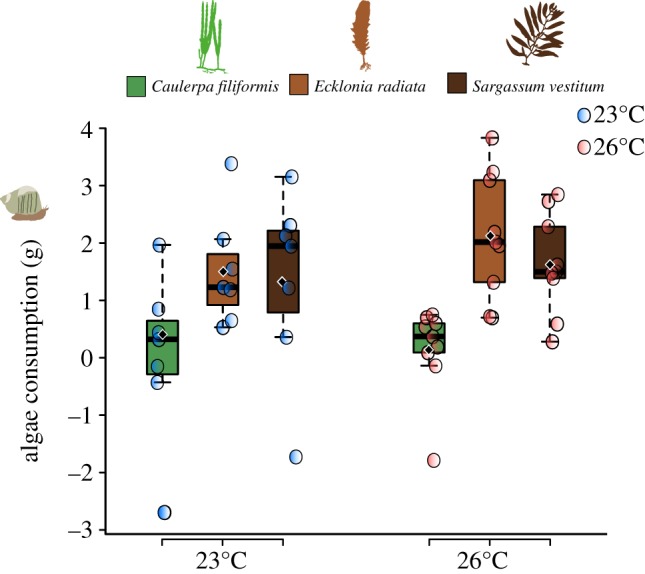
Preference of Turbo militaris for Caulerpa filiformis, Ecklonia radiata and Sargassum vestitum at 23°C and 26°C. Box plot represents the median, Q1, Q3, minimum and maximum values, and outliers, black diamonds represent mean values; each circle represents a mesocosm.
4. Discussion
Climate change is modifying trophic interactions among range expanding native species [36,70], but the interactions with invasion in a changing climate are not yet fully understood. Here, we determined the interactive effects of ocean warming and invasion on consumption (grazing) of native algae and found that ocean warming increased consumption of two common native algal taxa through greater grazing intensity. However, for herbivory on the dominant habitat forming kelp (Ecklonia) at warmer temperatures, low consumption was related to the presence of Caulerpa. The interactive effects of ocean warming and invasion thus had a positive feedback for kelp, reducing biomass loss from gastropod grazing. In certain instances, therefore, invasion may contribute to the future persistence of kelp in warming oceans.
Changes to trophic interactions under ocean warming can influence community structure and marine ecosystem function [36,37], threatening key habitat forming species, such as kelp. Our experiments showed that gastropods intensified grazing rates at elevated temperatures, increasing consumption of preferred native macrophytes in response to ocean warming. The metabolic theory of ecology [71] predicts that the metabolic rates of organisms increase with temperature owing to biochemical reactions constrained by thermodynamics [32,34]. Enhanced metabolic performance in near-future climate change scenarios probably stimulated algal consumption by Turbo militaris and, therefore, might have consequences for Ecklonia and Sargassum populations in future oceans. Additionally, elevated temperatures could also intensify the strength of herbivory through changes to the susceptibility of plant tissues [8], such as changing nitrogen content [72], or plants could allocate reduced energy to grazing defence mechanisms. Therefore, when the effects of ocean warming are considered independently, our results reinforce the contention that herbivory will increase with temperature [34]. In turn, this could lead to overgrazing of native macrophytes and even complete regime shifts to more simple systems states [70,73].
Combined effects of warming and invasive species interacting in additive or synergetic ways have been demonstrated [74,75]. Mostly previous studies suggest that warming temperatures could favour invasive mechanisms leading to dominance over native species (i.e. owing to efficiency to compete or avoid predation) intensifying impacts of climate change on ecosystem proprieties. By contrast, we show that these combined effects can be antagonistic [26]. Indeed, our results demonstrated that consumption of Ecklonia was lower at elevated temperature under an invasion scenario (the presence of Caulerpa). Probably, the biologically active secondary metabolites (caulerpenynes) present in Caulerpa species act as a feeding deterrent that mediates grazing activity [47,48]. Presence of caulerpenyne in the water when Caulerpa is present probably inhibits the biological activity of gastropods, reducing feeding intensity on Ecklonia. Evidence that Caulerpa presence reduces gastropod grazing activity also comes through the fact that grazing was reduced even in the absence of native algae. Thus, our results show that invasion indirectly benefits Ecklonia by reducing herbivory and suggests that under certain scenarios, antagonistic effects between climate change and invasive species can favour Ecklonia persistence as oceans warm in the future.
