Abstract
With climate change, the effect of global warming on snow cover is expected to cause range expansion and enhance habitat suitability for species at their northern distribution limits. However, how this depends on landscape topography and sex in size-dimorphic species remains uncertain, and is further complicated for migratory animals following climate-driven seasonal resource fluctuations across vast landscapes. Using 11 years of data from a partially migratory ungulate at their northern distribution ranges, the red deer (Cervus elaphus), we predicted sex-specific summer and winter habitat suitability in diverse landscapes under medium and severe global warming. We found large increases in future winter habitat suitability, resulting in expansion of winter ranges as currently unsuitable habitat became suitable. Even moderate warming decreased snow cover substantially, with no suitability difference between warming scenarios. Winter ranges will hence not expand linearly with warming, even for species at their northern distribution limits. Although less pronounced than in winter, summer ranges also expanded and more so under severe warming. Summer habitat suitability was positively correlated with landscape topography and ranges expanded more for females than males. Our study highlights the complexity of predicting future habitat suitability for conservation and management of size-dimorphic, migratory species under global warming.
Keywords: climate change, IPCC, home range, landscape composition, migration, species distribution models
1. Introduction
Climate change is a substantial threat to biodiversity and ecosystems worldwide [1]. Increasing temperatures are affecting a wide range of taxa, leading to phenological mismatch across trophic levels [2], and shifting, contracting or expanding distribution ranges [3–5]. Climate change is particularly topical for migratory species [6,7], who follow seasonal resource fluctuations in time and space [8]. These resources are highly affected by climate, making management and conservation of migratory species increasingly challenging [6]. In seasonal environments, animals migrate between separate seasonal ranges, e.g. summer and winter ranges or wet and dry season ranges, and these ranges are typically situated at different latitudes and/or elevations. With the predicted increasing temperatures and lack of snow cover in the decades to come [9], it is of particular interest to derive predictions of how migratory species will respond to changes in their seasonal ranges.
A widespread method to make predictions about future habitat availability for different species under climate change is species distribution models (SDMs) and estimation of habitat suitability maps [10]. SDMs have been used to predict future ranges of a variety of organisms such as plants, amphibians, reptiles, birds and mammals (e.g. [11,12]), including non-migratory ungulates such as Svalbard reindeer (Rangifer tarandus platyrhynchus [13]) and mountain goat (Oreamnos americanus [14]). However, this becomes more complicated for migratory animals with two disparate seasonal ranges, as climatic factors interact with topography and determine the weather ultimately affecting migratory animals and their food resources [15]. The migration patterns of ungulates in temperate environments are largely driven by snow levels during autumn, forcing them to stay at low elevation/low latitude during winter. In spring, they expand their ranges and increase access to early forage maturation in summer ranges at higher elevation or latitude [8]. Thus, habitat suitability varies for migratory species depending on season, i.e. with snow levels determining the available habitat in winter, and temperature influencing the quality of summer habitats.
We aim to investigate how the current available habitat for a migratory species depends on season, sex and landscape topography, and to predict how winter and summer ranges will change under two alternative future emission scenarios (medium and severe), both in terms of size and habitat suitability. We use 11 years of data (2005–2015) from a total of 192 global positioning system (GPS) marked red deer (Cervus elaphus) in Norway, a partially migratory species [16]. This study system is particularly useful, as it spans over a long south–north and coast-inland gradient, and an extensive (approximately 800 m) elevation gradient including a range of different landscapes [8,15]. Snow levels are the limiting factor for winter range availability [17,18]. Global warming predicts increased temperatures, less precipitation falling as snow and a prolonged growing season, but the magnitude depends on landscape topography [9]. We therefore predict an overall range expansion and increase in red deer habitat suitability for both seasons and emission scenarios, with a more pronounced response in the severe scenario. We expect a larger range expansion and increase in habitat suitability with increasing elevation, as snow levels and temperature are limiting factors for current use of high elevation areas. Finally, polygynous species such as the red deer show sex-specific use of the elevation gradient, with males using higher elevation areas to a larger extent since they have no dependent offspring requiring protective forest habitat [19]. We therefore explore differences between the sexes in future habitat suitability and range size.
2. Material and methods
(a). Study area
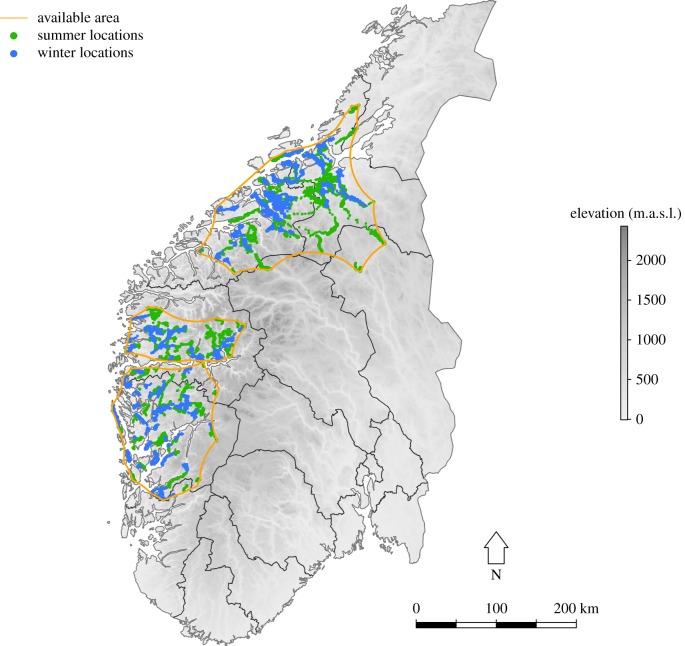
The study area comprises four counties (Sør-Trøndelag, Møre og Romsdal, Sogn og Fjordane and Hordaland) situated in the core area for red deer on the western part of southern Norway, where approximately 90% of red deer in Norway are harvested yearly (http://www.ssb.no; figure 1). The vegetation is mostly in the boreonemoral zone dominated by Scots pine (Pinus sylvestris) and deciduous forests, with increasing domination of birch (Betula sp.) northwards. Norway spruce (Picea abies) has been planted on a large scale. Temperature and precipitation generally decrease from coast to inland and from south to north, while snow depth and number of days with snow increases along the same gradients. The topography is characterized by diverse elevation gradients, with generally steeper terrain and higher elevations inland.
