Abstract
All animals use sensory systems to monitor external events and have to decide whether to move. Response times are long and variable compared to reflexes, and fast escape movements. The complexity of adult vertebrate brains makes it difficult to trace the neuronal circuits underlying basic decisions to move. To simplify the problem, we investigate the nervous system and responses of hatchling frog tadpoles which swim when their skin is stimulated. Studying the neuron-by-neuron pathway from sensory to hindbrain neurons, where the decision to swim is made, has revealed two simple pathways generating excitation which sums to threshold in these neurons to initiate swimming. The direct pathway leads to short, and reliable delays like an escape response. The other includes a population of sensory processing neurons which extend firing to introduce noise and delay into responses. These neurons provide a brief, sensory memory of the stimulus, that allows tadpoles to integrate stimuli occurring within a second or so of each other. We relate these findings to other studies and conclude that sensory memory makes a fundamental contribution to simple decisions and is present in the brainstem of a basic vertebrate at a surprisingly early stage in development.
Keywords: decisions, locomotion, response times, sensory memory
1. Introduction
In 100 m sprint races, men take 120–165 ms to start moving [1]. Such response times (RTs) fascinated experimental psychologists since Helmholtz [2] described methods to measure them. Coordinated movements, like eye movements towards a new target, start after variable delays of 50–200 ms [3]. Why are these responses slow and variable when reflex movements and the rapid escape responses of animals like squid, crayfish and fishes, have short and constant RTs (less than 20 ms) [4–6]?
Studies of RTs have focused on eye movements of people and other mammals, especially monkeys and on the complex neuronal circuits in the pre-frontal motor cerebral cortex which control them [7–9]. In the simplest experiments, subjects gaze at a light spot and are required to shift their gaze to another light spot when it comes on. Recordings of neuronal activity in mammals have shown that sensory information travels from the eyes to the cerebral cortex where it influences the ongoing firing of neurons. When the light flashes briefly, a ‘sensory memory’ of the stimulus is needed and this too has been studied primarily in the cerebral cortex. The memory only lasts for a second or so, and is proposed to be based on mutual re-excitation within small populations of neurons [10–12]. During the lead-up to the decision to move the eyes, recordings in the primate and rat pre-frontal cortex have shown that the stimulus results in a slow and noisy build-up of firing frequency. In some neurons, peak firing rates correlate with the decision to move.
Many theories underlying decisions to move the eyes propose that an excitatory ‘decision signal’ builds noisily to a threshold in cortical neurons and, when this is reached, the movement occurs (figure 1a) [3,13–15]. Variability in RTs results from noise in the decision. The complexity of the cortex makes the neuron-by-neuron circuits and synaptic properties of decision-making impossible to define. Furthermore, the advantages of slowing down decisions to move are unclear. Do they allow other stimuli and central states to be taken into account before a response is made?
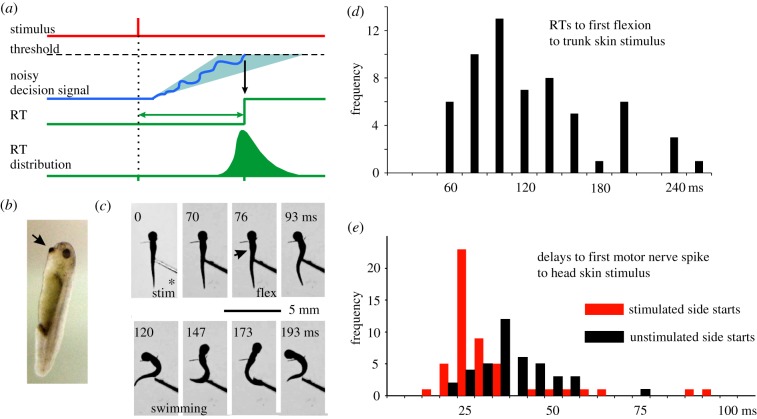
Figure 1.
A simple theory for cortical decision circuits and tadpole responses to a brief skin stimulus. (a) Diagram to illustrate general theory for variable delay generation (based on [3]). (b) A resting hatchling Xenopus tadpole, 5 mm long, hangs from mucus secreted by its cement gland (arrow). (c) Video frames of tadpole (dorsal view) flexing (arrowhead), then swimming following a current pulse to the right trunk (*). (d,e) RTs to the first flexion of swimming and first motor nerve activity of fictive swimming in immobilized tadpoles. (Online version in colour.)
