Abstract
Maintaining sustainable populations in captivity without supplementation through wild-capture is a major challenge in conservation that zoos and aquaria are working towards. However, the capture of wild animals continues for many purposes where conservation is not the primary focus. Wild-capture hinders long-term conservation goals by reducing remaining wild populations, but the direct and long-term indirect consequences of wild-capture for captive population viability are rarely addressed using longitudinal data. We explored the implications of changes in wild-capture on population dynamics in captivity over 54 years using a multi-generational studbook of working Asian elephants (Elephas maximus) from Myanmar, the largest remaining captive elephant population. Here we show that population growth and birth rates declined between 1960 and 2014 with declines in wild-capture. Importantly, wild-caught females had reduced birth rates and a higher mortality risk. However, despite the disadvantages of wild-capture, the population may not be sustainable without it, with immediate declines owing to an unstable age-structure that may last for 50 years. Our results highlight the need to assess the long-term demographic consequences of wild-capture to ensure the sustainability of captive and wild populations as species are increasingly managed and conserved in altered or novel environments.
Keywords: individual-based model, population viability, demography, ex situ conservation, life history
1. Introduction
Captive management and conservation are considered to be important stop-gap measures in efforts to ensure that wild animal populations are sustainable [1,2]. Although ex situ conservation strategies have been implemented successfully (e.g. [3]), captively managed populations are often small, and fail to be representative of the species as a whole, genetically robust or self-sustaining [1]. Many studies have found that zoo populations are unsustainable [4,5]. An assessment of 87 mammalian zoo populations revealed that only half were breeding to replacement rate [5]. Although an increased effort is now being placed into maintaining sustainable captive populations through captive breeding and reproductive technology [4,6], captive populations in zoos and aquaria have long been supplemented through wild-capture [7]. However, capture from the wild may impose long-term demographic consequences for captive populations [8], and therefore its implications for population viability must be explored.
Importantly but often overlooked, animals are also removed from the wild and kept in partially free-ranging or semi-captive conditions for many reasons other than conservation [9,10], most notably as research animals or for economic purposes as working animals [11–13]. Large numbers of individuals may be captured from the wild and conservation is not the primary goal of many semi-captive populations, but conservation management must still be considered, particularly where International Union for Conservation of Nature protection is in place [14]. However, few systems enable the assessment of how variation in wild-capture rates influences demography and population viability in captivity. One such species is Asian elephants (Elephas maximus), which are endangered but have a captive population of over 16 000 individuals, up to a third of the total population, increasing the importance of captive management [15]. Asian elephants are slow reproducers, exceptionally long-lived (mean lifespan = 38.4 ± 11.6 years and age at first reproduction = 19.8 ± 5.7 years [16]) and have a matriarchal social structure that has a large impact on survival [17], making them sensitive to anthropogenic disturbance and slow to recover [18]. In the wild, although there have been global population estimates and some assessments that indicate large population declines [15,19,20], we have a poor understanding of population dynamics [21]. In captivity, many studies have emphasized that elephant populations managed in zoos are not self-sustaining [7,22,23], but this constitutes only a small number of individuals globally (approx. 1000 individuals; [24]). The vast majority of captive Asian elephants are partially free-ranging, semi-captive working animals in range countries, used primarily for timber logging, tourism and ceremonial purposes [12,24]. Traditionally, wild elephants were captured to supplement the working population, which has been monitored in countries such as Myanmar for over a century [12,25]. Although the majority of captive elephants are primarily managed for economic purposes, conservation measures for the working population have also been incorporated in to local action plans, e.g. in Myanmar [14]. The case study of working elephants, therefore, provides a unique opportunity to understand how wild-capture influences population-dynamics in captivity.
Here, we aim to assess how long-term variation in wild-capture has influenced population viability in the largest captive population of the long-lived Asian elephant. To address this issue, we use a detailed longitudinal studbook of government-owned female timber elephants (n = 3585) that were captive-born or wild-caught in Myanmar between 1960 and 2014. There has been substantial variation in wild-capture during this period; Aung [26] estimated that at least 2000 individuals were caught from the wild between 1970 and 1993. Furthermore, systematic wild-capture was formally banned in 1994 [27]. Thus, this unique dataset enables us to capture detailed variation in wild-capture and vital rates across several decades, which provides rare insight into the demographic challenges faced by vulnerable species in captivity as a result of capture from the wild. From these extensive demographic records, we address two key questions: (i) between 1960 and 2014, how much did wild-capture contribute to observed annual changes in the number of individuals in the population? and (ii) now that systematic wild-capture is no longer practised, and given observed variation in demographic rates, will the current population decline in the future? To address the first question, we captured historic trends in age-specific life-history traits in wild-caught and captive-born female elephants, and related observed changes in population size to wild-capture rates in each year from 1960 to 2014. For the second question, using age-specific demographic rates from years after capture was banned, we constructed individual-based, stochastic projection models to assess long-term population viability over 250 years. We explored population viability under model uncertainty of life-history rates, observed variation in the environment and demographic stochasticity. Finally, we performed sensitivity analyses of the projection models under different scenarios of changes to life-history rates, to provide targets for sustainable management in semi-captive elephants.