Figure 1.
Map of southern Norway showing the study area with available polygons (orange), red deer winter (blue) and summer (green) locations.
(b). Red deer global positioning system data
Adult red deer (females ≥ 1.5 and males ≥ 2.5 years old) were fitted with GPS collars (Followit, Sweden, and Vectronic, Germany) between 2005 and 2015 [16,17]. The individuals were darted on winter feeding grounds in winter (January–March [20]), following a standard procedure approved by the Norwegian Animal Research Authority. The GPS collars were pre-programmed to record a position every 1–2 h, and the individuals were followed between 1 and 3 years, depending on the GPS collar battery life, technical difficulties, mortalities and recaptures. GPS locations from the first 24 h after marking were removed, and the remaining raw location data were screened for outliers following Bjørneraas et al. ([21]; less than 0.01%). The rate of successful GPS locations obtained by the collars and the magnitude of the GPS location error vary with canopy cover of habitat and topography (e.g. lower success rate in areas with steep mountains [22]). In our study area, these sources of errors were quantified in using the same collar types as that in red deer [23]. Variable success rate may influence the models, and to avoid bias, we simulated missing GPS locations using a map of the study area with an associated probability of obtaining a GPS location in each pixel, based on the models built by Godvik et al. [23] and according to Frair et al. [22]. For details on the simulation of missing locations, see Godvik et al. [23] and Loe et al. [24].
We used the model-fitting approach developed by Bunnefeld et al. [25], and sophisticated by Bischof et al. [8] and Rivrud et al. [17], to determine red deer migration patterns. We retained individuals with clear migratory movement patterns. Migration is rapid and merely a transit between the separate summer and winter areas [8,17], and we therefore removed locations between migration onset and end for each individual. See the electronic supplementary material, table S1 and figure S1 for a summary of migration characteristics. Owing to the marking/drop-off schedule, many individuals were missing data for parts of the winter season. Individuals usually use the same ranges every year (A. Mysterud, EL Meisingset 2005–2015, unpublished data), and partial winter ranges were retained if overlapping with past or subsequent complete winter ranges. Seasonal ranges where individuals spent less than two weeks were removed (nobs = 38 ranges).
A total of 62 male and 127 female individual red deer covering 89 and 163 seasons, respectively, were available for summer analyses (nobs = 670 328), and 53 male and 110 female red deer covering 119 and 244 seasons, respectively, were available for winter analyses (nobs = 842 238).
(c). Environmental variables
We derived all environmental variables from maps prepared and rasterized using ArcGIS 10.3 (ESRI, USA), with a resolution of 100 × 100 m. Elevation metres above sea level (m.a.s.l.), slope (degrees) and aspect (radians) were derived from a digital elevation model. Aspect was cosine transformed to northness, a continuous variable ranging from 1 (north) to −1 (south). Layers with roads and coastline (scale 1 : 50 000) were used to calculate the shortest linear distance (m) to roads and the coast for each pixel in the study area. Digital land resource maps (scale 1 : 5000) were obtained from Norwegian Institute of Bioeconomy Research, with information on four functional habitat types relevant for our study species (agricultural areas, forests, mountains and marshland), in addition to non-relevant habitats (inhabited areas, glaciers, water bodies and uncharted areas). All locations sampled as used and available in non-relevant categories were removed before running models, to assure balanced data. As the use of pastures depends on the availability in our study area [23], we calculated the proportion of available pasture pixels to relevant habitat types within a circle for each location with a season-specific radius corresponding to the median sizes of 95% seasonal adaptive local convex hull (a-LoCoH) home ranges (summer; 799 m, winter; 732 m).
Daily 1 × 1 km grids of snow water equivalent (SWE; used as proxy of snow depth) and temperature covering the study area and period were provided by the Norwegian Water Resources and Energy Directorate and the Norwegian Meteorological Institute (NMI), respectively. These grids were made using statistical downscaling predicting SWE and temperature based on observed values of temperature and precipitation recorded by NMI weather stations in the area. Tests have shown close correlation with observed data, but some overestimation of SWE occurred during snow melt in spring [26]. The Norwegian Centre for Climate Services provided daily 1 × 1 km grids with future predictions of SWE and temperature covering Norway [27]. The 1 × 1 km predictions were based on regional 12 × 12 km HIRHAM simulations provided by the Danish Meteorological Institute and EURO-CORDEX (COordinated Regional climate Downscaling EXperiment [28]), which again were based on global predictions from the Earth system model (EC-EARTH [29]). Future predictions of red deer habitat suitability were made for two alternative emission pathways, based on the Intergovernmental Panel on Climate Change's Representative Concentration Pathway (RCP) 4.5 and 8.5 [9,30]. A medium emission scenario is represented by RCP4.5, where emission increases until approximately 2040, before a reduction and stabilization from approximately 2080. This scenario results in about 2.5°C increase in global temperature around year 2100, compared to 1850–1900. RCP8.5 represents a severe emission scenario, with emissions following the same trajectory as during the last decade. Global temperatures are expected to increase about 4°C in year 2100 relative to 1850–1900 in this scenario. Maps of monthly means for summer and winter used in the analyses can be seen in the electronic supplementary material, figure S2.
All extraction of environmental variables and coupling to the red deer locations was done in R.
(d). Estimation of use and availability
The use and availability were estimated on the home range and landscape scale, respectively, corresponding to second-order selection [31].
(i). Availability
We divided all red deer GPS locations into three regions defined by natural barriers in the landscape such as great fiords (nobs = 899 153, 321 343 and 446 578 from north to south; figure 1). As the study area is on the western coast of Norway, locations were bounded by open sea in the west and high elevation areas in the east, which resulted in the total GPS locations taking a banana-shape. Thus, regular kernel- or minimum convex polygon methods did not perform well. The available area in each region was therefore estimated by calculating the α-convex polygons, which is more flexible in shape, using the ‘alphahull’ package in R [32]. A range of α-values were tested searching for a value of α encompassing all locations, but minimizing large areas of inaccessible habitats at high elevations. A radius α = 70 000 m yielded the best estimate for all regions. Larger values included obvious inaccessible areas, while smaller values resulted in fragmented areas and excluded locations from the polygon. Available locations were sampled randomly within the regional polygons. We sampled an excess of locations to be able to remove locations located in non-habitat (e.g. water), and still keep a 1 : 1 relationship between used and available locations. After removal of these locations, we retained the same amount of available and used locations (n = 1000 individual−1 yr−1). We also sampled 1000 random dates for each individual within their individual monitoring period for extraction of SWE and temperature, which were coupled with the sampled GPS locations representing availability.
