Abstract
Freeze tolerance, the ability to survive internal ice formation, facilitates survival of some insects in cold habitats. Low-molecular-weight cryoprotectants such as sugars, polyols and amino acids are hypothesized to facilitate freeze tolerance, but their in vivo function is poorly understood. Here, we use a combination of metabolomics and manipulative experiments in vivo and ex vivo to examine the function of multiple cryoprotectants in the spring field cricket Gryllus veletis. Cold-acclimated G. veletis are freeze-tolerant and accumulate myo-inositol, proline and trehalose in their haemolymph and fat body. Injecting freeze-tolerant crickets with proline and trehalose increases survival of freezing to lower temperatures or for longer times. Similarly, exogenous myo-inositol and trehalose increase ex vivo freezing survival of fat body cells from freeze-tolerant crickets. No cryoprotectant (alone or in combination) is sufficient to confer freeze tolerance on non-acclimated, freeze-intolerant G. veletis. Given that each cryoprotectant differentially impacts survival in the frozen state, we conclude that small cryoprotectants are not interchangeable and likely function non-colligatively in insect freeze tolerance. Our study is the first to experimentally demonstrate the importance of non-colligative cryoprotectant function for insect freeze tolerance both in vivo and ex vivo, with implications for choosing new molecules for cryopreservation.
Keywords: freeze tolerance, cold tolerance, acclimation, cryoprotectants, cryopreservation, Gryllus veletis
1. Introduction
Ectotherms overwintering in temperate regions risk freezing of their body fluids, which is lethal in most cases [1]. Most insects are chill-susceptible and are killed by low temperatures regardless of whether ice forms, while other insects are freeze-avoidant and will survive sub-zero temperatures unless their body fluids freeze [2]. Remarkably, some insects are freeze-tolerant and survive internal ice formation [2]. Many freeze-tolerant insects have a high supercooling point (SCP; the temperature at which ice formation begins) and will survive in the frozen state until cooled below their lower lethal temperature (LLT) or kept frozen beyond their lethal time (Lt) [3]. Despite observations of insect freeze tolerance dating back nearly 300 years, we have a limited understanding of the physiological mechanisms that underlie the ability to withstand internal ice formation [3].
Most cold-tolerant insects accumulate low-molecular-weight cryoprotectants such as polyols (e.g. glycerol), sugars (e.g. trehalose) or amino acids (e.g. proline) [2,3], which can also be used in cryopreservation of mammalian cells and tissues. However, no single cryoprotectant or group of cryoprotectants is accumulated by all freeze-tolerant insects. For example, most orders of freeze-tolerant insects accumulate more than 100 mM glycerol [3–5], but glycerol is absent or at low (less than 20 mM) concentrations in the drosophilid fly Chymomyza costata [6] and freeze-tolerant orthopterans (crickets and their relatives) [7,8]. Thus, it is difficult to draw inferences about whether these cryoprotectants are necessary or sufficient for freeze tolerance based on comparisons among species. Cryoprotectant manipulation in vivo has demonstrated that proline and trehalose likely function in freeze tolerance. Elevated whole body proline concentrations (via a high proline diet) improve survival of frozen C. costata larvae to lower temperatures [6] and confer freeze tolerance on (normally chill-susceptible) Drosophila melanogaster larvae [9]. Similarly, injection of trehalose into larvae of the Antarctic midge Belgica antarctica improves survival of freezing stress [10]. However, it is not clear whether these different cryoprotectants contribute to freeze tolerance via similar or unique mechanisms.
We have previously hypothesized that freeze-tolerant insects must withstand several challenges associated with low temperatures and internal ice formation [3]. As temperature decreases, so does the stability of membranes and proteins, while ice content (and associated dehydration stress) increases, potentially damaging cells and their components [3,11,12]. Once ice content equilibrates at a stable temperature, ice content does not change, but ice crystals may grow via recrystallization, which could cause mechanical damage [3,4]. In addition, cellular metabolism may continue in the frozen state, resulting in a build-up of harmful metabolites [3,13]. Mortality at the LLT and Lt may be caused by these temperature- and time-dependent processes, respectively. Cryoprotectants may facilitate freeze tolerance mechanisms by mitigating some of these challenges.
There are two main (but not mutually exclusive) hypotheses for the function of low-molecular-weight cryoprotectants in freeze tolerance: (1) cryoprotectants minimize damage in a concentration-dependent (colligative) manner, for example, by reducing ice content; and (2) cryoprotectants improve cell survival via non-colligative means (e.g. by directly protecting macromolecules such as proteins and membranes [2]). In support of hypothesis 1, high cryoprotectant concentrations (e.g. approx. 300 mM glycerol in the woolly bear caterpillar Pyrrharctia isabella) are correlated with high haemolymph osmolality and low ice content at mild sub-zero temperatures (less than 50% of body water converted to ice [3,14]). Hypothesis 2 is supported by in vitro and ex vivo experiments. In vitro, trehalose and proline reduce membrane disruption during freezing [15]; and sugars [16], amino acids [17] and glycerol [18] can help maintain protein structure by stabilizing their hydration shell. Glycerol also improves survival of Chilo suppressalis fat body [19] and P. isabella foregut cells [20] frozen ex vivo. However, there is currently limited support for either hypothesis in vivo.