Although our findings show that effects between climate change and invasive species mediate positive feedbacks for Ecklonia, other studies have demonstrated both direct and indirect negative effects of Caulerpa on native macrophytes [46]. Caulerpa, once established, accumulates sediment favouring algal turfs which may then inhibit colonization by kelp [44]. Additionally, Caulerpa can affect the abundance of fauna associated with neighbouring macroalgal habitats [76]. Although theoretical evidence predicts that a warming climate will generally increase rates of invasion and negatively impact biodiversity [22,74,75], our results demonstrate that biotic interactions are more difficult to predict.
When considering direct and indirect effects of ocean warming, the future of kelp forests in many systems appears bleak independently of invasions [36,37,77–79]. Modelling projections suggest major climate-mediated changes to the suitable habitats for kelp forests, producing significant retreat of kelp forest around the Australian coast [78] and in many other parts of the world [80–82]. Elevated temperatures can directly reduce complex canopies [77] or decrease consumption at higher trophic levels, releasing pressure on consumers and indirectly increasing herbivory on kelp [37]. However, we demonstrate that the simple presence of an invasive macrophyte has the potential to alter existing relationships involving native herbivores and algae, possibly changing the predicted trajectories of kelp forests compared to scenarios where only ocean temperature is considered.
Overall, our results suggest that the current range expansions of Caulerpa may contribute to the persistence of kelp as oceans warm by reducing herbivory. However, such effects may not extend to other native macrophytes, as we demonstrate no effect of invasion on grazing on Sargassum. In a changing climate, it will be increasingly difficult to predict the impacts of invasive species on ecosystems. Nonetheless, understanding such complex ecological relationships will be key to predicting and managing marine environments under future ecological scenarios. It is clear that future management actions based on single-threat drivers will often be too simplistic and must be reconsidered in connection with interactive effects from climate change and other stressors.
Supplementary Material
Acknowledgements
We thank NMSC staff for technical advice, E. Provost, J.Z. Sippo, J. Fouliard and S. Pryor for assistance with collection. We thank Dr K. Benkendorff for contributions to the discussion.
Data accessibility
The datasets supporting this article are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.761js4b [83].
Authors' contributions
R.J.M., B.P.K., M.A.C. and F.B. designed the study. R.J.M., A.T., M.S.R., L.T.M. performed the research. R.J.M. and B.P.K. analysed the data. All authors contributed to the writing of the manuscript.
Competing interests
The authors declare that they have no conflict of interests.
Funding
Financial support was received through the ARC Project no. DP150104263 and NSW Environmental Trust Grant RD 0113. R.J.M. was supported by a Doctoral Exchange Program PhD scholarship through CAPES (Brazilian Federal Agency for Support and Evaluation of Graduate Education) scholarship no. 88881.132702/2016-01.