(ii). Use
Used areas were estimated by calculating the 95% a-LoCoH home ranges for each animal each season using the package ‘adehabitatHR’ in R [33]. This method also performs very well when dealing with linear home ranges or home ranges bounded by elements such as shorelines or steep mountains. An a-value larger than the two longest distances between individual locations should always give the 100% isopleth, but also keep a small radius of LoCoH elements in areas of high use [34]. We therefore used this a-value when possible, and increased to the sum of the three, four or five longest distances if the a-LoCoH did not converge. The mean number of locations used for estimation of individual summer home ranges was 2618 (s.d. = 1098) and 2211 (s.d. = 1686) for winter ranges. Used locations were sampled within the individual 95% home range polygons, and the number of locations sampled for each individual corresponded to the number of available locations sampled to ensure a balanced dataset. The individual dates sampled above were coupled to the GPS locations representing use for extraction of SWE and temperature.
(e). Statistical analyses
Resource selection functions were estimated using generalized linear mixed effects models (GLMMs) with a use-availability design, and random intercept for year to account for yearly sampling variation. The response is binomial, where used locations are coded 1 and available locations are coded 0. We ran four separate models, split by season (summer and winter) and sex (electronic supplementary material, table S2). The landscape variables elevation, slope, northness, distance to coast, distance to roads, proportion of pasture (arcsine-square root transformed), SWE (mm; winter models) and temperature (°C; summer models), were all checked for correlations before initial model building. Distance to roads and elevation were correlated with r > |0.6|, and thus, only elevation was retained as this was more relevant. We did not include the categorical variable habitat type, as mountainous habitat was highly correlated with elevation. All variables except proportion of pasture and northness were rescaled by centring on their mean and dividing by their standard deviation to avoid convergence issues. Generalized additive models were used to check for nonlinearity. Temperature/SWE were included in interaction with elevation, and we included the interaction between pasture availability and pasture use following Godvik et al. [23] and Loe et al. [24] to account for trade-offs in pasture use. GLMMs including all covariates and interactions listed above were estimated with the ‘glmer’ function in the ‘lme4’ package [35] in R. The candidate GLMMs were subjected to backwards fixed-effect model selection using likelihood ratio tests [36].
The most parsimonious models were extrapolated into habitat suitability maps by stacking the individual environmental maps into a multi-layered raster map, and predicting from the GLMMs the relative probability of detecting individual red deer in each pixel. Changes in future range size and habitat suitability were then quantified separately for each of the three regions. Three habitat suitability maps were estimated for each model; current (average temperature (July) and SWE (February) from 2005 to 2014), RCP4.5 and RCP8.5 year 2100 (both with average temperature (July) and SWE (February) from 2100). February and July averages were chosen as these months represent the most snow-rich (February) and warmest (July) months in Norway. All habitat suitability maps were made in R using the ‘raster’ package [37]. Finally, we calculated the niche overlap between present and future habitat suitability maps using Schoener's D [38].
(f). Effects of sample size and measures of model quality
To ensure that the sample sizes were appropriate, we investigated how sample size (number of individuals) affected model quality. For each of the four models, we divided the data into a training set and a test set as follows: individuals were sampled randomly (range 2–70) with replacement and the dataset was subset based on these individuals, representing the training data. We sampled with replacement as these individuals could represent other unsampled individuals with identical habitat selection strategies [24]. The rest of the dataset represented the test data. The models were then fitted with the training data, and model predictions were made based on the test data, from which we calculated the area under the receiver operating characteristics curve (AUC) and Cohen's κ to assess prediction accuracy [39]. The process was repeated 100 times for each sample size. Model quality of the four final models was assessed using k-fold cross-validation [40]. The dataset was randomly split into fivefolds, of which 80% were assigned as training data and 20% as test data. The model was fitted with the training data, and then evaluated on the test data by estimating the overall prediction accuracy using the ‘caret’ package in R [41]. The process was repeated 20 times, yielding 100 model runs for each of the four models.
3. Results
(a). Effects of sample size and measures of model quality
The mean prediction accuracy stabilized at a sample size of 15 individuals for winter models (both AUC and Cohen's κ), and for 25–30 individuals for summer models depending on the quality measure used (see the electronic supplementary material, figure S3). Overall prediction accuracy for the final models based on k-fold cross-validation was high, and winter models (mean ± s.d. = 0.79 ± 0.001 and 0.80 ± 0.0003 for males and females, respectively) performed better than summer models (mean ± s.d. = 0.71 ± 0.003 and 0.72 ± 0.002 for males and females, respectively).
(b). Seasonal patterns of current habitat selection
The final summer models for both sexes included elevation, slope, distance to coast (squared), northness (squared), temperature and the interaction between elevation and temperature, and pasture trade-off term were included in female summer models only (table 1a). The final winter models for both sexes included elevation, slope (squared), distance to coast, northness, pasture trade-off, SWE and the interaction between elevation and SWE (table 1b).
Table 1.
Parameter estimates from the final resource selection functions for both sexes during (a) summer and (b) winter, with year as random intercept. s.e., standard error. (Standard deviation for the random effects for summer was 0.088 (females) and 0.127 (males) and for winter 0.136 (females) and 0.072 (males).)