2. Methods
(a). Study population
The Union of Myanmar has the largest working population of Asian elephants, with more than 5000 individuals, and approximately 2700 are state-owned and used for timber extraction processes [24,25]. The timber elephant population is managed centrally by the state forestry commission, the Myanma Timber Enterprise (MTE), and keeping systems (including workload regulations) are consistent across Myanmar [12]. Although MTE elephants are held in captivity, we describe them as semi-captive: (i) they are free-roaming outside of working hours and in the three-month annual rest period and forage naturally without supplementation, (ii) there is no reproductive management of the population and individuals mate freely with captive or wild conspecifics, (iii) there is no human intervention with the weaning of calves, which are cared for by the mother until training at the age of five [12], and (iv) culling is not practised, and elephants only have access to basic veterinary care. Veterinarians diagnose disease and record deaths and their causes following broad post-mortem exams, increasing the reliability of mortality estimates [28]. Despite workload and work-related stress having the potential to influence life-history traits, population vital rates are more comparable to those of wild African elephants [22] and Asian elephants [19] than to those held in zoos [29]. Timber elephants have been monitored by the state for over a century, and the current studbook has been collated from individual elephant log-books and annual MTE reports. To our knowledge, the studbook covers most individuals in the working population between 1960 and 2000, but we had access to approximately 13% less demographic records between 2001 and 2014. The final studbook was a female-only dataset (n = 3585, wild-caught = 1215) with individuals from 11 out of the 14 regional divisions (or states) of Myanmar, including Ayeyarwady, Bago, Chin, Kachin, Magway, Mandalay, Rakhine, Sagaing, Shan, Tanintharyi, Yangon and Unknown regions (for data selection details, see the electronic supplementary material, S1, S2 and figure S1). This female-only dataset was used in all analyses of life-history traits and population projections.
Wild individuals were systematically captured in Myanmar until 1994 to supplement the working population, after which they were protected [12,27]. However, individuals are still taken from the wild into captivity in instances of human-elephant conflict, but this occurs at much lower levels than historically [12]. For wild-caught individuals, specific birth date is unknown, and therefore age is estimated at the time of capture using shoulder height and comparison of body condition with elephants of known age [12]. In addition, the extent of pigmentation on the face (including trunk and temporal areas), folding of the upper ear, tail hair and wrinkliness of the skin are used to estimate age in wild-caught individuals [8]. The exact error in age-estimation for wild-caught individuals is unknown, but thought to be within a couple of years for individuals that continue to grow (up to approx. 25 years old; [30]), which constitutes the majority (72%) of those captured [8]. Using records of wild-caught females, we included a measure of wild-capture, which broadly assessed the number of individuals captured in each year. However, this does not necessarily include all individuals captured for two reasons. First, an estimated 5–30% of individuals die during capture [31], and the studbook only includes individuals remaining in the working population [8]. Second, we only included wild-caught females caught before an estimated age of 25, when their age-estimation is likely to be most accurate. We have no estimate on the level of poaching in the wild population, and to our knowledge, only very few individuals in the captive population are removed after they were born/captured. We restricted the studbook to a female-only dataset because we could not reliably estimate paternity and thus reproductive rates for male elephants from demographic records. There are differences in life-history traits between male and female elephants [8], and this is a limitation of the current studbook, but we could not include the dynamics of males in this study. However, a female-only design was appropriate for the current study because reproduction was not limited by the number/frequency of males, with a mean sex ratio of 1.34 across the study period (females : males, range = 1.23–1.45; electronic supplementary material, figure S6) and 50.54% of births to male calves. Females also mate with both wild and captive bulls [12]. Thus, population growth and decline can be assessed reliably using the dynamics of females.
(b). Long-term trends in the age-specific vital rates of wild-caught and captive-born females
Mortality and birth events within the studbook were used to quantify population vital rates through time for individuals of different birth origins, to parameterize population projection models. Age-specific rates of mortality and birth were estimated from the raw data using a generalized additive mixed modelling framework, run using the gam function in the R package mgcv [32,33]. The raw data were smoothed using an additive modelling approach because there was a large variation in the density of life-history data spatio-temporally and across ages. Thus, raw age-specific data in a given year may not be representative of general population-level trends of life history. An additive modelling approach also enables us to flexibly capture nonlinear trends in vital rates across an individual's lifespan and through time. All analyses were carried out in R [33].
For every year of a female's life from birth/capture (or any years of a female's life after 1960 if entering before 1960) to death/censoring, we coded the mortality and birth events of each individual as binary response variables (fitted with binomial error structures and a logit link function), where a 1 indicated an event (death or birth) in a given observation year. Individuals exited the analysis at death or at their last known age alive (censor date). The time-series dataset contained 66 528 (wild-caught = 30 287) year-age observations from the 3585 females. We then modelled the probability of death and birth separately as functions of age (numeric integer), observation year (numeric integer, years from 1960 to 2014), and birth origin (binary factor, captive-born versus wild-caught). Using model selection, we explored the predictive performance of 18 models, which incorporated age as a linear predictor or smoothing term, and observation year as a linear term, factor (decade or half-decade), smoothing term and random effect smoothing term. We also explored interactions between age, observation year and birth origin, included as thin plate regression spline smoothers for each birth origin, or as tensor product interaction smoothing terms [34,35]. Models were selected based on the Akaike information criterion (AIC) [36,37] (for full details of model selection see the electronic supplementary material, S2 and table S1).
We assessed the distributional assumptions of the best models by testing the under/overdispersion of scaled model residuals. Scaled model residuals were calculated from the DHARMa package of R, which uses a simulation-based approach to create readily interpretable scaled residuals for mixed effects models [38]. We tested for under/overdispersion and uniformity in simulated residuals using 1000 simulations (electronic supplementary material, figure S2). Then, we quantified the uncertainty in birth and mortality rate predictions from the best models. This enabled us to assess how much parameter uncertainty influenced variation in population size in future projections. Parameter uncertainty was quantified using posterior simulation of the best model, with 1000 replicates of model coefficients from the posterior mean and covariance matrix of the model. Posterior simulation was selected ahead of other bootstrapping techniques as it prevented the need to re-fit models, which would risk under-smoothing.
(c). How was past population growth influenced by wild-capture?
To explore how past trends in population growth were influenced by wild-capture, we calculated realized changes in the number of females from demographic data. For each year between 1960 and 2014, we calculated the number of females alive and the realized annual growth rate was calculated as , where N is the number of individuals in year t. Population changes from 2000 to 2001 were ignored because there was a decrease in the number of demographic records available to us between 2000 and 2001. We partitioned out population change effects owing to wild-capture and to annual vital rates alone by subtracting the observed annual wild-capture rate from the change in the number of individuals and re-calculating the realized annual population growth rate. We tested the difference in population growth rate with and without wild-capture when capture was still practised systematically (before 1995) using a linear model, with realized annual growth rate as the response variable and both year (numeric integer) and capture presence (binary factor) as predictor terms.