Many freeze-tolerant insects accumulate multiple cryoprotectants, and our ability to detect these molecules has improved with advances in metabolomics [5]. For example, the goldenrod gall fly Eurosta solidaginis accumulates glycerol, sorbitol and trehalose [21], and the New Zealand alpine weta Hemideina maori accumulates proline and trehalose [22]. We can use these multi-cryoprotectant systems to test hypotheses about cryoprotectant function. If cryoprotectants predominantly contribute to freeze tolerance via colligative mechanisms, we expect different cryoprotectants to be functionally interchangeable. For example, high in vivo concentrations of any cryoprotectant would reduce ice content as a ‘compatible osmolyte’, reducing cellular dehydration [23] and thereby facilitating survival of freeze-tolerant insects to lower temperatures. Conversely, if cryoprotectants have non-colligative roles in freeze tolerance, we predict that the impact of increased cryoprotectant concentrations on freeze tolerance could differ depending on the identity of that molecule.
We are developing the spring field cricket Gryllus veletis (Alexander & Bigelow) (Orthoptera: Gryllidae) as a model for studying the mechanisms underlying insect freeze tolerance [24,25]. A six-week laboratory acclimation to autumn-like temperature and photoperiod induces freeze tolerance in late instar nymphs [25]. Acclimated crickets elevate their haemolymph osmolality, modify the temperature at which ice formation begins and survive freezing for up to one week at −8°C (Lt), and to temperatures as low as −12°C (LLT) [25]. Freeze-tolerant G. veletis protect fat body cells from freeze injury in vivo (except at the lethal limits), and fat body tissue from freeze-tolerant crickets survives brief freeze treatments in vivo and ex vivo [25]. Conversely, fat body cells from freeze-intolerant G. veletis do not survive freezing [25]. Here, we use G. veletis to test hypotheses concerning the function of cryoprotectants in whole animal and cellular freeze tolerance. We identify putative low-molecular-weight cryoprotectants by comparing the metabolome of freeze-tolerant and freeze-intolerant G. veletis haemolymph and fat body tissue. We then test the cryoprotective function of multiple metabolites on crickets and their fat body cells by manipulating their concentrations in vivo and ex vivo. We hypothesized that cryoprotectants would confer freeze tolerance on freeze-intolerant G. veletis and their tissues and facilitate survival of freeze-tolerant crickets or their tissues frozen to lower temperatures or for longer times. The effects of each cryoprotectant on organismal and tissue freeze tolerance differed, and we provide evidence that these cryoprotectants probably contribute to freeze tolerance via non-colligative mechanisms.
2. Material and methods
(a). Rearing and acclimation conditions
Our laboratory colony of G. veletis originated from individuals collected in 2010 from the University of Lethbridge campus, Alberta, Canada, and was reared as described recently [25]. We haphazardly assigned fifth-instar male G. veletis approximately eight weeks post-hatch to remain in rearing (control) conditions for six weeks or to undergo a six-week acclimation with decreasing temperature and photoperiod, mimicking autumn conditions in London, Ontario, Canada [25]. Crickets in control conditions were maintained at a constant temperature of 25°C and 14 L : 10 D photoperiod. Control crickets do not survive a moderate freeze treatment approximately 1°C below their SCP (−8°C for 1.5 h [25]) and are hereafter referred to as freeze-intolerant. Crickets were acclimated in a Sanyo MIR 154 incubator (Sanyo Scientific, Bensenville, IL, USA) with temperature fluctuating daily between 12 h at high and 12 h at low temperatures. The high and low temperature decreased from 16/12°C to 1/0°C (high/low) over six weeks, and photoperiod decreased by 36 min per week (from 11.5 L : 12.5 D to 7.9 L : 16.1 D). Acclimated crickets survive a moderate freeze treatment [25] and are hereafter referred to as freeze-tolerant.