| variable | females |
males |
||||||
|---|---|---|---|---|---|---|---|---|
| estimate | s.e. | z | p-value | estimate | s.e. | z | p-value | |
| (a) summer | ||||||||
| intercept | −0.079 | 0.029 | −2.72 | 0.006 | −0.008 | 0.044 | −0.19 | 0.853 |
| elevation | −1.309 | 0.007 | −194.80 | <0.001 | −1.297 | 0.009 | −143.12 | <0.001 |
| slope | 0.603 | 0.005 | 128.14 | <0.001 | 0.541 | 0.006 | 85.16 | <0.001 |
| distance to coast | 0.378 | 0.009 | 41.13 | <0.001 | 0.666 | 0.012 | 55.58 | <0.001 |
| northness | −0.247 | 0.006 | −42.49 | <0.001 | −0.158 | 0.008 | −20.94 | <0.001 |
| temperature | 0.152 | 0.005 | 31.78 | <0.001 | −0.021 | 0.006 | −3.55 | <0.001 |
| distance to coast2 | −0.085 | 0.003 | −30.92 | <0.001 | −0.240 | 0.005 | −48.04 | <0.001 |
| northness2 | 0.231 | 0.012 | 19.85 | <0.001 | 0.328 | 0.016 | 21.07 | <0.001 |
| pasture availability × pasture use | −0.904 | 0.028 | −32.45 | <0.001 | ||||
| elevation × temperature | 0.275 | 0.006 | 47.20 | <0.001 | 0.142 | 0.007 | 20.85 | <0.001 |
| (b) winter | ||||||||
| intercept | −0.382 | 0.041 | −9.21 | <0.001 | −0.401 | 0.026 | −15.31 | <0.001 |
| elevation | −2.089 | 0.010 | −213.51 | <0.001 | −1.814 | 0.013 | −143.97 | <0.001 |
| slope | 0.596 | 0.005 | 112.14 | <0.001 | 0.530 | 0.008 | 69.16 | <0.001 |
| distance to coast | −0.766 | 0.010 | −80.62 | <0.001 | −0.934 | 0.014 | −65.72 | <0.001 |
| northness | −0.273 | 0.005 | −49.62 | <0.001 | −0.259 | 0.008 | −33.06 | <0.001 |
| snow water equivalent | −0.467 | 0.009 | −54.29 | <0.001 | −0.269 | 0.010 | −26.63 | <0.001 |
| slope2 | −0.172 | 0.003 | −55.94 | <0.001 | −0.132 | 0.004 | −30.87 | <0.001 |
| pasture availability × pasture use | 0.452 | 0.020 | 22.72 | <0.001 | 0.282 | 0.028 | 10.15 | <0.001 |
| elevation × snow water equivalent | −0.712 | 0.018 | −40.36 | <0.001 | −0.315 | 0.017 | −18.23 | <0.001 |
(i). Summer
During summer, habitat selection in the elevation gradient depended on temperature for both sexes, with increased selection of high elevation areas when temperatures increased (table 1a). In general, high temperatures resulted in females showing stronger selection of high elevation than males (table 1a). Selection for distance to coast and northness were both nonlinear (table 1a). Both sexes selected for intermediate distances from coast, with females using a larger range of distances than males, and both avoided areas close to or very far from the coastline. There was strong selection for southfacing aspect for both sexes, with decreasing selection towards northfacing aspects. Females showed stronger avoidance of northfacing aspects than males. Males and females both selected for steeper slopes and avoided flat terrain (table 1a).
(ii). Winter
Selection in the elevation gradient during winter depended on snow levels for both sexes. In general, red deer avoided high elevation areas, and avoidance increased with increasing snow depths (table 1b). The relationship was stronger for females than for males. Males and females both selected for areas closer to the coast and for southfacing aspects (table 1b). Selection of slopes was nonlinear for both sexes with selection for intermediate slopes and higher selection for steeper slopes for males than females (table 1b). Finally, females showed stronger selection for pastures than males (table 1b).
(c). Current habitat suitability
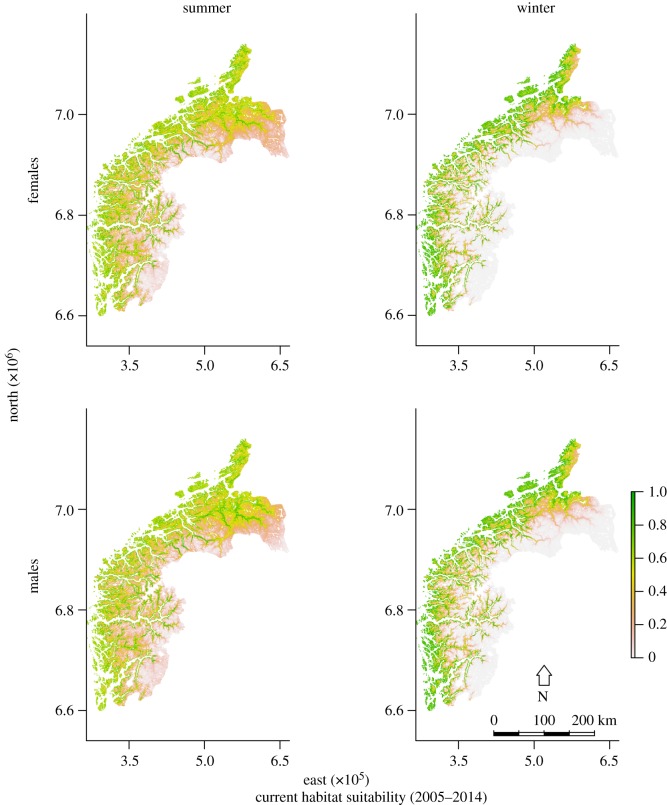
Habitat suitability maps during the study period showed larger sex differences in winter than in summer (figure 2). During winter, the most suitable habitat was constricted to coastal areas, and more so for females than males (figure 2). The differences also varied across the south–north gradient (figure 3). Males had consistently larger suitable ranges than females, and the range size difference varied as a function of topography and distance to coast (figures 2 and 3). Range size differed less between the sexes in flatter regions with higher summer temperatures and less snow (Sør-Trøndelag) than in steeper regions with lower summer temperatures (Sogn og Fjordane; figure 3; electronic supplementary material, table S3).
Figure 2.
Predicted habitat suitability for red deer in western Norway during 2005–2014 for females (a,b) and males (c,d) in summer (a,c) and winter (b,d).
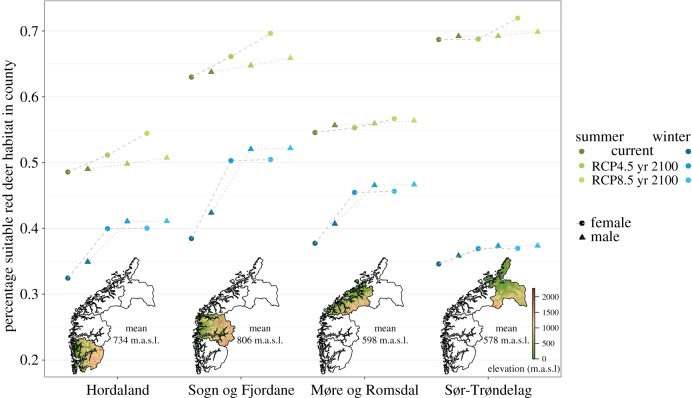
Figure 3.