(d). Population projection models for a future without wild-capture
To assess the future viability of the timber elephant population, we built female-only, stochastic individual-based projection models using predicted age-specific birth and mortality rates for years after systematic wild-capture was banned (1995–2014) (more details in the electronic supplementary material, S3, figures S9 and S10; [39]). We opted to use an individual-based modelling framework to incorporate demographic stochasticity. All projection models were run on predicted values from the Kachin regional division; Kachin had a large number of life-history records, while having average predicted vital rates most consistent with the overall mean vital rates across all divisions. We did not incorporate density dependence in projection models, as we found that population size did not improve model performance (electronic supplementary material, table S1). Finally, we removed individuals over the age of 70 in each year of each simulation (i.e. mortality of 1 at age 70), as there was a large variation in life-history parameters at these ages and very few individuals. For each year in all projections, birth and death events were randomly sampled from a Bernoulli distribution according to age-specific probabilities from the best models. For all projections, we assumed that all births were to females.
We first constructed a projection model for the average vital rates across observation years in this period (1995–2014), without incorporating parameter uncertainty or environmental stochasticity (electronic supplementary material, S3). Thus, the first model was intended to explore the average long-term dynamics of the population with demographic stochasticity alone. The projection began with the age-structure present in 2014 (n = 1369; electronic supplementary material, figure S10). Over 500 iterations, we projected 250 years into the future, which was selected to capture long-term trends over 10–12.5 generations in the future (generation time 20–25 years from [40]). This ensured that we captured stable long-term dynamics based on the average vital rates between 1995 and 2014.
We then performed a hierarchical population viability analysis under three levels of uncertainty; (i) parameter uncertainty from the best model, (ii) environmental stochasticity (variation across years 1995–2014) and (iii) demographic stochasticity (electronic supplementary material, figure S11). (i) Parameter uncertainty was incorporated using posterior simulation of the best birth and mortality models, from which we calculated 200 sets of predicted values. Each set of predicted values included interannual (environmental) variation with observation year included as both a smoothing term and random effect (electronic supplementary material, table S1). (ii) Environmental stochasticity was incorporated by resampling both the random effect and smoothing term of observation year from the best models. We randomly sampled years for both the smoothing term and random effect term, and adjusted birth and mortality rates together according to the sampled years. We sampled 10 sets of years for each of the 200 sets of predicted values generated through posterior simulation. (iii) Demographic stochasticity was incorporated by repeating each set of years 10 times. The total number of simulations when assessing population dynamics over a 50 year period with different levels of uncertainty was 20 000. We then projected 50 years into the future from the starting population size and age-structure in 2014 (n = 1369). Finally, we investigated the relative importance of the three different levels of uncertainty on population size in the population projection. We used nested hierarchical mixed effects models for each year in the projection, implemented in the lme4 package [41], to partition the variance in ln population size attributable to demographic stochasticity within environmental stochasticity within parameter uncertainty (electronic supplementary material, figure S11).
(e). Identifying demographic targets for population management
To assess how age-specific rates influence population growth to identify demographic targets for population management, we performed numeric sensitivity analyses on the average long-term dynamics of the population excluding environmental stochasticity or parameter uncertainty. We first split age-specific demographic parameters of captive-born females into four main stages for life-history: juvenile (0–4 years of age before weaning), pre-reproductive (5–12 years old), adolescent (13–20 years old), reproductive adult (21–44 years old), senescent adult (45–70 years old). Life-history stages were selected based on previous findings of life-history patterns in timber elephants and raw age-specific data [42,43] (electronic supplementary material, figure S7). Then, for each life-history stage, we increased birth rates by 10% or decreased mortality rates by 10%, perturbing birth and mortality separately. We selected 10% because it represented a realistic potential change in management for a given life-history stage, laying beneath the variation in life-history rates that was observed in the raw data between 1960 and 2014 (s.d. 19% and 14% for total birth and death rates, respectively). To assess population viability, we performed population projections for each scenario, performing 1000 simulations over 200 years, randomly assigning births and deaths to each individual in each year, with birth and death probabilities adjusted for each scenario. Finally, we compared population dynamics in each scenario to the baseline under current conditions, to identify targets for management.
3. Results
The average annual birth rate was 3.1% (range = 1.2–5.4%) and the average annual mortality rate was 2.1% (range = 0.3–4.2%) for female elephants (n = 3585) between 1960 and 2014 (electronic supplementary material, figure S5a). Our measure of wild-capture for females entering the final studbook occurred at an average rate of 20.6 individuals per year, with the maximum number of individuals captured in a single year being 117 in 1972 (electronic supplementary material, figure S5b). Capture rates between 1965 and 1975 were higher than other years within the study period, with 56% of all captures taking place within this 10 year period (electronic supplementary material, figure S5b).
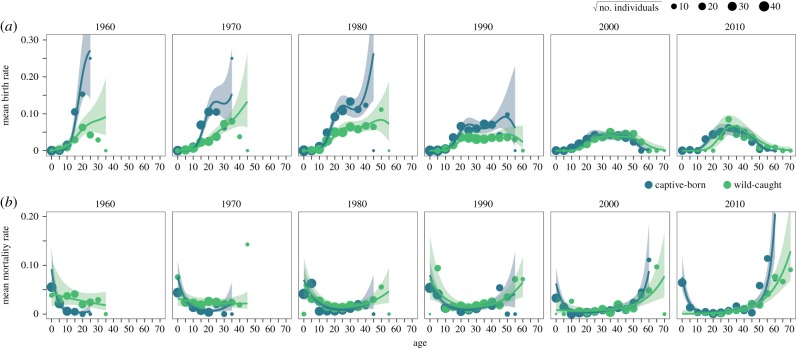
Birth rates varied across lifespan and years for both captive-born and wild-caught females (figure 1a). For captive-born females, birth rates increased at the age of 12 up to an average initial peak of approximately 10% between the ages of 20 and 22, after which generally there was a decline later in life (figure 1a; electronic supplementary material, figure S7a). In earlier years before 1970, there were fewer old-aged individuals and the population was smaller, and so predicted birth rates increased later into life, but on average birth rates declined beyond the age of 44 (figure 1a; electronic supplementary material, figure S7a). Birth rates were consistently lower on average in wild-caught females across ages, increasing more slowly from age 12 and reaching a maximum annual predicted birth rate of only 7%. However, at older ages, wild-caught females exhibited higher birth rates, but also declined after the age of 50 (electronic supplementary material, figure S7a). Overall, birth rates declined between 1960 and 2014, particularly for captive-born females (figure 1a; electronic supplementary material, figure S8a). The best model for birth rates included a tensor product interaction smoothing term between age, year and birth origin, and an additional term for annual variation with year as a random factor (electronic supplementary material, table S1). We did not find evidence for an effect of population size on birth rate as it did not improve predictive performance; the AIC difference between the best model and the model with population size was 0.42, but the more parsimonious model with fewer parameters was selected (electronic supplementary material, table S1 and figure S4a). There was no evidence of overdispersion or non-uniformity in the simulated residuals (electronic supplementary material, figure S2a). Furthermore, there was no observed covariance between simulated model residuals and explanatory variables (electronic supplementary material, figure S3a,c).