(b). Identifying putative cryoprotectants
We used targeted GC/LC-MS and GC-FID to quantify haemolymph and fat body concentrations of major low-molecular-weight metabolites (sugars, polyols and acidic metabolites) during six weeks of acclimation or control conditions [26]. We isolated fifth-instar male nymphs into individual 180 ml plastic cups (Polar Plastics, Summit Food Distributors, London, ON, Canada) with mesh covering and shelters made from egg cartons and transferred them to acclimation or control conditions described above. We extracted 4 µl of haemolymph and dissected fat body tissue from crickets at zero, three and six weeks post-isolation. We briefly blotted fat body samples on tissue paper to remove haemolymph and then flash-froze samples in liquid nitrogen. Each biological replicate consisted of haemolymph or fat body tissue pooled from five crickets. We extracted metabolites from haemolymph and fat body samples in 70% ethanol [6]. Acidic metabolites (amino acids, organic acids, fatty acids) were derivatized by ethylchloroformate in pyridine/ethanol, extracted in chloroform (for GC-MS) or 30% methanol (for LC-MS) and quantified as described previously [26]. Samples for sugar and polyol quantification were derivatized by oximation and methylsilylation, dissolved in iso-octane and analysed by GC-FID [26].
We compared the haemolymph and fat body metabolomes of crickets maintained under control or acclimation conditions using principal components analysis (PCA) in R version 3.4.1 [6,27]. We centred and scaled metabolite concentrations prior to analysis by subtracting the mean and dividing by the standard deviation of each metabolite. We identified potential cryoprotectants as metabolites that were (1) influential in the PCA (i.e. had the largest loadings) or (2) abundant (greater than 10 mM or nmol mg−1 tissue) in freeze-tolerant crickets. We compared concentrations of potential cryoprotectants between freeze-tolerant and freeze-intolerant crickets using one-way ANOVAs. In addition, we validated haemolymph concentrations in freeze-tolerant and freeze-intolerant crickets after six weeks of control or acclimation conditions (respectively) using spectrophotometric assays (see electronic supplementary material, methods) and compared these concentrations using one-tailed Welch's t-tests in R.
(c). In vivo manipulations of putative cryoprotectants
To determine the cryoprotective potential of myo-inositol, proline and trehalose, we tested whether elevated haemolymph concentrations of these metabolites affected freeze tolerance. We determined whether putative cryoprotectants could confer freeze tolerance by injecting freeze-intolerant crickets with metabolites and subjecting them to a moderate freeze treatment (−8°C for 1.5 h) that freeze-tolerant crickets typically survive [25]. We also tested whether putative cryoprotectants could improve freeze tolerance in combination with other changes during acclimation by injecting freeze-tolerant crickets with metabolites and freezing them to conditions that are normally lethal for freeze-tolerant crickets: the LLT (−12°C for 1.5 h) or Lt (−8°C for 7 days) [25].
To increase haemolymph concentrations of putative cryoprotectants, we injected 5 µl of metabolite in G. veletis Ringer's solution (160 mM NaCl, 11 mM KCl, 8.4 mM CaCl2, 5.9 mM MgCl2, 5 mM HEPES, pH 7.6) under the pronotum of freeze-intolerant and freeze-tolerant crickets using a 10 µl gastight Hamilton syringe with a 30-gauge disposable needle (BD Canada, Mississauga, ON, Canada). Metabolite solutions included: 0.5 M myo-inositol, 2.5 M proline, 1.3 M trehalose and a combination of 0.5 M myo-inositol, 2 M proline and 1 M trehalose (Sigma Aldrich, Mississauga, ON, Canada). As a vehicle control, we injected crickets with 5 μl G. veletis Ringer's solution. To determine which properties of metabolites (e.g. size, functional groups, permeability to cells) were important for cryoprotective function, we also injected other polyols (5 M glycerol and 15% w/v PEG-8000 polyethylene glycol) and 2.5 M glucose, the monosaccharide monomer of trehalose (Sigma Aldrich). To verify successful injection of metabolites, we extracted 2 µl haemolymph samples 10–15 min post-injection, flash-froze these samples in liquid nitrogen and stored them at −80°C until analysis via spectrophotometric assays (see electronic supplementary material, methods). Most metabolite solutions were saturated to maximize the dose administered to crickets, with the exception of glycerol and PEG solutions, which were diluted to a viscosity suitable for injection.
We froze crickets 20–30 min post-injection using recently described methods [25]. Briefly, crickets were cooled to the target temperature (−8 or −12°C) at 0.25°C min−1, held at the target temperature for the time described above (1.5 h or 7 days) and rewarmed to 6°C at 0.25°C min−1. After thawing, crickets were transferred to individual mesh-covered 180 ml transparent cups for recovery at 15°C. We assessed survival as the ability of crickets to move in response to gentle prodding within 48 h of recovery. We compared the survival of cryoprotectant-injected crickets to Ringer's-injected (control) crickets from the same freeze treatment using a generalized linear model with a binomial distribution and we tested the fit with Wald's χ2 using the package MASS in R [28].