Percentage of area consisting of suitable red deer habitat (habitat suitability threshold 0.2) in each county per season and sex based on resource selection functions. Predictions are made for the study period (current), and for year 2100 under alternative future emission scenarios medium (RCP4.5) and severe (RCP8.5). Maps on the x-axis show the elevation gradient in each county with elevation mean.
(d). Predictions of future ranges and habitat suitability
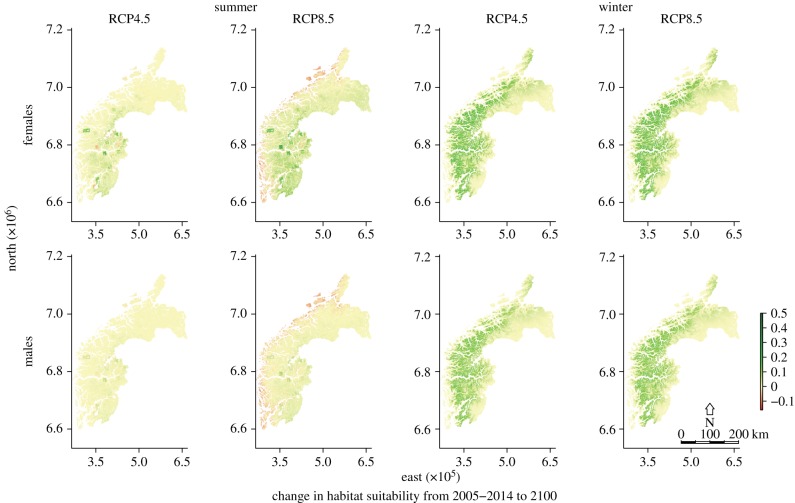
Habitat suitability increased strongly in winter under both scenarios, in particular in inland areas (figure 4). The predicted effect of summer warming was less pronounced but differed more between scenarios. In coastal areas, summer habitat suitability decreased for both sexes under severe emission (figure 4). The future increase in habitat suitability was mainly owing to poor or unsuitable habitat becoming suitable during both seasons (electronic supplementary material, figure S4). Present high-quality winter habitat improved further, while present high-quality summer habitat was unchanged or became less suitable (electronic supplementary material, figure S4). In order to investigate how changes in habitat suitability values affected range expansion, we need to set a threshold for suitable habitat. With a threshold value of 0.2, the future ranges expanded in both seasons and sexes, and the ranges expanded more for females than males (figure 3; electronic supplementary material, table S4). While females showed range expansion for all tested thresholds for habitat suitability (0.1–0.5), male summer ranges were predicted to contract at suitability thresholds higher than 0.5, implying that the currently best male summer habitat will deteriorate most (electronic supplementary material, table S4).
Figure 4.
Predicted change in habitat suitability from 2005–2014 to 2100 in western Norway for both sexes during summer (July) and winter (February). Predictions are made under the alternative future emission scenarios medium (RCP4.5) and severe (RCP8.5).
As predicted, future habitat suitability and range size was dependent on topography. Ranges expanded substantially during winter for both sexes in all counties, but more in the higher elevation counties (Hordaland and Sogn og Fjordane; figures 3 and 4). Contrary to our predictions, there were no differences in range size between the alternative emission scenarios medium and severe (figure 3), suggesting that even with medium emission, snow cover will be limited. Summer range size was more variable both with elevation, emission scenarios and sexes. There was a general future range expansion, with stronger increase under severe emission and in higher elevation counties, as predicted, and the range expansion was larger for females than males (figures 3 and 4). The niche overlap between present and future habitat suitability, assessed by Schoener's D, ranged from 0.887 to 0.994, and was higher in summer than in winter, and higher for males than females (see the electronic supplementary material, table S5).
4. Discussion
Northern latitudes are expected to experience the greatest climate change impacts [9], with potentially large consequences for migratory species following fluctuating resources across broad scales [6]. Studies showing range shifts, contractions or expansions under climate change are numerous, and represent many taxa (reviewed in [1]). However, few have done detailed investigations of how the response varies within species over different seasons and landscapes, and under alternative emission scenarios. We found three particularly important results in this context. First, there was no difference in range expansion or habitat suitability between emission scenarios during the winter season. This is probably owing to the snow cover, which limit the winter distribution ranges, disappearing already with medium emissions. Second, the magnitude of range expansion and change in habitat suitability depended on landscape topography. Range expansion was smaller in areas with overall lower elevation, and habitat suitability in coastal areas even decreased in summer indicating possible range shifts. Finally, warmer summers resulted in larger range expansion and higher habitat suitability for females than males. Males remained less responsive to climate change, and even showed a marginal future range contraction of highly suitable habitat. The seasonal effects interacting with sex and landscape topography in response to climate change highlight the complexity of estimating future ranges for migratory species.
Species at their northern distribution limits are expected to expand their ranges northwards and to higher elevations with increasing global warming owing to decrease in snow cover, but this expansion will naturally reach a plateau when all snow cover is lost. In the Alps, predictions show that an increase of 4°C in the mean temperature will reduce the duration of snow cover by 50% and 95% at 2000 and 1000 m.a.s.l., respectively [42]. In our case, the predicted decrease in snow cover is reflected in the large expansion of future winter ranges, but without large range size differences between emission scenarios, indicating nonlinear effects of global warming on snow cover and in turn habitat suitability. Range expansions reaching a plateau, or range contraction, is a commonly documented pattern in species dependent on high elevation habitat [1]. The magnitude of future winter range expansion differed with landscape composition, where the northernmost county (Sør-Trøndelag) showed a lower increase than the remaining counties. The future available red deer habitat under climate change is probably limited in this region, as there is less high elevation area to expand into as snow levels decrease. Hence, the global warming effects on habitat suitability are not necessarily stronger further north as responses depend more on topography.