Figure 1.
Mean age-specific birth (a) and mortality (b) rates in wild-caught (green) and captive-born (blue) female timber elephants for each decade between 1960 and 2010. Points are mean age-specific vital rates for each 5-year age-class across all regional divisions in Myanmar for each decade, with the size depicting the square root of the sample size (range = 1–1815 individuals). Lines are the mean model predicted values from the best birth (a) and mortality (b) models across regional divisions, with 95% confidence intervals from posterior simulations. Model predictions between 1995 and 2014 were used to parameterize individual-based projections.
Mortality rates were high in young individuals, declining until the age of 10 and remaining low until 45, after which mortality rates rapidly increased into old age (figure 1b). Mortality rates were also higher in wild-caught females than captive-born females, but at extreme ages (greater than 50 years of age), there was some evidence that wild-caught females had reduced mortality owing to selective disappearance (figure 1b; electronic supplementary material, figure S7b; [8]). Predicted mortality risk at all ages also fluctuated across the study period for both captive-born and wild-caught females (figure 1b; electronic supplementary material, figure S8b). For mortality, the best model also included a tensor product interaction smoother between age, observation year and birth origin, with an additional random term of year. Again, we found no clear evidence of an effect of population size on mortality rate, with an AIC difference of 0.38 compared with the second-best explanatory model with more parameters (electronic supplementary material, table S1 and figure S4b). Furthermore, there was little evidence of non-uniformity, overdispersion or covariance with explanatory variables in the simulated model residuals (electronic supplementary material, figures S2b and S3b,d). For both birth and mortality models, the random effect of spatial division was accounted for in subsequent projections by using values from Kachin state, which was closest to the average birth and mortality values across divisions, with a large population size.
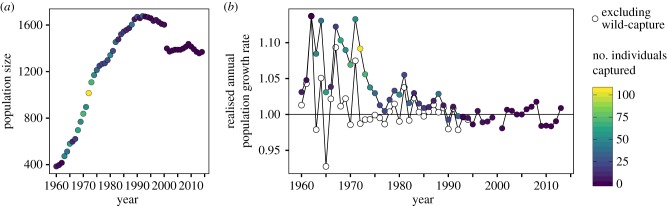
The number of individuals in the final female-only studbook dataset between 1960 and 2014 increased from 385 to 1369, with a maximum of 1677 individuals in 1992 (figure 2a). To investigate changes in population growth rate across the study period and to assess the implications of wild-capture for population growth, we calculated the observed annual population growth rate both with and without wild-capture from raw data. Realized annual growth rates were highly variable across observation years (figure 2b). Generally, growth rates declined between 1960 and 2014 (range = 0.93–1.14) (figure 2b) but remained above replacement rate (growth rate ≥ 1) before 1990 when capture was included. However, population growth rate was highly dependent on wild-capture, suggesting the population may not be sustainable, particularly as systematic wild-capture was banned in 1994. Growth rates excluding wild-capture before 1995 were 2.1% lower than those including wild-capture (F2,67 = 22.1, p < 0.001). Together, the historic changes in the female timber elephant population suggest that large population increases were accompanied by intensive wild-capture rates, and population growth rate has fluctuated around 1 beyond 1995, making the population vulnerable to population decline in the future.
Figure 2.
Historic trends in the female timber elephant population with wild-capture. (a) The number of female timber elephants in each year between 1960 and 2014. The decrease in the year 2001 is owing to a decrease in the number of demographic records. (b) Changes in the realized annual population growth rate, both including (coloured points) and excluding (open points) capture from the wild. Solid line indicates annual growth rate of 1, i.e. replacement rate. For both (a) and (b), the colour indicates the annual capture rate in each year.