References
- 1.Hoegh-Guldberg O, Bruno JF. 2010. The impact of climate change on the world's marine ecosystem. Science 328, 1523–1529. ( 10.1126/science.1189930) [DOI] [PubMed] [Google Scholar]
- 2.Vergés A, et al. 2016. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proc. Natl Acad. Sci. USA 113, 13 791–13 796. ( 10.1073/pnas.1610725113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenberg SU, Nagelkerken I, Marangon E, Bonnet A, Ferreira CM, Connell SD. 2018. Ecological complexity buffers the impacts of future climate on marine consumers. Nat. Clim. Chang. 8, 229–233. ( 10.1038/s41558-018-0086-0) [DOI] [Google Scholar]
- 4.Bradshaw WE, Holzapfel CM. 2001. Genetic shift in photoperiodic response correlated with global warming. Proc. Natl Acad. Sci. USA 98, 14 509–14 511. ( 10.1073/pnas.241391498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walther G, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh-Guldberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395. ( 10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 6.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 7.Alsterberg C, Eklof JS, Gamfeldt L, Havenhand JN, Sundback K. 2013. Consumers mediate the effects of experimental ocean acidification and warming on primary producers. Proc. Natl Acad. Sci. USA 110, 8603–8608. ( 10.1073/pnas.1303797110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poore AGB, Graba-landry A, Favret M, Sheppard H, Byrne M, Dworjanyn SA. 2013. Direct and indirect effects of ocean acidification and warming on a marine plant–herbivore interaction. Oecologia 173, 1113–1124. ( 10.1007/s00442-013-2683-y) [DOI] [PubMed] [Google Scholar]
- 9.Werner FJ, Matthiessen B. 2013. Temperature indirectly affects benthic microalgal diversity by altering effects of top-down but not bottom-up control. Oikos 122, 52–63. ( 10.1111/j.1600-0706.2012.19952.x) [DOI] [Google Scholar]
- 10.Dukes JS, Mooney HA. 1999. Does global change increase the success of biological invaders? Trends Ecol. Evol. 14, 135–139. ( 10.1016/S0169-5347(98)01554-7) [DOI] [PubMed] [Google Scholar]
- 11.Stachowicz JJ, Terwin JR, Whitlatch RB, Osman RW. 2002. Linking climate change and biological invasions: ocean warming facilitates nonindigenous species invasions. Proc. Natl Acad. Sci. USA 99, 15 497–15 500. ( 10.1073/pnas.242437499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrenfeld JG. 2010. Ecosystem consequences of biological invasions. Annu. Rev. Ecol. Evol. Syst. 41, 59–80. ( 10.1146/annurev-ecolsys-102209-144650) [DOI] [Google Scholar]
- 13.Strayer DL. 2012. Eight questions about invasions and ecosystem functioning. Ecol. Lett. 15, 1199–1210. ( 10.1111/j.1461-0248.2012.01817.x) [DOI] [PubMed] [Google Scholar]
- 14.Phillips BL, Brown GP, Shine R. 2010. Life-history evolution in range-shifting populations. Ecology 91, 1617–1627. ( 10.1002/ecm.1283) [DOI] [PubMed] [Google Scholar]
- 15.Merzouk A, Johnson LE. 2011. Kelp distribution in the northwest Atlantic Ocean under a changing climate. J. Exp. Mar. Biol. Ecol. 400, 90–98. ( 10.1016/j.jembe.2011.02.020) [DOI] [Google Scholar]
- 16.Filbee-Dexter K, Feehan CJ, Scheibling RE. 2016. Large-scale degradation of a kelp ecosystem in an ocean warming hotspot. Mar. Ecol. Prog. Ser. 543, 141–152. ( 10.3354/meps11554) [DOI] [Google Scholar]
- 17.Pessarrodona A, Foggo A, Smale DA. 2018. Can ecosystem functioning be maintained despite climate-driven shifts in species composition? Insights from novel marine forests. J. Ecol. 107, 91–104. ( 10.1111/1365-2745.13053) [DOI] [Google Scholar]