Increasing summer temperatures can affect migratory ungulates both directly and indirectly. Large-bodied herbivores inhabiting northern environments have been shown to shift to higher elevations, select habitats with more cover but lower forage quality and reduce foraging rates when temperatures increase to avoid heat stress [43]. Higher temperatures cause increased lignification of plant cell walls owing to rapid growth, reducing forage quality and digestibility [44] and affecting the animals indirectly. Faster snow melt may cause more rapid green-up, thus reducing time with high-quality forage at early phenological stages [45]. Although these are all predicted negative effects of a warming climate, increased temperatures can also be positive, i.e. by making high elevation areas earlier covered by snow accessible. We found that changes in future summer ranges and habitat suitability were less consistent than during winter, and depended on sex, landscape topography and emission scenario. Female summer ranges expanded more with increasing emission, and more than male ranges. Habitat suitability depended on topography for both sexes, with an increase in inland areas and a decrease in coastal areas. The red deer is a sexually size-dimorphic species, and the sexes also spend most of the year segregated [19]. Males already use more high elevation habitat during summer than females as they are not limited by offspring at heel [19]. Consequently, males will have less new available habitat to expand into. Studies in birds found that male great bustards (Otis tarda), the most sexually size-dimorphic bird species, selected areas with more shade than females during the warmest periods of the day, and males also migrated further north at high summer temperatures [46,47]. Hence, both direct and indirect sex-specific responses to climate may be common for dimorphic species in many taxa and should be considered when predicting future suitable ranges.
A limitation of SDM approaches [13,14] is that habitat changes likely to occur over long time scales are not taken into account. Warming temperatures and lack of snow are expected to move the tree line and vegetation upwards [48,49], and create new suitable habitats with a time lag. The tree line ecotone is a major effect causing a nonlinear impact of habitat use along the elevation gradient. The alpine tree line ecotone determines whether the ecosystem carbon stocks will be mainly above ground (forests) or in ground (soil). Strict forest living species such as roe deer (Capreolus capreolus) showed no change in elevation distribution over the last decades in the Alps, while species more tolerant to open habitat, such as red deer and ibex (Capra ibex), are now found at higher elevations [50]. Complicating this, large herbivores may influence the advance of the tree line through grazing [49], and possibly affect their own future habitat negatively. Although many species have already shifted to higher elevation or latitudes in response to global warming [3], the movement in elevation even for species using open habitat will eventually be limited by soil depth and quality. The soil in the high alpine zone is of poor quality or absent [51] and developing soil of sufficient depth takes more time than the projected upwards movement of vegetation caused by rapid climate change [52]. Other consequences of future climate change, such as more unpredictable and extreme weather events [53] and indirect effects on habitat suitability caused by humans through, i.e. changes in infrastructure and habitat fragmentation, are also expected to influence the future habitat suitability of species. In addition, different species can be affected differently by climate change, which may alter the competitive interactions between species, and in turn affect species distribution [54]. These complex interactions are hard to incorporate precisely, but are also likely to play a relatively minor role compared to the overall effect of climate change.
5. Conclusion
Our models predict range expansion and increase in habitat suitability for migratory deer populations at their northern distribution limits, with interesting interactions with season, sex and landscape topography. Annual habitat suitability predictions are therefore not sufficiently detailed to foresee consequences of climate change for future conservation and management of migratory species. With males and females displaying different tolerance levels to snow and temperature in sexually size-dimorphic species [47,55] and global warming affecting the weather differently during summer and winter [9], incorporating these factors in SDMs is clearly necessary to improve future range predictions for these species. In addition, landscape topography is crucial both for determining the speed of climate change effects and to buffer effects of global warming, thus creating possible refugia where species can persist [56].
Supplementary Material
Acknowledgements
Øystein Brekkum has been valuable for handling the red deer database, and Jess Anderson at the Norwegian Water Resources and Energy Directorate has kindly provided snow water equivalent grids.
Ethics
All capture and handling of red deer have been approved by the Norwegian Animal Research Authority.
Data accessibility
The datasets supporting this article can be accessed from the Dryad Digital Repository: https://doi.org/10.5061/dryad.8p003sg [57].
Authors' contributions
A.M. and I.M.R. designed the study, and E.L.M. and A.M. organized the data collection. I.M.R. analysed the data with input from L.E.L., and I.M.R. wrote the first draft of the manuscript. All authors contributed substantially to the final version.
Competing interests
The authors declare no competing interests.
Funding
The study was funded by The Research Council of Norway, Grant/Award Number: DeerUnit, Pr. no. 230275.