(d). Ex vivo manipulations of putative cryoprotectants
To test the function of putative cryoprotectants in cellular freeze tolerance, we froze fat body tissue ex vivo in a solution containing exogenous metabolites. We tested whether these metabolites could confer freeze tolerance on fat body tissue extracted from freeze-intolerant crickets. We froze this fat body tissue to −8°C for 10 min, a treatment that is survivable for fat body cells from freeze-tolerant crickets [25]. In addition, we determined whether each metabolite could improve freeze tolerance of fat body cells extracted from freeze-tolerant crickets; we froze the fat body cells to the cellular LLT (−16°C for 10 min) and for the cellular Lt (−8°C for 24 h) [25].
To expose fat body to exogenous cryoprotectants ex vivo, we dissected fat body tissue from freeze-tolerant and freeze-intolerant G. veletis and transferred the tissue into a 0.6 ml tube containing 2 µl silver iodide (AgI) and 10 µl Grace's Insect Medium (Sigma Aldrich) or 10 µl metabolite solution in Grace's Insect Medium. The AgI was added to ensure extracellular freezing began at a uniform temperature (approx. −4°C) for all samples. ‘Low’ metabolite concentrations mimicked freeze-tolerant G. veletis haemolymph concentrations of putative cryoprotectants (30 mM myo-inositol, 30 mM proline, 70 mM trehalose and a combination of 30 mM myo-inositol, 30 mM proline and 70 mM trehalose) or similar molecules (140 mM glucose, 30 mM glycerol, 0.3 mM PEG). Each metabolite was also tested at a ‘high’ concentration: 300 mM myo-inositol, proline, trehalose, glucose, glycerol, 3 mM PEG and a combination of 300 mM each myo-inositol, proline and trehalose.
Ex vivo freeze treatments began within 15 min of dissection, as described recently [25]. Samples were cooled at 0.25°C min−1 to the target temperature (−8 or −16°C), held at the target temperature for the time described above (10 min or 24 h) and rewarmed at 0.25°C min−1 to 6°C. After thawing, we transferred fat body samples to tubes containing 10 µl G. veletis Ringer's solution and determined cell viability by live–dead staining with DAPI and propidium iodide as described recently [25]. We calculated the average proportion of live cells from three replicates of fat body samples from eight crickets for each freeze treatment × cryoprotectant combination. We compared survival of cells frozen in cryoprotectant solutions to those frozen in Grace's Insect Medium (control) from the same freeze treatment in R using a generalized linear model with a binomial error distribution.
3. Results
(a). Potential cryoprotectants accumulate intracellularly and extracellularly during acclimation
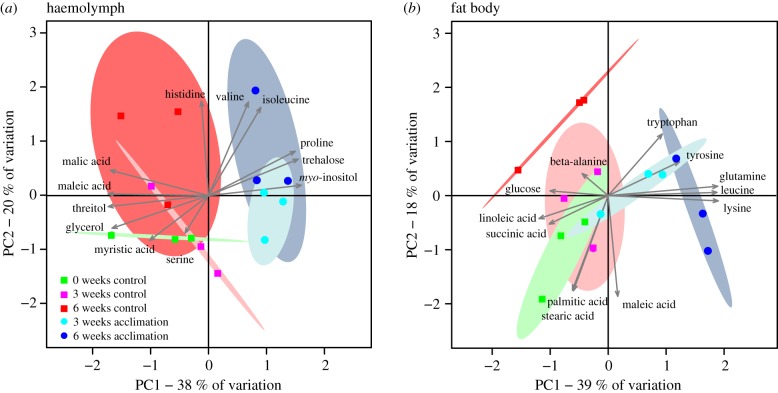
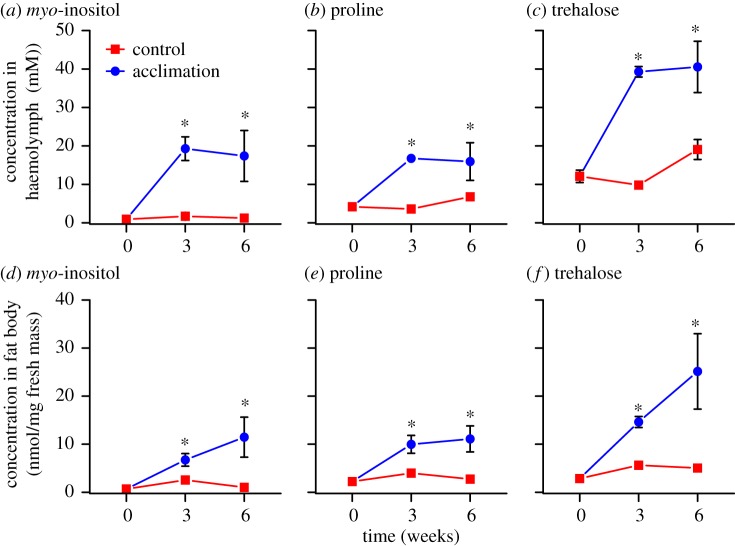
Acclimation altered the low-molecular-weight metabolite profile of both G. veletis haemolymph and fat body cells (figure 1). We detected 49 metabolites in haemolymph and 48 in fat body tissue (all 48 haemolymph metabolites except sucrose; electronic supplementary material, dataset). myo-inositol, proline and trehalose notably increased in concentration (greater than 10 mM or 10 nmol mg−1 tissue) in freeze-tolerant crickets during acclimation (figure 2), and we therefore identified them as potential cryoprotectants. With spectrophotometric assays, we verified that the concentration of these metabolites in haemolymph from acclimated crickets was 30.0 ± 2.6 mM (myo-inositol), 23.7 ± 2.9 mM (proline) and 70.3 ± 5.2 mM (trehalose). The concentrations of other haemolymph metabolites totalled approximately 30 mM in both freeze-tolerant and freeze-intolerant crickets (electronic supplementary material, dataset).