- 18.Lin ZH, Wu CH, Ho CK. 2018. Warming neutralizes host-specific competitive advantages between a native and invasive herbivore. Sci. Rep. 8, 1–8. ( 10.1038/s41598-018-29517-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diez JM, et al. 2012. Will extreme climatic events facilitate biological invasions? Front. Ecol. Environ. 10, 249–257. ( 10.1890/110137) [DOI] [Google Scholar]
- 20.García Molinos J, et al. 2016. Climate velocity and the future global redistribution of marine biodiversity. Nat. Clim. Chang. 6, 83–88. ( 10.1038/nclimate2769) [DOI] [Google Scholar]
- 21.Hellmann JJ, Byers JE, Bierwagen BG, Dukes JS. 2008. Five potential consequences of climate change for invasive species. Conserv. Biol. 22, 534–543. ( 10.1111/j.1523-1739.2008.00951.x) [DOI] [PubMed] [Google Scholar]
- 22.Rolls RJ, Hayden B, Kahilainen KK. 2017. Conceptualising the interactive effects of climate change and biological invasions on subarctic freshwater fish. Ecol. Evol. 7, 4109–4128. ( 10.1002/ece3.2982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahel FJ, Olden JD. 2008. Assessing the effects of climate change on aquatic invasive species. Conserv. Biol. 22, 521–533. ( 10.1111/j.1523-1739.2008.00950.x) [DOI] [PubMed] [Google Scholar]
- 24.Folt C, Chen C. 1999. Synergism and antagonism among multiple stressors. Limnol. Oceanogr. 44, 864–877. ( 10.4319/lo.1999.44.3) [DOI] [Google Scholar]
- 25.Strayer DL. 2010. Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol. 55, 152–174. ( 10.1111/j.1365-2427.2009.02380.x) [DOI] [Google Scholar]
- 26.Treasure AM, Chown SL. 2014. Antagonistic effects of biological invasion and temperature change on body size of island ectotherms. Divers. Distrib. 20, 202–213. ( 10.1111/ddi.12153) [DOI] [Google Scholar]
- 27.Darling ES, Côté IM. 2008. Quantifying the evidence for ecological synergies. Ecol. Lett. 11, 1278–1286. ( 10.1111/j.1461-0248.2008.01243.x) [DOI] [PubMed] [Google Scholar]
- 28.Eklöf JS, Alsterberg C, Havenhand JN, Sundbäck K, Wood HL, Gamfeldt L. 2012. Experimental climate change weakens the insurance effect of biodiversity. Ecol. Lett. 15, 864–872. ( 10.1111/j.1461-0248.2012.01810.x) [DOI] [PubMed] [Google Scholar]
- 29.Sampaio E, Rodil IF, Vaz-Pinto F, Fernández A, Arenas F. 2017. Interaction strength between different grazers and macroalgae mediated by ocean acidification over warming gradients. Mar. Environ. Res. 125, 25–33. ( 10.1016/j.marenvres.2017.01.001) [DOI] [PubMed] [Google Scholar]
- 30.Morelissen B, Harley CDG. 2007. The effects of temperature on producers, consumers, and plant-herbivore interactions in an intertidal community. J. Exp. Mar. Biol. Ecol. 348, 162–173. ( 10.1016/j.jembe.2007.04.006) [DOI] [Google Scholar]
- 31.Kordas RL, Harley CDG, Connor MIO. 2011. Community ecology in a warming world: the influence of temperature on interspecific interactions in marine systems. J. Exp. Mar. Biol. Ecol. 400, 218–226. ( 10.1016/j.jembe.2011.02.029) [DOI] [Google Scholar]
- 32.Lemoine NP, Burkepile DE. 2012. Temperature-induced mismatches between consumption and metabolism reduce consumer fitness. Ecology 93, 2483–2489. ( 10.1890/12-0375.1) [DOI] [PubMed] [Google Scholar]
- 33.Gutow L, Petersen I, Bartl K, Huenerlage K. 2016. Marine meso-herbivore consumption scales faster with temperature than seaweed primary production. J. Exp. Mar. Biol. Ecol. 477, 80–85. ( 10.1016/j.jembe.2016.01.009) [DOI] [Google Scholar]
- 34.O'Connor MI. 2009. Warming strengthens an herbivore: plant interaction. Ecology 90, 388–398. ( 10.1890/08-0034.1) [DOI] [PubMed] [Google Scholar]
- 35.Goldenberg SU, Nagelkerken I, Ferreira CM, Ullah H, Connell SD. 2017. Boosted food web productivity through ocean acidification collapses under warming. Glob. Chang. Biol. 23, 4177–4184. ( 10.1111/gcb.13699) [DOI] [PubMed] [Google Scholar]