Analysis of simpler neuronal circuits should help to understand the details of how neuronal circuits control the initiation of coordinated motor responses to a sensory stimulus. It is disheartening that the only cases where the neuron-by-neuron pathway from a sensory stimulus to a response have been traced are those for the rapid escape movements controlled by giant neurons in crayfish [5,16] and in fishes [17]. This is a consequence of the ease of study of giant neurons and the difficulties imposed by the small size of most neurons.
2. Why use tadpoles to study the initiation of movements?
Larval fishes and amphibians provide useful models for studying the foundations of vertebrate nervous development, organization and function [18,19]. We have investigated the neurons, and networks that allow hatchling Xenopus laevis tadpoles (figure 1b) to behave and survive [20–22]. This has led to some surprising new discoveries about the organization of sensory systems, sensory memory and the neurons where the decisions to move are made [23–25].
As in adult animals, tadpole RTs for swimming following skin stimulation are long and variable. If touched on one side of the trunk with a fine hair, tadpoles flex unpredictably to left or right and then swim off [26]. The RTs to swimming following a brief current pulse (figure 1c) are long and variable (median 102 ms, interquartile range 81–136 ms; figure 1d; [25]).
Tadpoles also have to decide which side will flex first. We have studied this by immobilizing tadpoles so that recordings can be made from motor nerves and one or two individual neurons in the spinal cord and brain. When a near threshold current pulse is given to the skin on one side of the head, the first motor nerve burst has an approximately 0.5 probability of being on the stimulated or unstimulated side (figure 1e; [24]). Reaction times are shorter than behavioural responses but are still long and variable (stimulated side starts: median 25 ms, range 15–87; unstimulated side starts: median 35 ms, range 20–71) compared to reflexes (less than 10 ms; [27]). When the trunk skin is stimulated, the median delay to the first motor nerve firing on the unstimulated side was 40 ms (inter-quartile range 33–61; [25]). In this review, we discuss where and how the delays and their variabilities arise in the tadpole's decision to swim.
3. Overview of the tadpole skin sensory swimming initiation pathway
To find out about the different types of neuron, their anatomy, activity and synaptic connections, we have used dye-filled electrodes to record from pairs of neurons in the brain and spinal cord of immobilized tadpoles. This has allowed us to build a broad picture of how the neuronal networks they form are organized [20,21]. The tadpole skin touch to motor pathway has five functional stages (figure 2), as does the leech [28].
-
(1)
Touch sensory neurons for the head skin lie in the trigeminal ganglia and for the trunk skin in the spinal cord [29,30]. A single action potential (AP) in one or two sensory neurons can initiate swimming. Their peripheral axons carry APs into the central nervous system where their central axons project to distribute excitation longitudinally.
-
(2)
Sensory pathway neurons are excited by sensory neurons and distribute sensory signals to both sides of the brain [23,30]. Single sensory neurons excite many sensory pathway neurons and in this way the sensory signal is amplified [27]. Trigeminal sensory pathway neurons are only excited by head skin stimuli and make direct connections to level 4 reticulospinal neurons [23].
-
(3)
Sensory processing neurons are a new and important proposal for the movement initiation pathway [25]. The preliminary evidence and definition of these neurons suggests that they extend firing (possibly by mutual re-excitation) and introduce noise and variability. They transform the short or nearly synchronous firing in sensory pathway neurons (less than 30 ms) into longer lasting and variable firing (approx. 1000 ms) which is different in each neuron of their population. They are excited by sensory pathway neurons and excite reticulospinal neurons. Their firing acts as a sensory memory of any brief sensory stimulus to the skin.
-
(4)
Reticulospinal neurons lie in the hindbrain and project to the spinal cord. They sum the input from different sensory pathways and modalities, like skin touch and light [31]. It is here that excitation from sensory processing neurons builds up noisily and can reach firing threshold. It is these reticulospinal neurons that effectively make the decision to swim because, when they fire, motoneurons are activated and initiate the first flexion of swimming. During swimming, they generate the rhythm by firing once on each cycle.
-
(5)
Motoneurons and reciprocal inhibitory neurons are excited strongly by the reticulospinal neurons so they fire once on each cycle of swimming [32]. Motoneuron firing leads to muscle contraction and flexion of the body. Alternation of firing is organized by reciprocal inhibition [33], re-enforced by post-inhibitory rebound firing organized by the same reciprocal inhibition [21,34]. The result is alternating waves of contraction and swimming.
Figure 2.