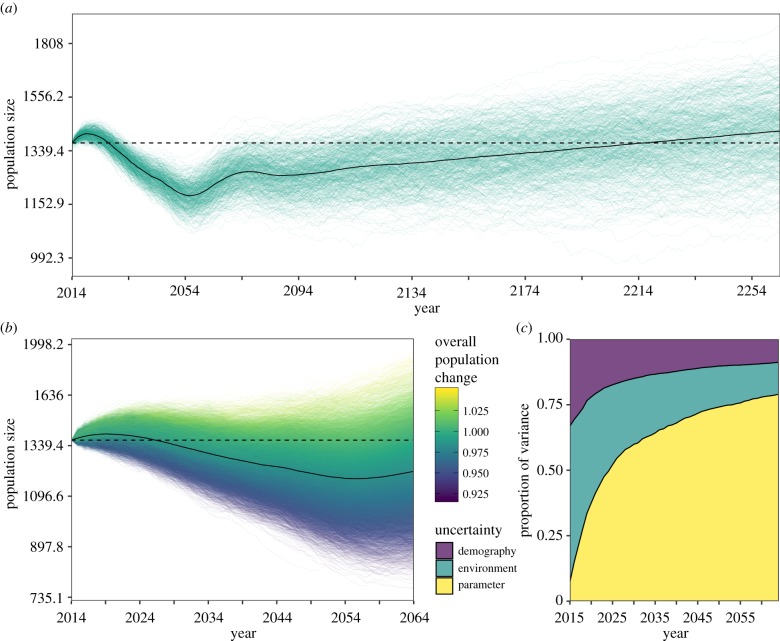
To assess the future outlook for timber elephants in a world excluding wild-capture, we performed individual-based, stochastic projection models of the population beginning with the starting age-structure in 2014. We first investigated long-term (250 years) dynamics over 500 simulations in a scenario excluding model parameter uncertainty or environmental stochasticity. Generally, as with historic population changes, the average change in the population was close to a population growth rate of 1, indicating little change over 250 years (figure 3a). However, the model projection had a long-lasting transient phase of fluctuation in the population of approximately 70 years, in which the population declined down to 1176 individuals in 2056. After this transient phase up to approximately 2080, the population reached a steady, but small stable annual growth rate of approximately 1.005 (figure 3a). Although population growth was predicted in the long term, the proximity of the growth rate to 1 indicates that the population is susceptible to decline given changes in the environment. As expected, the variation in population viability was far greater when environmental stochasticity and parameter uncertainty were included (figure 3b). Including uncertainty in the environment and parameter uncertainty, we again found an average population decline of approximately 150 individuals over 50 years. However, decomposition of the different sources of uncertainty revealed that although demographic and environmental stochasity are drivers of variation in population viability, model parameter uncertainty was the most important driver of observed population changes (figure 3c). After 50 years, parameter uncertainty explained approximately 75% of the variance in population size (figure 3c). This suggests that understanding long-term variation in demographic rates is particularly crucial in this long-lived species.
Figure 3.
Population projections for female timber elephants in a world without wild-capture. (a) Population projection over 250 years and 500 simulations representing the average dynamics of the population excluding model uncertainty in parameters and environmental stochasticity. Green lines represent the change in population size for each simulation, and the solid black line indicates the geometric mean. (b) Short-term changes (50 years) in the timber elephant population incorporating varying levels of uncertainty (parameter uncertainty and demographic/environmental stochasticity). Coloured lines indicate each simulation (20 000), and the colour denotes the overall population change in that simulation. Solid black line indicates the geometric mean of population size. For population projections, population size is on the natural log scale, and the dashed line indicates the starting population of 1369. (c) The proportion of variance in ln(population size) explained by uncertainty in model parameters (yellow), and with both environmental (green) and demographic (purple) stochasticity over 50 years for 20 000 simulations.
We investigated which age-specific demographic rates had the largest impact on population growth by performing population projections under scenarios with changes to demographic rates at key life-history stages and comparing them to the baseline scenario. We investigated the sensitivity of population viability to 10% changes in each life-history stage (increase for birth rates, decrease for mortality). The majority of changes to age-specific rates had relatively little effect on population viability relative to the baseline scenario (electronic supplementary material, figure S12). However, both a 10% increase to the birth rates of adult reproducers (21–44) and a 10% decrease in the mortality rates of juveniles (0–4) had a substantial influence on population viability and resulted in a more rapid population increase (figure 4). Population increases of 5% and 2% were observed under adult birth rate and juvenile mortality rate scenarios, respectively, compared to a 0.01% increase under the baseline scenario over the 200 year period. Notably, increases in birth rates at older ages (45–70) and in early reproducers (13–20) also had an influence on population growth (electronic supplementary material, figure S12).
Figure 4.
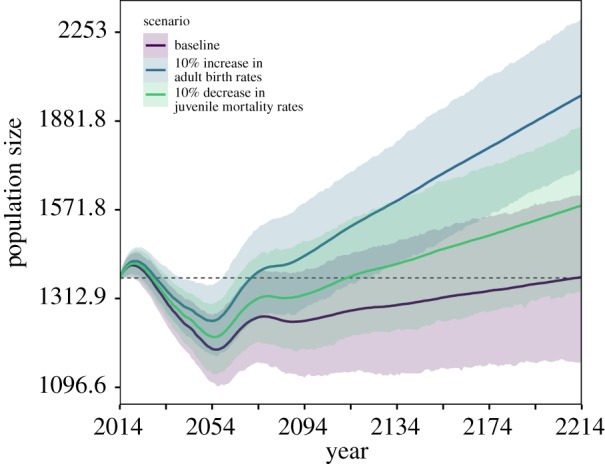
Increases to juvenile survival represent a realistic and meaningful target for conservation management. Individual-based, stochastic projections excluding parameter and environmental stochasticity over 200 years under three scenarios; baseline (average observed dynamics, purple), a 10% increase to adult birth rate (age 20–44, blue) and a 10% decrease in juvenile mortality (age 0–4, green). Solid lines are the population size on the natural log scale, with 95% confidence interval across 1000 simulations, dashed line indicates the starting population size of 1369 females in 2014.
4. Discussion
Our results challenge the prospect of maintaining viable populations of captive elephants without the capture of individuals from the wild. Historic trends in population dynamics using demographic data spanning 54 years revealed that population growth rate was highly dependent on wild-capture. Given this dependence on wild-capture and an accompanied decline in birth rates between 1960 and 2014, the outlook for captive elephants excluding wild-capture is uncertain. Long-term population projections predict immediate population declines, but long-term stable population growth rates that are close to replacement rate, suggesting that the working population is vulnerable to environmental disturbance. However, owing to an unstable age-structure, immediate transient population declines may last for approximately half a century, suggesting that management must be tailored to the slow life history of Asian elephants. Although population viability excluding wild-capture is uncertain, our results also suggest that there are long-term demographic consequences for individuals that are caught from the wild; wild-caught females have lower lifetime birth rates and higher death rates than captive-born females. Wild-capture reduces remnant wild populations, but also has a long-lasting demographic impact on the demography of the captive population, and we must focus on managing the demography of captive populations to prevent future declines.