- 36.Vergés A, et al. 2014. The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B 281, 1–10. ( 10.1098/rspb.2014.0846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Provost EJ, Kelaher BP, Dworjanyn SA, Russell BD, Connell SD, Ghedini G, Gillanders BM, Figueira W, Coleman MA. 2017. Climate-driven disparities among ecological interactions threaten kelp forest persistence. Glob. Chang. Biol. 23, 353–361. ( 10.1111/gcb.13414) [DOI] [PubMed] [Google Scholar]
- 38.Farrell EG, Critchley AT, Aken ME. 1993. The intertidal algal flora of Isipingo Beach, Natal, South Africa, and its phycogeographical affinities. Helgoländer Meeresun. 47, 145–160. ( 10.1007/BF02430355) [DOI] [Google Scholar]
- 39.Silva PC, Basson PW, Moe RL. 1996. Catalogue of the benthic marine algae of the Indian ocean. Berkeley, CA: University of California Press. [Google Scholar]
- 40.Glasby TM, Gibson PT, West G, Davies P, Voerman S. 2015. Range and habitat associations of the native macroalga Caulerpa filiformis in New South. Mar. Freshw. Res. 66, 1018–1026. ( 10.1071/MF14282) [DOI] [Google Scholar]
- 41.Jiménez A, Pingo S, Alfaro-Shigueto J, Mangel JC, Hooker Y. 2017. Feeding ecology of the green turtle Chelonia mydas in northern Peru. Lat. Am. J. Aquat. Res. 45, 585–596. ( 10.3856/vol45-issue3-fulltext-8) [DOI] [Google Scholar]
- 42.May V. 1976. Changing dominance of an algal species (Caulerpa filiformis). Telopea 1, 136–138. ( 10.7751/telopea19763204) [DOI] [Google Scholar]
- 43.Voerman E, Glasby TM, Gladstone W, Gribben PE. 2017. Habitat associations of an expanding native alga. Mar. Environ. Res. 131, 205–214. ( 10.1016/j.marenvres.2017.09.019) [DOI] [PubMed] [Google Scholar]
- 44.Bulleri F, Balata D, Bertocci I, Tamburello L, Benedetti-Cecchi L. 2010. The seaweed Caulerpa racemosa on Mediterranean rocky reefs: from passenger to driver of ecological change. Ecology 91, 2205–2212. ( 10.1890/09-1857.1) [DOI] [PubMed] [Google Scholar]
- 45.Gribben PE, Wright JT, O'Connor WA, Doblin MA, Eyre B, Steinberg PD. 2009. Reduced performance of native infauna following recruitment to a habitat-forming invasive marine alga. Oecologia 158, 733–745. ( 10.1007/s00442-008-1181-0) [DOI] [PubMed] [Google Scholar]
- 46.Zhang D, Glasby TM, Ralph PJ, Gribben PE. 2014. Mechanisms influencing the spread of a native marine alga. PLoS ONE 9, 1–11. ( 10.1371/journal.pone.0094647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis AR, Benkendorff K, Ward DW. 2005. Responses of common SE Australian herbivores to three suspected invasive Caulerpa spp. Mar. Biol. 146, 859–868. ( 10.1007/s00227-004-1499-z) [DOI] [Google Scholar]
- 48.Cummings DO, Williamson JE. 2008. The role of herbivory and fouling on the invasive green alga Caulerpa filiformis in temperate Australian waters. Mar. Freshw. Res. 59, 279–290. ( 10.1071/MF06238) [DOI] [Google Scholar]
- 49.Carey MP, Sanderson BL, Barnas KA, Olden JD. 2012. Native invaders: challenges for science, management, policy, and society. Front. Ecol. Environ. 10, 373–381. ( 10.1890/110060) [DOI] [Google Scholar]
- 50.Valéry L, Fritz H, Lefeuvre J, Simberloff D. 2009. Invasive species can also be native. Trends Ecol. Evol. 24, 585 ( 10.1016/j.tree.2009.06.017) [DOI] [PubMed] [Google Scholar]
- 51.Valéry L, Fritz H, Lefeuvre J, Simberloff D. 2008. In search of a real definition of the biological invasion phenomenon itself. Biol. Invasions 10, 1345–1351. ( 10.1007/s10530-007-9209-7) [DOI] [Google Scholar]