References
- 1.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 2.Kudo G, Ida TY. 2013. Early onset of spring increases the phenological mismatch between plants and pollinators. Ecology 94, 2311–2320. ( 10.1890/12-2003.1) [DOI] [PubMed] [Google Scholar]
- 3.Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026. ( 10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 4.Zhu K, Woodall CW, Clark JS. 2012. Failure to migrate: lack of tree range expansion in response to climate change. Glob. Change Biol. 18, 1042–1052. ( 10.1111/j.1365-2486.2011.02571.x) [DOI] [Google Scholar]
- 5.Thomas CD, Bodsworth E, Wilson RJ, Simmons AD, Davies ZG, Musche M, Conradt L. 2001. Ecological and evolutionary processes at expanding range margins. Nature 411, 577 ( 10.1038/35079066) [DOI] [PubMed] [Google Scholar]
- 6.Robinson RA, et al. 2009. Travelling through a warming world: climate change and migratory species. Endanger. Species Res. 7, 87–99. ( 10.3354/esr00095) [DOI] [Google Scholar]
- 7.Middleton AD, Kauffman MJ, McWhirter DE, Cook JG, Cook RC, Nelson AA, Jimenez MD, Klaver RW. 2013. Animal migration amid shifting patterns of phenology and predation: lessons from a Yellowstone elk herd. Ecology 94, 1245–1256. ( 10.1890/11-2298.1) [DOI] [PubMed] [Google Scholar]
- 8.Bischof R, Loe LE, Meisingset EL, Zimmermann B, Van Moorter B, Mysterud A.. 2012. A migratory northern ungulate in the pursuit of spring: jumping or surfing the green wave? Am. Nat. 180, 407–424. ( 10.1086/667590) [DOI] [PubMed] [Google Scholar]
- 9.IPCC. 2013. Climate change 2013: the physical science basis. In Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change (eds Stocker TF, et al.), p. 1535 Cambridge, UK: IPCC. [Google Scholar]
- 10.Elith J, Leathwick JR. 2009. Species distribution models: ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 40, 677–697. ( 10.1146/annurev.ecolsys.110308.120159) [DOI] [Google Scholar]
- 11.Thomas CD, et al. 2004. Extinction risk from climate change. Nature 427, 145 ( 10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 12.McClean CJ, et al. 2005. African plant diversity and climate change. Ann. MO Bot. Gard. 92, 139–152. [Google Scholar]
- 13.Hansen BB, Aanes R, Herfindal I, Kohler J, Saether B-E. 2011. Climate, icing, and wild arctic reindeer: past relationships and future prospects. Ecology 92, 1917–1923. ( 10.1890/11-0095.1) [DOI] [PubMed] [Google Scholar]
- 14.White KS, Gregovich DP, Levi T. 2018. Projecting the future of an alpine ungulate under climate change scenarios. Glob. Change Biol. 24, 1136–1149. ( 10.1111/gcb.13919) [DOI] [PubMed] [Google Scholar]
- 15.Pettorelli N, Mysterud A, Yoccoz NG, Langvatn R, Stenseth NC. 2005. Importance of climatological downscaling and plant phenology for red deer in heterogeneous landscapes. Proc. R. Soc. B 272, 2357–2364. ( 10.1098/rspb.2005.3218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mysterud A, Loe LE, Zimmermann B, Bischof R, Veiberg V, Meisingset E. 2011. Partial migration in expanding red deer populations at northern latitudes: a role for density dependence? Oikos 120, 1817–1825. ( 10.1111/j.1600-0706.2011.19439.x) [DOI] [Google Scholar]
- 17.Rivrud IM, Bischof R, Meisingset EL, Zimmermann B, Loe LE, Mysterud A. 2016. Leave before it's too late: anthropogenic and environmental triggers of autumn migration in a hunted ungulate population. Ecology 97, 1058–1068. ( 10.1002/ecy.1596) [DOI] [PubMed] [Google Scholar]
- 18.Nelson ME. 1995. Winter range arrival and departure of white-tailed deer in northeastern Minnesota. Can. J. Zool. 73, 1069–1076. ( 10.1139/z95-127) [DOI] [Google Scholar]
- 19.Bonenfant C, Loe LE, Mysterud A, Langvatn R, Stenseth NC, Gaillard JM, Klein F. 2004. Multiple causes of sexual segregation in European red deer: enlightenments from varying breeding phenology at high and low latitude. Proc. R. Soc. Lond. B 271, 883–892. ( 10.1098/rspb.2003.2661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sente C, Meisingset EL, Evans AL, Wedul SJ, Zimmermann B, Arnemo JM. 2014. Reversible immobilization of free-ranging red deer (Cervus elaphus) with xylazine-tiletamine-zolazepam and atipamezole. J. Wildl Dis. 50, 359–363. ( 10.7589/2012-10-267) [DOI] [PubMed] [Google Scholar]
- 21.Bjørneraas K, Van Moorter B, Rolandsen CM, Herfindal I.. 2010. Screening global positioning system location data for errors using animal movement characteristics. J. Wildl. Manage. 74, 1361–1366. ( 10.1111/j.1937-2817.2010.tb01258.x) [DOI] [Google Scholar]
- 22.Frair JL, Nielsen SE, Merrill EH, Lele SR, Boyce MS, Munro RH.M., Stenhouse GB, Beyer HL. 2004. Removing GPS collar bias in habitat selection studies. J. Appl. Ecol. 41, 201–212. ( 10.1111/j.0021-8901.2004.00902.x) [DOI] [Google Scholar]
- 23.Godvik IM.R., Loe LE, Vik JO, Veiberg V, Langvatn R, Mysterud A. 2009. Temporal scales, trade-offs, and functional responses in red deer habitat selection. Ecology 90, 699–710. ( 10.1890/08-0576.1) [DOI] [PubMed] [Google Scholar]
- 24.Loe LE, Bonenfant C, Meisingset EL, Mysterud A. 2012. Effects of spatial scale and sample size in GPS-based species distribution models: are the best models trivial for red deer management? Eur. J. Wildl. Res. 58, 195–203. ( 10.1007/s10344-011-0563-5) [DOI] [Google Scholar]
- 25.Bunnefeld N, Boerger L, van Moorter B, Rolandsen CM, Dettki H, Solberg EJ, Ericsson G.. 2011. A model-driven approach to quantify migration patterns: individual, regional and yearly differences. J. Anim. Ecol. 80, 466–476. ( 10.1111/j.1365-2656.2010.01776.x) [DOI] [PubMed] [Google Scholar]
- 26.Saloranta T. 2012. Simulating snow maps for Norway: description and statistical evaluation of the seNorge snow model. Cryosphere 6, 1323–1337. ( 10.5194/tc-6-1323-2012) [DOI] [Google Scholar]
- 27.Wong WK, Haddeland I, Lawrence D, Beldring S. 2016. Gridded 1×1 km climate and hydrological projections for Norway. NVE report no. 59/2016.
- 28.Jacob D, et al. 2014. EURO-CORDEX: new high-resolution climate change projections for European impact research. Reg. Environ. Change 14, 563–578. ( 10.1007/s10113-013-0499-2) [DOI] [Google Scholar]
- 29.Hazeleger W, et al. 2012. EC-Earth V2. 2: description and validation of a new seamless earth system prediction model. Clim. Dyn. 39, 2611–2629. ( 10.1007/s00382-011-1228-5) [DOI] [Google Scholar]
- 30.Van Vuuren DP, et al. 2011. The representative concentration pathways: an overview. Clim. Change 109, 5 ( 10.1007/s10584-011-0148-z) [DOI] [Google Scholar]
- 31.Johnson DH. 1980. The comparison of usage and availability measurements for evaluating resource preference. Ecology 61, 65–71. ( 10.2307/1937156) [DOI] [Google Scholar]
- 32.Pateiro-Lopez B, Rodriguez-Casal A. 2015. alphahull: generalization of the convex hull of a sample of points in the plane. R package version 2.0.