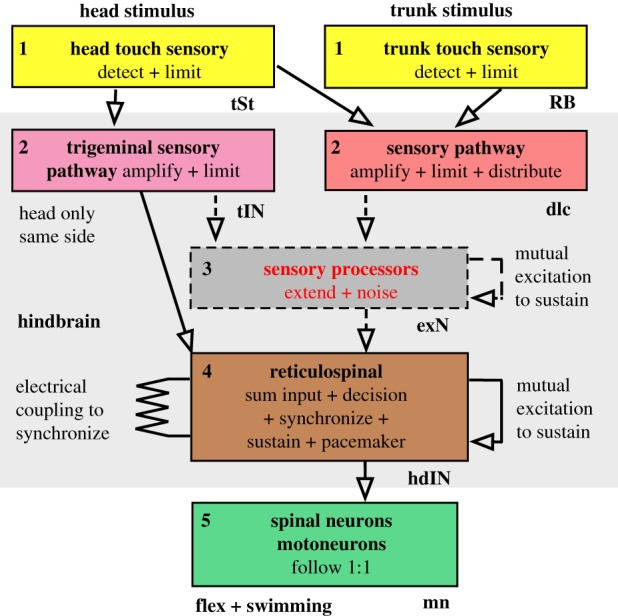
Organization of neuronal pathways from head and trunk skin stimulation to swimming in the hatchling frog tadpole. The boxes represent populations of similar excitatory neurons in the five functional levels between a skin stimulus and a motor response. Arrows show direct excitatory synaptic connections established by recording from pre- and post-synaptic neurons. Dashed boxes and arrows are populations and connections proposed to explain current evidence. For simplicity, the pathways are only shown for one side of the body and inhibitory neurons are omitted. Letters near boxes are the abbreviations of neuron names they contain. (Online version in colour.)
4. Evidence on the operation of the tadpole touch sensory system
Sensory neurons innervate the skin in all vertebrates with fine unspecialized ‘free’ nerve endings which wrap around the skin cells. Tadpole sensory neurons (tSt) for head skin lie in the trigeminal ganglia and their central axons reach the brain in the fifth cranial nerve [29]. The trunk skin has similar endings coming from sensory Rohon-Beard (RB) neurons in the spinal cord [30,35]. Recordings from sensory neurons showed that they fire one to three spikes when stimulated in their receptive field.
Spinal sensory pathway neurons are excited by synapses from the longitudinal axons of skin sensory neurons. In the spinal cord, recordings from pairs of neurons show that the RB neurons directly and strongly excite two types of sensory pathway neuron (figure 3a; [27,36,37]). These dorsolateral commissural (dlc) and ascending (dla) neurons only fire very briefly.
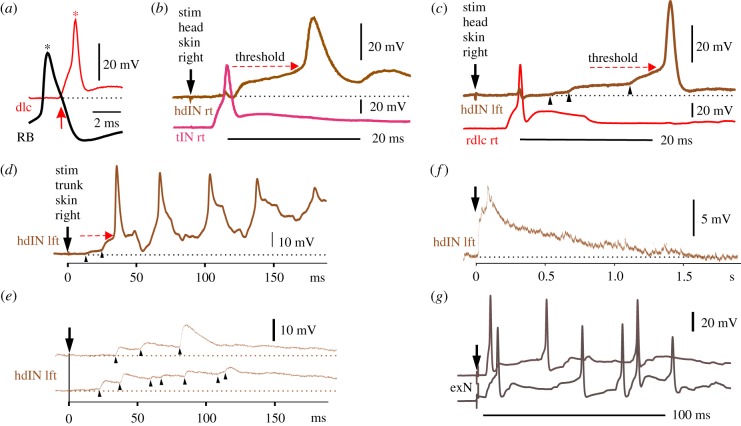
Figure 3.
Recordings show the activity and connections of neurons in the swimming response pathway: (a) current injection into a sensory RB neuron leads to an action potential (*) and direct excitation of a sensory pathway dlc neuron (excitatory post-synaptic potential (EPSP) starts at arrow) which then evokes an action potential in the dlc (*). (b,c) Paired recordings showing responses to a 1 ms head skin current stimulus which evoked swimming. (b) A sensory pathway tIN responds with a single spike. The excitation from this and other tIN spikes excites the hdIN on the same side which depolarizes smoothly to threshold (dashed arrow) and fires a spike. (c) A rostral dlc on the right side also fires a single spike but this cannot explain the slow, noisy build-up of excitation (arrowheads) to threshold (dashed arrow) and firing in the hdIN on the opposite side. (d–f) Trunk skin stimulation also leads to variable excitation of hdINs. (d) A stronger stimulus leads to two jumps (arrowheads) in hdIN which reach threshold (arrow) and firing is followed by rhythmic firing during swimming. (e) Two examples of responses subthreshold for swimming show noisy excitation (arrowheads) summing but not reaching firing threshold. (f) The long duration of subthreshold excitation in hdINs is revealed by averaging five recordings at a slower time-scale. (g) Two examples of responses of a possible sensory processing neuron (exN) in the hindbrain to a trunk skin stimulus (at arrow). Each record shows an early spike and variable later firing. (Online version in colour.)