- 52.Smoothey AF. 2013. Habitat-associations of turban snails on intertidal and subtidal rocky reefs. PLoS ONE 8, 1–9. ( 10.1371/journal.pone.0061257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Worthington DG, Fairweather PG. 1989. Shelter and food: interactions between Turbo undulatum (Archaeogastropoda: Turbinidae) and coralline algae on rocky seashores in New South Wales. J. Exp. Mar. Biol. Ecol. 129, 61–79. ( 10.1016/0022-0981(89)90063-4) [DOI] [Google Scholar]
- 54.Cooling K, Smith SD. 2015. Population dynamics of Turbo militaris (Gastropoda: Turbinidae) on rocky shores in a subtropical marine park: implications for management. Molluscan Res. 35, 173–181. ( 10.1080/13235818.2015.1052035) [DOI] [Google Scholar]
- 55.Foster GG, Hodgson AN. 1998. Consumption and apparent dry matter digestibility of six intertidal macroalgae by Turbo sarmaticus (Mollusca: Vetigastropoda: Turbinidae). Aquaculture 167, 211–227. ( 10.1016/S0044-8486(98)00315-9) [DOI] [Google Scholar]
- 56.Ciais P, et al. 2013. The physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. In Climate change 2013: The physical science basis (eds Stocker T, et al.), pp. 465–570. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 57.Collins M, et al. 2013. Long-term climate change: projections, commitments and irreversibility. In Climate change 2013: The physical science basis (eds Stocker T, et al.), pp. 1029–1136. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 58.Coleman MA, Wernberg T. 2017. Forgotten underwater forests: the key role of fucoids on Australian temperate reefs. Ecol. Evol. 7, 8406–8418. ( 10.1002/ece3.3279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bennett S, Wernberg T, Connell SD, Hobday AJ, Johnson CR, Poloczanska ES. 2015. The ‘Great Southern Reef’: social, ecological and economic value of Australia's neglected kelp forests. Mar. Freshw. Res. 67, 47–56. ( 10.1071/MF15232) [DOI] [Google Scholar]
- 60.Kirkman H. 1981. The first year in the life history and the survival of the juvenile marine macrophyte Ecklonia radiata (Turn.) J. Agardh. J. Exp. Mar. Biol. Ecol. 55, 243–254 ( 10.1016/0022-0981(81)90115-5) [DOI] [Google Scholar]
- 61.Genty B, Briantais JM, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990, 87–92. ( 10.1016/S0304-4165(89)80016-9) [DOI] [Google Scholar]
- 62.van Kooten O, Snel JFH. 1990. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 25, 147–150. ( 10.1007/BF00033156) [DOI] [PubMed] [Google Scholar]
- 63.Thompson RC, Johnson LE, Hawkins SJ. 1997. A method for spatial and temporal assessment of gastropod grazing intensity in the field: the use of radula scrapes on wax surfaces. J. Exp. Mar. Biol. Ecol. 218, 63–76. ( 10.1016/S0022-0981(97)00068-3) [DOI] [Google Scholar]
- 64.Forrest RE, Chapman MG, Underwood AJ. 2001. Quantification of radular marks as a method for estimating grazing of intertidal gastropods on rocky shores. J. Exp. Mar. Biol. Ecol. 258, 155–171. ( 10.1016/S0022-0981(01)00212-X) [DOI] [PubMed] [Google Scholar]
- 65.Lotze HK, Worm B. 2000. Variable and complementary effects of herbivores on different life stages of bloom-forming macroalgae. Mar. Ecol. Prog. Ser. 200, 167–175. ( 10.3354/meps200167) [DOI] [Google Scholar]
- 66.Wernberg T, White M, Vanderklift MA. 2008. Population structure of turbinid gastropods on wave-exposed subtidal reefs: effects of density, body size and algae on grazing behaviour. Mar. Ecol. Prog. Ser. 362, 169–179. ( 10.3354/meps07416) [DOI] [Google Scholar]
- 67.Dworjanyn SA, Pirozzi I, Liu W. 2007. The effect of the addition of algae feeding stimulants to artificial diets for the sea urchin Tripneustes gratilla. Aquaculture 273, 624–633. ( 10.1016/j.aquaculture.2007.08.023) [DOI] [Google Scholar]