Figure 1.
Haemolymph and fat body metabolite composition changes during acclimation. Principal components analysis (PCA) based on (a) haemolymph or (b) fat body concentrations of 49 or 48 low-molecular-weight metabolites (respectively) in fifth-instar male G. veletis held under control or acclimation conditions for six weeks. Each point represents one sample of haemolymph or fat body pooled from five crickets. Ellipses represent the 95% confidence interval of each group of points (coloured to match the points). The 12 metabolites with the largest loadings (represented by arrow length) along PC1 or PC2 are labelled.
Figure 2.
Putative cryoprotectants accumulate in haemolymph and fat body during acclimation. Concentrations of (a,d) myo-inositol, (b,e) proline and (c,f) trehalose in haemolymph or fat body of fifth-instar male G. veletis held under control or acclimation conditions for six weeks. Each point represents the mean concentration ± s.e. of three samples, each containing haemolymph or fat body pooled from five individuals. Small error bars are obscured by symbols. Asterisks indicate that the mean concentration is different from zero-week control (haemolymph ANOVAs: inositol: F4,10 = 8.00, p = 0.004; proline: F4,10 = 8.21, p = 0.003; trehalose: F4,10 = 19.18, p < 0.001; fat body ANOVAs: inositol: F4,10 = 5.21, p = 0.016; proline: F4,10 = 7.53, p = 0.005; trehalose: F4,10 = 6.73, p = 0.007). (Online version in colour.)
(b). Injection increases haemolymph metabolite concentrations
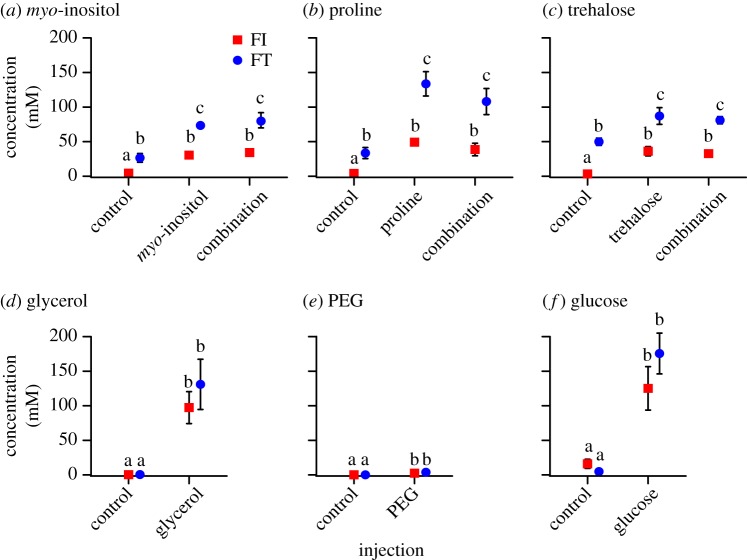
Injection of myo-inositol, proline or trehalose solutions (or a combination of all three) into freeze-intolerant crickets elevated concentrations to those measured in freeze-tolerant cricket haemolymph, or higher, within 30 min of injection (figure 3a–c). Injection of these solutions into freeze-tolerant crickets elevated their haemolymph concentrations by at least 40 mM (figure 3a–c). When we injected metabolites that freeze-tolerant G. veletis do not accumulate, glucose and glycerol concentrations increased above 100 mM in both freeze-tolerant and freeze-intolerant crickets, while PEG (which at 8000 g mol−1 is a very large molecule) increased to approximately 3 mM (figure 3d–f). Injection increased haemolymph metabolite concentrations by 33–100% more in freeze-tolerant than freeze-intolerant crickets (figure 3), suggesting that freeze-tolerant G. veletis have lower haemolymph volumes of that freeze-intolerant G. veletis metabolize the cryoprotectants more rapidly.