Trigeminal sensory pathway neurons (tINs) form a small trigeminal nucleus of around 25 neurons on each side of the hindbrain and have a descending axon [23]. Head skin sensory neurons directly excite tINs and more rostral spinal sensory pathway neurons (rdlcs; [24]).
Sensory pathway neurons: distribute signals from skin sensory neurons to targets on both sides of the brain; amplify sensory signals so one or two spikes in a few sensory neurons elicit a single spike in many pathway neurons (the number firing may depend on the number of sensory neurons firing and crudely code the stimulus strength); set a limit on the excitation reaching the brain because there is a fixed number of them.
5. Evidence on the activation of the swimming control system
Movements of the body and limbs in vertebrates are thought to be controlled by reticulospinal neurons in the hindbrain which project to the spinal cord [38–42]. Tadpole swimming starts when a population of hindbrain reticulospinal neurons (hdINs) becomes active [20,23,24,32]. These neurons are electrically coupled [43], so when a few fire, the whole population on one side is recruited and they all fire once in synchrony [44]. They then play a critical role in driving swimming [21,45]. As well as exciting motoneurons and other spinal neurons, these electrically coupled neurons excite each other chemically by releasing glutamate [32]. Mutual activation of their own N-methyl-d-aspartate receptors turns on pacemaker properties which sustains their own regular, rhythmic firing and swimming [44,46–48].
It was therefore surprising when recordings from these reticulospinal hdINs showed that the only sensory pathway neurons to make strong and direct synaptic connection to them were the hindbrain tINs excited by head skin stimulation (figure 3b). This pathway could evoke hdIN firing and swimming starting on the stimulated side at relatively short latencies (hdINs: median delay of 12 ms; motor nerve: median 25 ms; [24]). To our knowledge, this is the first simple, non-giant neuron pathway for the initiation of locomotion defined in the vertebrates.
How does a stimulus to one side lead to swimming starting on the opposite side? Spinal sensory pathway dlc neurons carry excitation to the opposite side when the head or trunk skin is stimulated. Surprisingly, recordings from these neurons and hdINs on the opposite side showed no direct, short latency excitation (figure 3c; [24]) but revealed a very variable pattern of longer latency excitation [25]. This could build up over time to reach the hdIN threshold, so they fired at long and variable latencies (figure 3c,d; median 57 ms). The RTs of the first hdIN firing matched the delays to the start of swimming in motor nerve recordings (figure 1e).
What are the characteristics of the variable pattern of excitation recorded in reticulospinal dINs following a brief skin stimulus? Even when the stimulus was too weak to evoke swimming, hdINs on each side of the body received long-lasting excitation with clear excitatory post-synaptic potentials (EPSPs) occurring for many hundreds of milliseconds after the stimulus (figure 3e,f) [25]. It is difficult to see how this pattern of EPSPs could be produced directly by the brief burst of spikes fired early by sensory pathway dlc neurons. The EPSPs must be generated by unidentified neurons firing irregularly for some time after skin stimulation. We obtained information about the timing of the APs in these unidentified neurons by measuring the timing of the EPSPs they evoked in hdINs following skin stimulation (figure 3e). The novel conclusion was that the sensory pathway neurons must excite undefined populations of sensory processing neurons on each side of the brain whose function was to extend sensory firing for about 1 s, act as a sensory memory of brief stimuli and produce variable excitation of the hdINs to initiate swimming. We call them extension neurons (exNs) and have candidate recordings of hindbrain neurons with suitable prolonged and variable firing to skin stimulation (figure 3g).
6. A model of sensory memory to extend brief sensory firing
How could sensory pathway neuron firing and excitation be extended by sensory processing exN neurons in the hindbrain? A simple proposal is that exN neurons excite each other to form a small recurrent network and sustain their own activity for a short time [10]. To test this idea, we built a network of 30 model exN neurons excited by brief excitation from sensory pathway dlc neurons. The exNs were connected to a model hdIN [48] to monitor the synaptic excitation they produced. With variability in the synaptic strength of all excitatory synapses in the network (maximum conductance of each synapse scaled by a randomly chosen value: 0.8, 0.6, 0.4, 0.2 or 0), the variable patterns of exN firing it generated could be qualitatively matched to the data on the timing of EPSPs in hdINs. These results show that it is simple to prolong firing in a small model network of unspecialized neurons if there is variable and weak recurrent excitation among them even without any inhibition [25]. If such a network was connected to hdINs in a model swimming network, it should start swimming after variable and unpredictable delays.