Between 24% and 29% of the global Asian elephant population is held in captivity [15,40], of which Myanmar's timber elephant population may constitute as much as a third. Thus, although this working population is often overlooked as a unit of conservation, sustainable management is crucial for the viability of this endangered species. However, our study shows that for decades, this has not been achievable without the capture of wild individuals. Wild-capture in Myanmar has been detrimental for the wild population, which is important for both Asian elephants and their surrounding ecosystem [44]. Leimgruber et al. [20] postulated that capture rates of 100 individuals per year would result in the extinction of the wild population in under half a century. However, the exact dynamics of Myanmar's wild population in relation to changes in wild-capture rates is unknown. As well as decreasing the size of the wild population, we found evidence that wild-caught females have lower birth rates and survival, which is most likely a result of the stress of the capture process [8]. However, despite the lower performance of wild-caught females in captivity, there were large declines in captive population birth rates with declines in wild-capture. Furthermore, historic rates of wild-capture do not necessarily take into account capture-related mortality itself, and many more elephants may have actually been removed from the wild than are used in the timber industry [8]. For example, the estimated instant mortality rate during the elephant capturing process in Myanmar is high, varying between 5% and 30% depending on the capture method [26,31]. The ongoing wild-capture of elephants is not limited to supporting Myanmar's timber elephants (which now continues in cases of human-elephant conflict, but not systematically): capture continues worldwide for both legal and illegal purposes (e.g. [45,46]). Asian elephant populations currently held in Western zoos, safari parks, and circuses are not self-sustaining [22,23], and 60% were wild-caught and imported from range countries [47]. The reliance of captive Asian elephant populations on wild-capture is alarming, and management must be addressed to ensure the sustainability of this species without continued capture.
Although population viability in captivity is under threat, population extinction was not predicted in long-term population projections. A handful of studies have also aimed to assess the viability of semi-captive elephant populations (e.g. [20,48]). Both studies forecasted that extinction was highly likely. Importantly, however, both studies impose carrying capacities on working elephant populations, which limits population growth [20,48]. We did not find evidence for a correlation between realized population size and age-specific vital rates in this extensive demographic dataset spanning 54 years. Furthermore, the notion of density dependence in semi-captive populations is not trivial; individuals are not always subjected to habitat limitation or competition as with fully wild populations, but because of human management. Another key difference in the current study was the incorporation of temporal variation in age-specific vital rates that were estimated directly from the demographic studbook, rather than static age-specific rates. Historic annual population growth rates displayed a large variation between 1960 and 2014. Understanding temporal differences in demography and life history are therefore crucial for population dynamics. However, temporal differences in vital rates have been absent in previous projections in Asian elephants [20,22,48]. Previous work has suggested that the quality of demographic and life-history data needs to be addressed in viability analyses [49], but our results suggest that this may be accentuated in long-lived species, where many decades of data are needed to quantify vital rates. Slow intrinsic growth rates and life history in species such as elephants may exacerbate external pressures, resulting in further population declines [18]. Indeed, we observed transient population dynamics that last several decades in long-term projections, and previous work has found long-lasting mortality effects in working elephants [8]. This result is important for the conservation of long-lived species; an unstable age-structure can lead to long-lasting transient dynamics with more rapid population declines. However, these changes may occur on significant timescales, increasing the importance of long-term monitoring and conservation strategies that reflect the life history of target species.
Although our results suggest that captive elephants in Myanmar may not be sustainable without wild-capture, we are not suggesting that reinstating the capture of wild individuals is a potential solution, because it is clearly detrimental for the wild population [20]. Instead, we suggest that management should be focused on sustaining the current individuals in the captive population. Specifically, our results suggest that increased survival in juveniles may be an important driver of population growth in long-lived species, which are characterized by low annual reproductive rates. Although, as expected, birth rates in adult females had the biggest influence on population viability, increasing adult birth rates does not necessarily present a tractable target for population management, particularly as adult females are working animals. Targeting juvenile mortality, however, provides a clear and tractable target for population management in this captive population. Currently, juvenile elephants are tamed around the age of five in order to learn commands and begin light carrying work [12,25,50]. Elephants are removed from the mother at this stage to undergo training, and this stress may have a negative impact on survival [12]. Furthermore, mortality is highest in neonatal, pre-weaning elephants [51,52]. This phenomenon is common in other populations and in African elephants, particularly in captivity [29,53]. Further to previous findings, our results suggest that targeting the factors influencing juvenile mortality may have a disproportionately beneficial effect on population growth. This could be achieved by adjusting management to reduce stress during the taming process and for peak reproductive-aged females, and to target neonatal mortality.
Ex situ conservation is now common to prevent extinction in wildlife populations, but removal of individuals from the wild may be detrimental to both populations in situ, and those in captivity. With human-managed populations becoming increasingly common, there is a need for an increased understanding of how human intervention influences demography and life history.
Supplementary Material
Acknowledgements
We thank the Ministry of Natural Resources and Environment Conservation, the Government of the Union of Myanmar for giving us permission to work with the MTE. We also thank all the vets and officers involved in data collection as well as the Myanmar Timber Elephant Project members for help and support, and M. Lahdenperä, R. Cristofari and C. Lynsdale for useful comments on the manuscript. We would like to extend a special thank you to Khin Than Win, Thu Zar Thwin and Mumu Thein, for their tireless work on the project, which enabled this work to be carried out.
Data accessibility
The data and code supporting our results are archived in the Dryad Digital Repository: https://doi.org/10.5061/dryad.rj237db [54].
Authors' contributions
K.U.M. and W.H. collected the data. J.J., D.Z.C. and V.L. designed the study. J.J. carried out analysis with support from D.Z.C. and V.L. J.J. wrote the manuscript, with contributions from V.L. and D.Z.C. All authors approved the manuscript for publication.
Competing interests
We have no conflicts of interest.
Funding
This work was supported by European Research Council and the Natural Environment Research Council.