Figure 3.
Metabolite injection alters haemolymph composition. Haemolymph concentration of (a) myo-inositol, (b) proline, (c) trehalose, (d) glycerol, (e) polyethylene glycol (PEG) or (f) glucose 15–30 min after freeze-intolerant (FI) or freeze-tolerant (FT) crickets were injected with a vehicle control (G. veletis Ringer's solution), the indicated metabolite or a combination of myo-inositol, proline and trehalose. Each point represents the mean ± s.e. metabolite concentration of 24 crickets. Small error bars are obscured by symbols. Different letters indicate significantly different concentrations of that metabolite among injection treatments (ANOVA, Tukey's post hoc test p < 0.05). (Online version in colour.)
(c). Exogenous metabolites enhance survival of freeze-tolerant G. veletis or their tissues
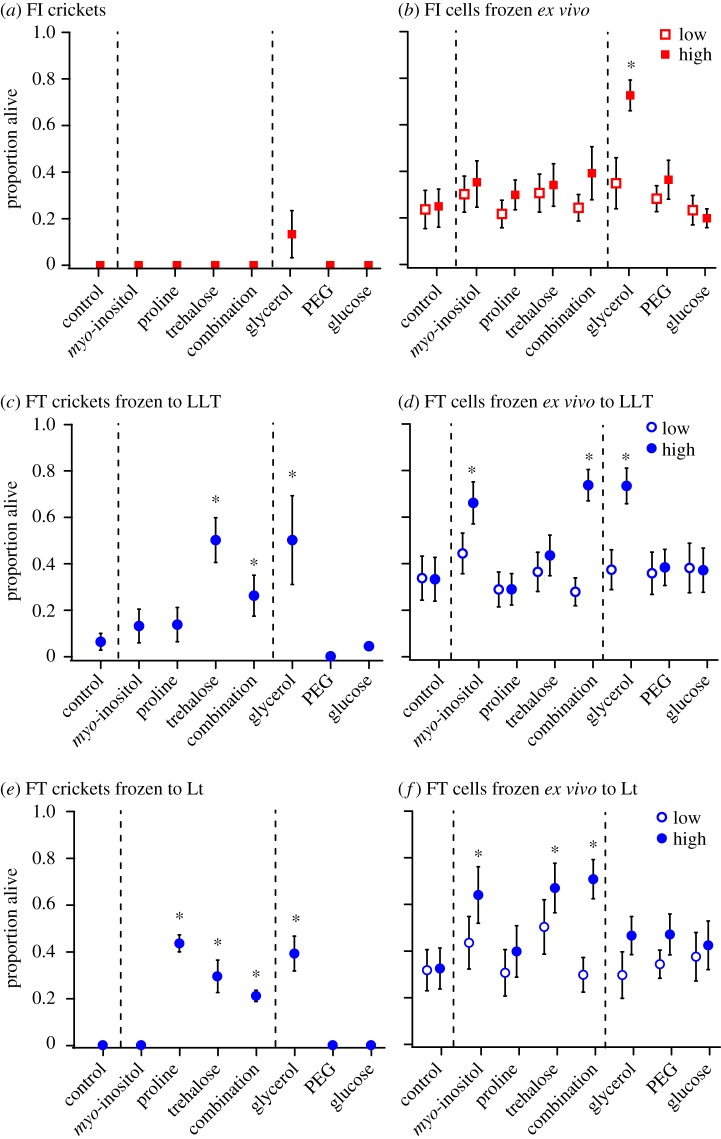
No freeze-intolerant crickets survived a moderate freeze treatment following injection with myo-inositol, proline, trehalose or a combination of all three (figure 4a). Similarly, glucose and PEG injection had no impact on survival of freeze-intolerant G. veletis (figure 4a). However, a small proportion (less than 20%) of freeze-intolerant crickets injected with glycerol survived freezing (figure 4a). Fat body dissected from freeze-intolerant G. veletis had low cell survival when frozen ex vivo with exogenous myo-inositol, proline, trehalose, PEG or glucose (figure 4b). High concentrations (300 mM) of exogenous glycerol reduced freeze injury of freeze-intolerant cricket fat body frozen ex vivo (figure 4b).
Figure 4.