7. Discussion
All animals have to make decisions about whether to respond to sensory stimuli. Studying simpler animals can uncover the detailed neuronal pathways for decisions to initiate simple movements, like swimming [28]. In crayfish and fish escape responses, sensory neurons excite giant neurons, bringing them rapidly to firing threshold. They fire a single spike, and the first flexion of swimming is initiated at a short and constant latency by the direct excitation of motoneurons [16,17].
In hatchling tadpoles, tracing the neuronal pathway from a brief skin touch to the initiation of swimming has revealed two ways in which populations of non-giant reticulospinal neurons (hdINs) can control the first flexion of swimming (figure 2). Stronger head skin stimulation excites tIN neurons on the same side of the hindbrain [23] and they directly excite the hdIN population and swimming follows at fairly short and constant latencies [24]. This pathway provides a simple and direct way to initiate locomotion. Weaker stimuli to the head or trunk have revealed unexpected complexity. Instead of a simple reflex-like pathway, we propose that small populations of sensory processing neurons (exNs) in the hindbrain are excited by sensory pathway neurons and extend firing for approximately 1 s (see figure 2 level 3; [25]). This process could introduce noise into the sensory signal in the brain and then provide prolonged excitation to the reticulospinal hdINs to bring them to firing threshold, so swimming is initiated after variable delays on either side of the body. The exNs therefore combine two functions: they hold a roughly 1 s sensory memory of recent stimuli and generate a noisy ‘decision signal’ to brainstem hdINs. The hdIN firing is the ‘decision’ which initiates the first flexion of swimming after variable delays.
8. What is the significance of extending sensory excitation and inserting delay and noise into the movement initiation pathway?
-
(i)
Integration of sensory inputs is made possible by the long-lasting excitation exNs produce in reticulospinal hdINs. If an initial stimulus does not lead to a motor response, then this excitation remains present and could sum with excitation from subsequent stimuli to the same location (temporal summation) or to other parts of the body (spatial summation). In addition, other sensory modalities like water currents [49] and light dimming [31] can initiate swimming. Extended excitation in hdINs acts as a sensory memory and could sum with other sensory inputs if they occur within the 1 s time window. Neurons with extended excitation can therefore act as integrators, so responses are ‘considered’ in the context of all stimuli reaching the animal.
-
(ii)
Motor coordination is essential for the production of effective movements. When stimulated, the tadpole must decide whether or not to make the first body flexion of swimming, and which side flexes first. This is a very organized process in response to head skin stimulation [24]. The hdIN firing on each side falls into discreet alternating time windows, so synchronous firing of the two sides is avoided. It is very likely that reciprocal inhibition is important here [33,50]. However, in Xenopus immobilized preparations synchronous firing of both sides can occur [51,52] as in simple model networks with reciprocal inhibition [34,53]. Slowing down hdIN excitation and making it noisy probably reduces the chance of hdINs reaching firing threshold simultaneously on both sides, thus reducing the probability of synchronous firing.
-
(iii)
Introducing variability into responses may make responses less predictable and help animals to avoid predation [54]. The slowly rising excitation in hdINs following skin stimulation introduces variability into: the delay before hdINs reach their firing threshold and swimming starts; the side which fires first to gentle stimulation; and the direction of swimming. There is one exception to this general picture. Stronger stimuli to the head skin lead to short latency and predictable flexion towards the stimulated side [24] and the biological significance of this behaviour is not clear.
9. Overview of a simple decision to move
Can the complete neuron-to-neuron pathway from a sensory stimulus to a coordinated motor response be defined? At present, the only examples are those specialized for fast responses [16,17,23]. The latencies to most skin stimuli in tadpoles are long and variable, like eye movements and other coordinated motor responses in adult animals [3]. Our new evidence on tadpole swimming initiation is still incomplete but has a surprising new sensory processing stage (figure 2). We propose that neurons at this stage extend and add noise to the signal reaching the brainstem reticulospinal neurons which drive swimming. The evidence supports the basic proposals from studies on eye movements where variable excitation leads to long and variable RTs. The evidence that some hindbrain neurons produce variable excitation is strong but characterization of the neurons involved is at present weak. Our expectation is that neurons performing this extension or sensory memory function will be a fundamental component of movement initiation pathways in most animals. The slowing down of responses allows animals to integrate multiple sensory inputs and to make ‘considered’ rather than ‘rash’ responses!