References
- 1.Redford KH, et al. 2011. What does it mean to successfully conserve a (vertebrate) species? Bioscience 61, 39–48. ( 10.1525/bio.2011.61.1.9) [DOI] [Google Scholar]
- 2.IUCN. 2016. The IUCN Red List of threatened species. Gland, Switzerland: IUCN.
- 3.Toone WD, Wallace MP. 1994. The extinction in the wild and reintroduction of the California condor (Gymnogyps californianus). In Creative conservation (ed. PJS Olney), pp. 411–419. Dordrecht, Netherlands: Springer. [Google Scholar]
- 4.Lacy RC. 2013. Achieving true sustainability of zoo populations. Zoo Biol. 32, 19–26. ( 10.1002/zoo.21029) [DOI] [PubMed] [Google Scholar]
- 5.Lees CM, Wilcken J. 2009. Sustaining the Ark: the challenges faced by zoos in maintaining viable populations. Int. Zoo Yearb. 43, 6–18. ( 10.1111/j.1748-1090.2008.00066.x) [DOI] [Google Scholar]
- 6.Saragusty J, et al. 2016. Rewinding the process of mammalian extinction. Zoo Biol. 35, 280–292. ( 10.1002/zoo.21284) [DOI] [PubMed] [Google Scholar]
- 7.Faust LJ, Thompson SD, Earnhardt JM. 2006. Is reversing the decline of Asian elephants in North American zoos possible? An individual-based modeling approach. Zoo Biol. 25, 201–218. ( 10.1002/zoo.20054) [DOI] [Google Scholar]
- 8.Lahdenperä M, Mar KU, Courtiol A, Lummaa V. 2018. Differences in age-specific mortality between wild-caught and captive-born Asian elephants. Nat. Commun. 9, 3544 ( 10.1038/s41467-018-06032-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dierenfeld ES, McCann CM. 1999. Nutrient composition of selected plant species consumed by semi free-ranging lion-tailed macaques (Macaca silenus) and ring-tailed Lemurs (Lemur catta) on St. Catherines Island, Georgia, U.S.A. Zoo Biol. 494, 481–494. () [DOI] [Google Scholar]
- 10.Golosova OS, Volodin IA, Isaeva IL, Volodina EV. 2017. Effects of free-ranging, semi-captive and captive management on the acoustics of male rutting calls in Siberian wapiti Cervus elaphus sibiricus. Mamm. Res. 62, 387–396. ( 10.1007/s13364-017-0322-4) [DOI] [Google Scholar]
- 11.Vors LS, Boyce MS. 2009. Global declines of caribou and reindeer. Glob. Chang. Biol. 15, 2626–2633. ( 10.1111/j.1365-2486.2009.01974.x) [DOI] [Google Scholar]
- 12.Mar KU. 2007. The demography and life history strategies of timber elephants in Myanmar. PhD thesis, University College London, UK. [Google Scholar]
- 13.Mason G, Burn CC, Ahloy J, Kroshko J, Mcdonald H, Jeschke JM. 2013. Plastic animals in cages: behavioural flexibility and responses to captivity. Anim. Behav. 85, 1113–1126. ( 10.1016/j.anbehav.2013.02.002) [DOI] [Google Scholar]
- 14.Win O. 2018. Myanmar elephant conservation action plan (MECAP): 2018-2027. MONREC, Myanmar.
- 15.Sukumar R. 2003. The living elephants: evolutionary ecology, behavior, and conservation. New York, NY: Oxford University Press. [Google Scholar]
- 16.Lahdenperä M, Mar KU, Lummaa V. 2014. Reproductive cessation and post-reproductive lifespan in Asian elephants and pre-industrial humans. Front. Zool. 11, 1–14. ( 10.1186/s12983-014-0054-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahdenperä M, Mar KU, Lummaa V. 2016. Nearby grandmother enhances calf survival and reproduction in Asian elephants. Sci. Rep. 6, 1–10. ( 10.1038/srep27213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turkalo AK, Wrege PH, Wittemyer G. 2016. Slow intrinsic growth rate in forest elephants indicates recovery from poaching will require decades. J. Appl. Ecol. 54, 153–159. ( 10.1111/1365-2664.12764) [DOI] [Google Scholar]
- 19.De Silva S, Elizabeth Webber C, Weerathunga US, Pushpakumara TV, Weerakoon DK, Wittemyer G.. 2013. Demographic variables for wild Asian elephants using longitudinal observations. PLoS ONE 8, e82788 ( 10.1371/journal.pone.0082788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leimgruber P, Senior B, Aung M, Songer MA, Mueller T, Wemmer C, Ballou JD. 2008. Modeling population viability of captive elephants in Myanmar (Burma): implications for wild populations. Anim. Conserv. 11, 198–205. ( 10.1111/j.1469-1795.2008.00172.x) [DOI] [Google Scholar]
- 21.Blake S, Hedges S. 2004. Sinking the flagship: the case of forest elephants in Asia and Africa. Conserv. Biol. 18, 1191–1202. ( 10.1111/j.1523-1739.2004.01860.x) [DOI] [Google Scholar]
- 22.Clubb R, Rowcliffe M, Lee P, Mar KU, Moss C, Mason GJ. 2009. Fecundity and population viability in female zoo elephants: problems and possible solutions. Anim. Welf. 18, 237–247. [Google Scholar]
- 23.Myroniuk P. 2004. Population viability analysis of captive Asian elephants in Australia: a conservation assessment. Wildl. Conserv. Serv. Int. 42, 1–11. [Google Scholar]
- 24.Sukumar R. 2006. A brief review of the status, distribution and biology of wild Asian elephants. Int. Zoo 40, 1–8. ( 10.1111/j.1748-1090.2006.00001.x) [DOI] [Google Scholar]
- 25.Toke GU. 1971. Burmese timber elephants. Yangon, Burma: Trade Corporation. [Google Scholar]
- 26.Myint A. 1997. On the distribution, status and conservation of wild elephants in Myanmar. Gajah. 18, 47–55. [Google Scholar]
- 27.Uga U. 2000. Conservation and use of wild Asian elephants (Elephas maximus). Forestry Department, Ministry of Forestry, Government of the Union of Myanmar.