Elevated metabolite concentrations increase post-thaw survival of Gryllus veletis and their fat body cells frozen ex vivo. (a,c,e) Proportion of freeze-intolerant (FI) and freeze-tolerant (FT) crickets that survived a freeze treatment following injection with a vehicle control (G. veletis Ringer's solution) or the indicated metabolite(s). Freeze treatments included (a) −8°C for 1.5 h, (c) lower lethal temperature (LLT; −12°C for 1.5 h) or (e) lethal time (Lt; 7 days at −8°C). (b,d,f) Proportion of live fat body cells following freezing ex vivo in Grace's Insect Medium (control) or the indicated metabolite(s) in Grace's Insect Medium at ‘low’ (0.3 mM PEG, 30–140 mM for others) or ‘high’ (3 mM PEG, 300 mM for others) concentrations. Freeze treatments included (b) −8°C for 10 min, (d) cellular LLT (−16°C for 10 min) or (f) cellular Lt (−8°C for 24 h). ‘Combination’ includes myo-inositol, proline and trehalose. Each point represents the surviving proportion ± s.e. of 24 crickets or cells from 24 fat body samples. Small error bars are obscured by symbols. Asterisks denote that survival is higher than the control sample in that group (p < 0.05, generalized linear models in electronic supplementary material, table S1). (Online version in colour.)
Elevated haemolymph concentrations of both trehalose and glycerol improved survival of freeze-tolerant G. veletis frozen to the LLT (figure 4c). When we froze freeze-tolerant cricket fat body tissue ex vivo to the cellular LLT, ‘high’ concentrations (300 mM) of exogenous myo-inositol and glycerol improved cell survival (figure 4d). Injection of proline, trehalose and glycerol increased the proportion of freeze-tolerant crickets that survived being frozen for the Lt (figure 4e). High concentrations of exogenous myo-inositol and trehalose protected cells from freeze-tolerant crickets frozen for the cellular Lt (figure 4f). We did not see these effects of improved cell survival ex vivo with ‘low’ concentrations of any metabolite (figure 4d,f). Injecting a combination of myo-inositol, proline and trehalose had similar effects on survival of freeze-tolerant G. veletis to that of proline (Lt) or trehalose (Lt and LLT) alone (figure 4c,e). Survival of freeze-tolerant fat body cells frozen with a combination of myo-inositol, proline and trehalose (300 mM each) did not differ from the survival of fat body cells frozen with 300 mM myo-inositol (LLT and Lt) or trehalose (Lt) alone (figure 4d,f).
4. Discussion
(a). Freeze-tolerant G. veletis accumulate low-molecular-weight cryoprotectants
Acquisition of freeze tolerance in G. veletis is accompanied by the accumulation of putative low-molecular-weight cryoprotectants in both haemolymph and tissues, consistent with metabolomic observations from other cold-tolerant insects (e.g. [5,6]). In combination, the accumulation of myo-inositol, proline and trehalose in freeze-tolerant G. veletis could account for approximately 100 mOsm of approximately 250 mOsm increase in haemolymph osmolality associated with acclimation [25]. We predict that this cryoprotectant accumulation would have two colligative effects: (1) decreasing the temperature at which ice forms and (2) decreasing ice content. However, both of these effects will be small. An additional 100 mOsm from these cryoprotectants will depress the SCP by only 0.4–0.6°C, and the entire 250 mOsm change will depress the SCP by approximately 1.4°C [29]. Small increases in haemolymph osmolality can cause large differences in ice content at high sub-zero temperatures [30,31]. In theory, an additional 100 mOsm will reduce ice fraction by 13%, and 250 mOsm by 20%, at −2°C. However, this effect is diminished at lower temperatures: for instance, an approximately 100 mOsm increase in osmolality of larval D. melanogaster haemolymph reduces maximum ice fraction by only 3% at approximately −8°C [31]. Freeze-tolerant G. veletis freeze at approximately −6°C [25], and we therefore do not expect the small concentration increase to have a sufficient colligative effect on ice content to modify survival, unless freezing is inoculated at high temperatures (greater than −2°C) by external ice [30,31].
Trehalose and proline are low molecular weight cryoprotectants commonly accumulated by freeze-tolerant insects [3,5]. Gryllus veletis accumulated these metabolites at haemolymph concentrations similar to those found in other freeze-tolerant orthopterans; for example, H. maori (approx. 40–80 mM proline, 15 mM trehalose [7,22,32]) and the great grig Cyphoderris monstrosa (approx. 10 mM proline, 20 mM trehalose [8]). myo-Inositol has been reported in other cold-hardy insects [5] and at least one freeze-tolerant cockroach [33], but to our knowledge, this is the first report in a freeze-tolerant orthopteran. However, accumulation of these low-molecular-weight cryoprotectants is not unique to freeze-tolerant insects: chill-tolerant Drosophila spp. adults can accumulate myo-inositol, proline and/or trehalose [34,35], and many freeze-avoidant insects accumulate similar metabolites [5]. We therefore conducted functional assays to determine whether these cryoprotective metabolites specifically impact survival in the frozen state and the limits of cryoprotectant function.