Supplementary Material
Acknowledgements
We would like to thank Dr Robert Meech for comments on an earlier draft, Dr Iain Gilchrist for valuable discussion and the BBSRC for their support of our research.
Data accessibility
This article is a review and contains no data.
Competing interests
We declare we have no competing interests.
Funding
We received no funding to write this review.
References
- 1.Pilianidis T, Mantzouranis N, Kasabalis A. 2012. Start reaction time and performance at the sprint events in World Athletic Championships. Int. J. Perf. Anal. Spor. 12, 112–118. ( 10.1080/24748668.2012.11868587) [DOI] [Google Scholar]
- 2.Helmholtz H. 1850. Vorläufiger Bericht über die Fortpflanzungsgeschwindigkeit der Nervenreizung. Archiv für Anatomie, Physiologie und Wissenschaftliche Medicin 27, 71–73. [Google Scholar]
- 3.Noorani I, Carpenter RH. 2016. The LATER model of reaction time and decision. Neurosci. Biobehav. Rev. 64, 229–251. ( 10.1016/j.neubiorev.2016.02.018) [DOI] [PubMed] [Google Scholar]
- 4.Korn H, Faber DS. 2005. The Mauthner cell half a century later: a neurobiological model for decision-making? Neuron 47, 13–28. ( 10.1016/j.neuron.2005.05.019) [DOI] [PubMed] [Google Scholar]
- 5.Edwards DH, Heitler WJ, Krasne FB. 1999. Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. Trends Neurosci. 22, 153–161. ( 10.1016/S0166-2236(98)01340-X) [DOI] [PubMed] [Google Scholar]
- 6.Sillar KT, Picton LD, Heitler WJ. 2016. The neuroethology of predation and escape. Oxford, UK: Wiley-Blackwell. [Google Scholar]
- 7.Smith PL, Ratcliff R. 2004. Psychology and neurobiology of simple decisions. Trends Neurosci. 27, 161–168. ( 10.1016/j.tins.2004.01.006) [DOI] [PubMed] [Google Scholar]
- 8.Schall JD. 2003. Neural correlates of decision processes: neural and mental chronometry. Curr. Opin. Neurobiol. 13, 182–186. ( 10.1016/S0959-4388(03)00039-4) [DOI] [PubMed] [Google Scholar]
- 9.Hanes DP, Schall JD. 1996. Neural control of voluntary movement initiation. Science 274, 427–430. ( 10.1126/science.274.5286.427) [DOI] [PubMed] [Google Scholar]
- 10.Goldman-Rakic PS. 1995. Cellular basis of working memory. Neuron 14, 477–485. ( 10.1016/0896-6273(95)90304-6) [DOI] [PubMed] [Google Scholar]
- 11.Durstewitz D, Seamans JK, Sejnowski TJ. 2000. Neurocomputational models of working memory. Nat. Neurosci. 3, 1184–1191. ( 10.1038/81460) [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Yang Y, Wang C, Gamo N, Jin L, Mazer J, Morrison J, Wang X, Arnsten A. 2013. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron 77, 736–749. ( 10.1016/j.neuron.2012.12.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpenter RH, Williams ML. 1995. Neural computation of log likelihood in control of saccadic eye movements. Nature 377, 59–62. ( 10.1038/377059a0) [DOI] [PubMed] [Google Scholar]
- 14.Gold JI, Shadlen MN. 2007. The neural basis of decision making. Annu. Rev. Neurosci. 30, 535–574. ( 10.1146/annurev.neuro.29.051605.113038). [DOI] [PubMed] [Google Scholar]
- 15.Brody CD, Hanks TD. 2016. Neural underpinnings of the evidence accumulator. Curr. Opin. Neurobiol. 37, 149–157. ( 10.1016/j.conb.2016.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herberholz J, Marquart GD. 2012. Decision making and behavioral choice during predator avoidance. Front. Neurosci. 6, 125 ( 10.3389/fnins.2012.00125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fetcho JR. 1991. Spinal network of the Mauthner cell. Brain Behav. Evol. 37, 298–316. ( 10.1159/000114367) [DOI] [PubMed] [Google Scholar]
- 18.Kimura Y, Satou C, Fujioka S, Shoji W, Umeda K, Ishizuka T, Yawo H, Higashijima S. 2013. Hindbrain V2a neurons in the excitation of spinal locomotor circuits during zebrafish swimming. Curr. Biol. 23, 843–849. ( 10.1016/j.cub.2013.03.066) [DOI] [PubMed] [Google Scholar]
- 19.McLean DL, Dougherty KJ. 2015. Peeling back the layers of locomotor control in the spinal cord. Curr. Opin. Neurobiol. 33, 63–70. ( 10.1016/j.conb.2015.03.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W-C. 2011. Generation of locomotion rhythms without inhibition in vertebrates: the search for pacemaker neurons. Integr. Comp. Biol. 51, 879–889. ( 10.1093/icb/icr021) [DOI] [PubMed] [Google Scholar]