- 28.Lynsdale CL, Mumby HS, Hayward AD, Mar KU, Lummaa V. 2017. Parasite- associated mortality in a long-lived mammal: variation with host age, sex, and reproduction. Ecol. Evol. 7, 1–12. ( 10.1002/ece3.3559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clubb R, Rowcliffe M, Lee P, Mar KU, Moss C, Mason GJ. 2008. Compromised survivorship in zoo elephants. Science 322, 1649 ( 10.1126/science.1164298) [DOI] [PubMed] [Google Scholar]
- 30.Mumby HS, Chapman SN, Crawley JAH, Mar KU, Htut W, Thura Soe A, Aung HH, Lummaa V. 2015. Distinguishing between determinate and indeterminate growth in a long-lived mammal. BMC Evol. Biol. 15, 1–9. ( 10.1186/s12862-015-0487-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lair RC. 1997. Gone astray: the care and management of the Asian elephant in domesticity. Bangkok, Thailand: FAO Regional Office for Asia and the Pacific. [Google Scholar]
- 32.Wood SN. 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. B 73, 3–36. ( 10.1111/j.1467-9868.2010.00749.x) [DOI] [Google Scholar]
- 33.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- 34.Wood SN. 2006. Low-rank scale-invariant tensor product smooths for generalized additive mixed models. Biometrics 62, 1025–1036. ( 10.1111/j.1541-0420.2006.00574.x) [DOI] [PubMed] [Google Scholar]
- 35.Wood SN. 2003. Thin plate regression splines. J. R. Stat. Soc. B 65, 95–114. ( 10.1111/1467-9868.00374) [DOI] [Google Scholar]
- 36.Akaike H. 1987. Factor analysis and AIC. Psychometrika 52, 317–332. ( 10.1007/BF02294359) [DOI] [Google Scholar]
- 37.Burnham KP, Anderson DR. 2004. Multimodel inference: understanding AIC and BIC in model selection. Sociol. Methods Res. 33, 261–304. ( 10.1177/0049124104268644) [DOI] [Google Scholar]
- 38.Hartig F. 2018. DHARMa: residual diagnostics for hierarchical (multi-level/ mixed) regression models. See https://CRAN.R-project.org/package-DHARMa.
- 39.Grimm V, et al. 2006. A standard protocol for describing individual-based and agent-based models. Ecol. Modell. 198, 115–126. ( 10.1016/j.ecolmodel.2006.04.023) [DOI] [Google Scholar]
- 40.Choudhury A, et al. 2008. Elephas maximus . The IUCN Red List of threatened species 2008. See 10.2305/IUCN.UK.2008.RLTS.T7140A12828813.en. [DOI]
- 41.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 42.Hayward AD, Mar KU, Lahdenperä M, Lummaa V. 2014. Early reproductive investment, senescence and lifetime reproductive success in female Asian elephants. J. Evol. Biol. 27, 772–783. ( 10.1111/jeb.12350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crawley JAH, Mumby HS, Chapman SN, Lahdenperä M, Mar KU, Htut W, Thura Soe A, Aung HH, Lummaa V. 2017. Is biggger better? The relationship between size and reproduction in female Asian elephants. J. Evol. Biol. 30, 1836–1845. [DOI] [PubMed] [Google Scholar]
- 44.Leimgruber P, Gagnon JB, Wemmer C, Kelly DS, Songer MA, Selig ER. 2003. Fragmentation of Asia's remaining wildlands: implications for Asian elephant conservation. Anim. Conserv. 6, 347–359. ( 10.1017/S1367943003003421) [DOI] [Google Scholar]
- 45.Fernando P, Leimgruber P, Prasad T, Pastorini J. 2012. Problem-elephant translocation: translocating the problem and the elephant? PLoS ONE 7, e50917 ( 10.1371/journal.pone.0050917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nijman V. 2014. An assessment of the live elephant trade in Thailand. Report from TRAFFIC International, Cambridge, UK.
- 47.Clubb R, Mason G. 2002. A Review of the Welfare of Zoo Elephants in Europe. Report from RSPCA, Horsham, West Sussex, UK.
- 48.Suter I, Maurer G, Baxter G. 2014. Population viability of captive Asian elephants in the Lao PDR. Endanger. Species Res. 24, 1–7. ( 10.3354/esr00578) [DOI] [Google Scholar]
- 49.Coulson T, Mace GM, Hudson E, Possingham H. 2001. The use and abuse of population viability analysis. Trends Ecol. Evol. 16, 219–221. ( 10.1016/S0169-5347(01)02137-1) [DOI] [PubMed] [Google Scholar]
- 50.Crawley JAH, Lahdenperä M, Seltmann MW, Htut W, Aung HH, Nyein K, Lummaa V. 2019. Investigating changes within the handling system of the largest semi-captive population of Asian elephants. PLoS ONE 14, e0209701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mar KU, Lahdenperä M, Lummaa V. 2012. Causes and correlates of calf mortality in captive Asian elephants (Elephas maximus). PLoS ONE 7, 1–9. ( 10.1371/journal.pone.0032335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mumby HS, Courtiol A, Mar KU, Lummaa V. 2013. Birth seasonality and calf mortality in a large population of Asian elephants. Ecol. Evol. 3, 3794–3803. ( 10.1002/ece3.746) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weise RJ. 2000. Asian elephants are not self-sustaining in North America. Zoo Biol. 19, 299–309. () [DOI] [Google Scholar]
- 54.Jackson J, Childs DZ, Mar KU, Htut W, Lummaa V. 2019. Data from: Long-term trends in wild-capture and population dynamics point to an uncertain future for captive elephants Dryad Digital Repository. ( 10.5061/dryad.rj237db) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Jackson J, Childs DZ, Mar KU, Htut W, Lummaa V. 2019. Data from: Long-term trends in wild-capture and population dynamics point to an uncertain future for captive elephants Dryad Digital Repository. ( 10.5061/dryad.rj237db) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data and code supporting our results are archived in the Dryad Digital Repository: https://doi.org/10.5061/dryad.rj237db [54].