- 68.Prince JS, LeBlanc WG, Maciá S. 2004. Design and analysis of multiple choice feeding preference data. Oecologia 138, 1–4. ( 10.1007/s00442-003-1413-2) [DOI] [PubMed] [Google Scholar]
- 69.R Development Core Team. 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.r-project.org. [Google Scholar]
- 70.Wernberg T, et al. 2011. Impacts of climate change in a global hotspot for temperate marine biodiversity and ocean warming. J. Exp. Mar. Biol. Ecol. 400, 7–16. ( 10.1016/j.jembe.2011.02.021) [DOI] [Google Scholar]
- 71.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789. ( 10.1890/03-9000) [DOI] [Google Scholar]
- 72.Staehr PA, Wernberg T. 2009. Physiological responses of Ecklonia radiata (laminariales) to a latitudinal gradient in ocean temperature. J. Phycol. 45, 91–99. ( 10.1111/j.1529-8817.2008.00635.x) [DOI] [PubMed] [Google Scholar]
- 73.Wernberg T, Russell BD, Thomsen MS, Gurgel CFD, Bradshaw CJA, Poloczanska ES, Connell SD. 2011. Seaweed communities in retreat from ocean warming. Curr. Biol. 21, 1828–1832. ( 10.1016/j.cub.2011.09.028) [DOI] [PubMed] [Google Scholar]
- 74.Brook BW, Sodhi NS, Bradshaw CJA. 2008. Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460. ( 10.1016/j.tree.2008.03.011) [DOI] [PubMed] [Google Scholar]
- 75.Walther G-R, et al. 2009. Alien species in a warmer world: risks and opportunities. Trends Ecol. Evol. 24, 686–693. ( 10.1016/j.tree.2009.06.008) [DOI] [PubMed] [Google Scholar]
- 76.Lanham BS, Gribben PE, Poore AGB. 2015. Beyond the border: effects of an expanding algal habitat on the fauna of neighbouring habitats. Mar. Environ. Res. 106, 10–18. ( 10.1016/j.marenvres.2015.02.006) [DOI] [PubMed] [Google Scholar]
- 77.Steneck RS, Johnson CR. 2014. Kelp forests: dynamic patterns, processes, and feed-backs. In Marine community ecology and conservation (eds Bertness M, Bruno J, Silliman B, Stachowicz J), pp. 315–336. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 78.Martínez B, et al. 2018. Distribution models predict large contractions in habitat-forming seaweeds in response to ocean warming. Divers. Distrib. 24, 1350–1366. ( 10.1111/ddi.12767) [DOI] [Google Scholar]
- 79.Wernberg T, Coleman MA, Bennett S, Thomsen MS, Tuya F, Kelaher BP. 2018. Genetic diversity and kelp forest vulnerability to climatic stress. Sci. Rep. 8, 1–8. ( 10.1038/s41598-018-20009-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ. 2002. Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ. Conserv. 29, 436–459. ( 10.1017/S0376892902000322) [DOI] [Google Scholar]
- 81.Raybaud V, Beaugrand G, Goberville E, Delebecq G, Destombe C, Valero M, Davoult D, Morin P, Gevaert F. 2013. Decline in kelp in West Europe and climate. PLoS ONE 8, 1–10. ( 10.1371/journal.pone.0066044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tegner MJ, Dayton PK, Edwards PB, Riser KL. 1996. Is there evidence for long-term climatic change in southern California kelp forests? Calif. Coop. Ocean. Fish. Investig. Rep. 37, 111–126. [Google Scholar]
- 83.Miranda R, Coleman M, Tagliafico A, Rangel M, Mamo L, Barros F, Kelaher B. 2019. Data from: Invasion-mediated effects on marine trophic interactions in a changing climate: positive feedbacks favour kelp persistence Dryad Digital Repository. ( 10.5061/dryad.761js4b) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Miranda R, Coleman M, Tagliafico A, Rangel M, Mamo L, Barros F, Kelaher B. 2019. Data from: Invasion-mediated effects on marine trophic interactions in a changing climate: positive feedbacks favour kelp persistence Dryad Digital Repository. ( 10.5061/dryad.761js4b) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.761js4b [83].