- 21.Roberts A, Li W.-C, Soffe SR. 2010. How neurons generate behaviour in a hatchling amphibian tadpole: an outline. Front. Behav. Neurosci. 4, 1–11. ( 10.3389/fnbeh.2010.00016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts A, Conte D, Hull M, Merrison-Hort R, al Azad AK, Buhl E, Borisyuk R, Soffe SR. 2014. Can simple rules control development of a pioneer vertebrate neuronal network generating behavior? J. Neurosci. 34, 608–621. ( 10.1523/Jneurosci.3248-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buhl E, Roberts A, Soffe SR. 2012. The role of a trigeminal sensory nucleus in the initiation of locomotion. J. Physiol. 590, 2453–2469. ( 10.1113/jphysiol.2012.227934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buhl E, Soffe SR, Roberts A. 2015. Sensory initiation of a co-ordinated motor response: synaptic excitation underlying simple decision-making. J. Physiol. 593, 4423–4437. ( 10.1113/JP270792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koutsikou S, Merrison-Hort R, Buhl E, Ferrario A, Li WC, Borisyuk R, Soffe SR, Roberts A. 2018. A simple decision to move in response to touch reveals basic sensory memory and mechanisms for variable response times. J. Physiol. 596, 6219–6233. ( 10.1113/JP276356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boothby KM, Roberts A. 1995. Effects of site and strength of tactile stimulation on the swimming responses of Xenopus laevis embryos. J. Zool. (Lond.) 235, 113–125. ( 10.1111/j.1469-7998.1995.tb05132.x) [DOI] [Google Scholar]
- 27.Li W-C, Soffe SR, Roberts A. 2003. The spinal interneurons and properties of glutamatergic synapses in a primitive vertebrate cutaneous flexion reflex. J. Neurosci. 23, 9068–9077. ( 10.1523/JNEUROSCI.23-27-09068.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristan WB. 2008. Neuronal decision-making circuits. Curr. Biol. 18, R928–R932. ( 10.1016/j.cub.2008.07.081) [DOI] [PubMed] [Google Scholar]
- 29.Roberts A. 1980. The function and role of two types of mechanoreceptive ‘free’ nerve endings in the head skin of amphibian embryos. J. Comp. Physiol. A 135, 341–348. ( 10.1007/BF00657650) [DOI] [Google Scholar]
- 30.Clarke J.DW, Hayes BP, Hunt SP, Roberts A. 1984. Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J. Physiol. (Lond.) 348, 511–525. ( 10.1113/jphysiol.1984.sp015122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamieson D, Roberts A. 1999. A possible pathway connecting the photosensitive pineal eye to the swimming central pattern generator in young Xenopus laevis tadpoles. Brain Behav. Evol. 54, 323–337. ( 10.1159/000006632) [DOI] [PubMed] [Google Scholar]
- 32.Li W-C, Soffe SR, Wolf E, Roberts A. 2006. Persistent responses to brief stimuli: feedback excitation among brainstem neurons. J. Neurosci. 26, 4026–4035. ( 10.1523/jneurosci.4727-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dale N. 1985. Reciprocal inhibitory interneurones in the Xenopus embryo spinal cord. J. Physiol. (Lond.) 363, 61–70. ( 10.1113/jphysiol.1985.sp015695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrario A, Merrison-Hort R, Soffe SR, Li WC, Borisyuk R. 2018. Bifurcations of limit cycles in a reduced model of the Xenopus tadpole central pattern generator. J. Math. Neurosci. 8, 10 ( 10.1186/s13408-018-0065-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts A, Hayes BP. 1977. The anatomy and function of ‘free’ nerve endings in an amphibian skin sensory system. Proc. R. Soc. Lond. B 196, 415–429. ( 10.1098/rspb.1977.0048) [DOI] [PubMed] [Google Scholar]
- 36.Clarke J.DW, Roberts A. 1984. Interneurones in the Xenopus embryo spinal cord: sensory excitation and activity during swimming. J. Physiol. (Lond.) 354, 345–362. ( 10.1113/jphysiol.1984.sp015380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts A, Sillar KT. 1990. Characterization and function of spinal excitatory interneurons with commissural projections in Xenopus laevis embryos. Eur. J. Neurosci. 2, 1051–1062. ( 10.1111/j.1460-9568.1990.tb00017.x) [DOI] [PubMed] [Google Scholar]