- 33.Calenge C. 2006. The package ‘adehabitat’ for the R software: a tool for the analysis of space and habitat use by animals. Ecol. Modell. 197, 516–519. ( 10.1016/j.ecolmodel.2006.03.017) [DOI] [Google Scholar]
- 34.Getz WM, Fortmann-Roe S, Cross PC, Lyons AJ, Ryan SJ, Wilmers CC. 2007. LoCoH: nonparameteric kernel methods for constructing home ranges and utilization distributions. PLoS ONE 2, e207 ( 10.1371/journal.pone.0000207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: linear mixed-effects models using Eigen and S4. R package version 1.1-6. [Google Scholar]
- 36.Murtaugh PA. 2009. Performance of several variable-selection methods applied to real ecological data. Ecol. Lett. 12, 1061–1068. ( 10.1111/j.1461-0248.2009.01361.x) [DOI] [PubMed] [Google Scholar]
- 37.Hijmans RJ. 2018. raster: Geographic data analysis and modeling. R package version 2.8-4 edn.
- 38.Warren DL, Glor RE, Turelli M. 2008. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62, 2868–2883. ( 10.1111/j.1558-5646.2008.00482.x) [DOI] [PubMed] [Google Scholar]
- 39.Boyce MS, Vernier PR, Nielsen SE, Schmiegelow FKA. 2002. Evaluating resource selection functions. Ecol. Modell. 157, 281–300. ( 10.1016/S0304-3800(02)00200-4) [DOI] [Google Scholar]
- 40.Stone M. 1974. Cross-validatory choice and assessment of statistical predictions. J. R. Stat. Soc. Ser. B (Methodol.) 36, 111–133. ( 10.1111/j.2517-6161.1974.tb00994.x) [DOI] [Google Scholar]
- 41.Kuhn M. 2008. Building predictive models in R using the caret package. J. Stat. Softw. 28, 1–26. ( 10.18637/jss.v028.i05)27774042 [DOI] [Google Scholar]
- 42.Christensen JH, et al. 2007. Regional climate projections. In Climate change 2007: the physical science basis. Contributions of working group I to the fourth assessment report of the intergovernmental panel on climate change (eds Tignor M, Miller HL), pp. 847–940. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 43.Aublet JF, Festa-Bianchet M, Bergero D, Bassano B. 2009. Temperature constraints on foraging behaviour of male Alpine ibex (Capra ibex) in summer. Oecologia 159, 237–247. ( 10.1007/s00442-008-1198-4) [DOI] [PubMed] [Google Scholar]
- 44.Lenart EA, Bowyer RT, Hoef JV, Ruess RW. 2002. Climate change and caribou: effects of summer weather on forage. Can. J. Zool. 80, 664–678. ( 10.1139/z02-034) [DOI] [Google Scholar]
- 45.Pettorelli N, Pelletier F, von Hardenberg A, Festa-Bianchet M, Cote SD.. 2007. Early onset of vegetation growth vs. rapid green-up: impacts on juvenile mountain ungulates. Ecology 88, 381–390. ( 10.1890/06-0875) [DOI] [PubMed] [Google Scholar]
- 46.Alonso JC, Palacín C, Alonso JA, Martín CA. 2009. Post-breeding migration in male great bustards: low tolerance of the heaviest Palaearctic bird to summer heat. Behav. Ecol. Sociobiol. 63, 1705–1715. ( 10.1007/s00265-009-0783-9) [DOI] [Google Scholar]
- 47.Alonso JC, Salgado I, Palacín C. 2015. Thermal tolerance may cause sexual segregation in sexually dimorphic species living in hot environments. Behav. Ecol. 27, 717–724. ( 10.1093/beheco/arv211) [DOI] [Google Scholar]
- 48.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37 ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 49.Speed JD, Martinsen V, Mysterud A, Mulder J, Holand Ø, Austrheim G. 2014. Long-term increase in aboveground carbon stocks following exclusion of grazers and forest establishment in an alpine ecosystem. Ecosystems 17, 1138–1150. ( 10.1007/s10021-014-9784-2) [DOI] [Google Scholar]
- 50.Büntgen U, Greuter L, Bollmann K, Jenny H, Liebhold A, Galván JD, Stenseth NC, Andrew C, Mysterud A. 2017. Elevational range shifts in four mountain ungulate species from the Swiss Alps. Ecosphere 8, e01761. [Google Scholar]
- 51.Jobbágy EG, Jackson RB. 2000. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 10, 423–436. ( 10.1890/1051-0761(2000)010[0423:TVDOSO]2.0.CO;2) [DOI] [Google Scholar]
- 52.Jumpponen A, Brown SP, Trappe JM, Cázares E, Strömmer R. 2012. Twenty years of research on fungal–plant interactions on Lyman Glacier forefront: lessons learned and questions yet unanswered. Fungal Ecol. 5, 430–442. ( 10.1016/j.funeco.2012.01.002) [DOI] [Google Scholar]
- 53.Field CB, Barros V, Stocker TF, Dahe Q. 2012. Managing the risks of extreme events and disasters to advance climate change adaptation: special report of the intergovernmental panel on climate change Cambridge, UK: Cambridge University Press. [Google Scholar]
- 54.Araújo MB, Luoto M. 2007. The importance of biotic interactions for modelling species distributions under climate change. Glob. Ecol. Biogeogr. 16, 743–753. ( 10.1111/j.1466-8238.2007.00359.x) [DOI] [Google Scholar]
- 55.Conradt L, Clutton-Brock TH, Guinness FE. 2000. Sex differences in weather sensitivity can cause habitat segregation: red deer as an example. Anim. Behav. 59, 1049–1060. ( 10.1006/anbe.2000.1409) [DOI] [PubMed] [Google Scholar]
- 56.Ashcroft MB. 2010. Identifying refugia from climate change. J. Biogeogr. 37, 1407–1413. [Google Scholar]
- 57.Rivrud IM, Meisingset E, Loe LE, Mysterud A. 2019. Data from: Future suitability of habitat in a migratory ungulate under climate change Dryad Digital Repository. ( 10.5061/dryad.8p003sg) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Rivrud IM, Meisingset E, Loe LE, Mysterud A. 2019. Data from: Future suitability of habitat in a migratory ungulate under climate change Dryad Digital Repository. ( 10.5061/dryad.8p003sg) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets supporting this article can be accessed from the Dryad Digital Repository: https://doi.org/10.5061/dryad.8p003sg [57].