- 38.Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. 2008. Descending command systems for the initiation of locomotion in mammals. Brain Res. Rev. 57, 183–191. ( 10.1016/j.brainresrev.2007.07.019) [DOI] [PubMed] [Google Scholar]
- 39.Orlovsky GN, Deliagina TG, Grillner S. 1999. Neuronal control of locomotion, p. 322 Oxford, UK: Oxford University Press. [Google Scholar]
- 40.Viana Di Prisco G, Pearlstein E, Robitaille R, Dubuc R.. 1997. Role of sensory-evoked NMDA plateau potentials in the initiation of locomotion. Science 278, 1122–1125. ( 10.1126/science.278.5340.1122) [DOI] [PubMed] [Google Scholar]
- 41.Daghfous G, Green WW, Alford ST, Zielinski BS, Dubuc R. 2016. Sensory activation of command cells for locomotion and modulatory mechanisms: lessons from lampreys. Front. Neural Circuits 10, 18 ( 10.3389/fncir.2016.00018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deliagina TG, Zelenin PV, Orlovsky GN. 2002. Encoding and decoding of reticulospinal commands. Brain Res. Rev. 40, 166–177. ( 10.1016/S0165-0173(02)00199-6) [DOI] [PubMed] [Google Scholar]
- 43.Li W-C, Roberts A, Soffe SR. 2009. Locomotor rhythm maintenance: electrical coupling among premotor excitatory interneurons in the brainstem and spinal cord of young Xenopus tadpoles. J. Physiol. (Lond.) 587, 1677–1693. ( 10.1113/jphysiol.2008.166942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hull MJ, Soffe SR, Willshaw DJ, Roberts A. 2015. Modelling the effects of electrical coupling between unmyelinated axons of brainstem neurons controlling rhythmic activity. PLoS Comput. Biol. 11, e1004240 ( 10.1371/journal.pcbi.1004240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soffe SR, Roberts A, Li W-C. 2009. Defining the excitatory neurons that drive the locomotor rhythm in a simple vertebrate: insights into the origin of reticulospinal control. J. Physiol. 587, 4829–4844. ( 10.1113/jphysiol.2009.175208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W-C, Roberts A, Soffe SR. 2010. Specific brainstem neurons switch each other into pacemaker mode to drive movement by activating NMDA receptors. J. Neurosci. 30, 16 609–16 620. ( 10.1523/jneurosci.3695-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrario A, Merrison-Hort R, Soffe SR, Borisyuk R. 2018. Structural and functional properties of a probabilistic model of neuronal connectivity in a simple locomotor network. Elife 7, e33281 ( 10.7554/eLife.33281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hull MJ, Soffe SR, Willshaw DJ, Roberts A. 2016. Modelling feedback excitation, pacemaker properties and sensory switching of electrically coupled brainstem neurons controlling rhythmic activity. PLoS Comput. Biol. 12, e1004702 ( 10.1371/journal.pcbi.1004702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts A, Feetham B, Pajak M, Teare T. 2009. Responses of hatchling Xenopus tadpoles to water currents: first function of lateral line receptors without cupulae. J. Exp. Biol. 212, 914–921. ( 10.1242/jeb.027250) [DOI] [PubMed] [Google Scholar]
- 50.Messina JA, St Paul A, Hargis S, Thompson WE, McClellan AD. 2017. Elimination of left–right reciprocal coupling in the adult lamprey spinal cord abolishes the generation of locomotor activity. Front. Neural Circuits 11, 89 ( 10.3389/fncir.2017.00089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kahn JA, Roberts A. 1982. The central nervous origin of the swimming motor pattern in embryos of Xenopus laevis. J. Exp. Biol. 99, 185–196. [DOI] [PubMed] [Google Scholar]
- 52.Li WC, Merrison-Hort R, Zhang HY, Borisyuk R. 2014. The generation of antiphase oscillations and synchrony by a rebound-based vertebrate central pattern generator. J. Neurosci. 34, 6065–6077. ( 10.1523/JNEUROSCI.4198-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X.-J, Rinzel J. 1992. Alternating and synchronous rhythms in reciprocally inhibitory model neurons. Neural Comput. 4, 84–97. ( 10.1162/neco.1992.4.1.84) [DOI] [Google Scholar]
- 54.Domenici P, Blagburn JM, Bacon JP. 2011. Animal escapology I: theoretical issues and emerging trends in escape trajectories. J. Exp. Biol. 214, 2463–2473. ( 10.1242/jeb.029652) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article is a review and contains no data.