(b). Low-molecular-weight cryoprotectants facilitate survival in the frozen state
Although freeze-tolerant G. veletis do not accumulate glycerol, our results support its function as a cryoprotectant [2,5]. Increased concentrations of glycerol conferred freeze tolerance on a small proportion of freeze-intolerant G. veletis. Similarly, high exogenous glycerol concentrations increase freezing survival in less than 10% of (normally chill-susceptible) D. melanogaster larvae [9]. Glycerol also protected freeze-intolerant G. veletis fat body cells frozen ex vivo, similar to ex vivo experiments with C. suppressalis fat body cells frozen in 250–750 mM glycerol [19] and P. isabella foregut cells frozen with 1 M glycerol [20]. Our combination of in vivo and ex vivo functional assays suggest that, in insects that accumulate it, high concentrations of glycerol may contribute to whole animal freeze tolerance by improving cell survival. Furthermore, we demonstrate that—going forward—G. veletis may be a useful system for evaluating the in vivo or ex vivo cryoprotective function of other naturally occurring or synthetic cryoprotectants.
Our experiments in freeze-intolerant crickets demonstrated that none of the endogenous G. veletis cryoprotectants (myo-inositol, proline and trehalose) could confer freeze tolerance on freeze-intolerant crickets or their tissues. Thus, although the injection of these metabolites into freeze-intolerant crickets temporarily elevated haemolymph concentrations to those observed in freeze-tolerant G. veletis, these cryoprotectants had less impact on freeze tolerance than glycerol. However, trehalose and proline injection improved survival of freeze-tolerant crickets frozen to their lethal limits, suggesting that these molecules facilitate survival in the frozen state, but only when paired with other changes associated with acclimation. Similarly, myo-inositol and trehalose improved cell survival of fat body tissue frozen ex vivo from freeze-tolerant but not freeze-intolerant crickets. This is consistent with D. melanogaster larvae, where increased in vivo [proline] alone is not sufficient for freeze tolerance, but a combination of elevated [proline] and dormancy-inducing cold acclimation induces freeze tolerance [9,36]. We hypothesize that cryoprotectants such as myo-inositol, proline and trehalose only facilitate G. veletis freeze tolerance in concert with other changes induced during acclimation, such as upregulation of cryoprotectant transporters or other changes (e.g. cell membrane composition, cytoskeletal stability) that may improve cell survival [24,37–39].
(c). Cryoprotectants probably function via non-colligative mechanisms
The data from our functional experiments in G. veletis do not support the hypothesis that low-molecular-weight cryoprotectants facilitate survival in a solely colligative (concentration-dependent) manner, either in vivo or ex vivo. Each cryoprotectant that improved survival of freeze-tolerant G. veletis or their tissues did so under a unique combination of conditions, suggesting they are not functionally interchangeable: myo-inositol only enhanced fat body cell survival ex vivo, proline only increased whole cricket survival at the Lt, and trehalose and glycerol both improved whole animal and fat body cell survival. The metabolites that improved survival at the whole animal LLT or Lt (trehalose and glycerol) were at similar or lower haemolymph concentrations to cryoprotectants that did not impact survival (myo-inositol, proline and glucose), further suggesting that cryoprotectant function is not solely dictated by concentration. Similarly, a combination of myo-inositol, proline and trehalose did not improve survival beyond the effect of individual cryoprotectants (e.g. proline or trehalose at the Lt), despite the higher osmolality associated with the combination. Our results complement those from C. costata, where colligative reduction of ice content (by proline accumulation) does not explain their strong freeze tolerance [31]. Three (not mutually exclusive) hypotheses could account for the different effects of each cryoprotectant on survival in the frozen state: (1) each cryoprotectant may have a unique non-colligative function and contribute to freeze tolerance via mechanisms distinct from other cryoprotectants [40]; (2) cryoprotectants may have similar functions but different ‘potency’ in those functions; and (3) tissue permeability to cryoprotectants may differ, affecting their ability to protect cells and their components.
myo-inositol, proline and trehalose may contribute to freeze tolerance via a combination of unique and overlapping non-colligative mechanisms. These mechanisms could mitigate the challenges associated with ice, low temperature itself (irrespective of ice formation) or the stresses experienced during or after thawing [3]. For example, myo-inositol can impact hydrogen bonding dynamics of water, potentially regulating ice structure [41], a role that is distinct from the macromolecular stabilization roles attributed to trehalose and proline. Trehalose and proline can stabilize soluble macromolecules in vitro [16,17] and may improve survival by preventing macromolecule instability induced by low temperatures (e.g. cold denaturation of proteins) or damage over time by harmful metabolites. Trehalose is also a well-known anhydroprotectant [10,42,43] and may therefore mitigate dehydration stress associated with high ice content at low temperatures. Both trehalose and proline are readily catabolized [44,45] and may therefore contribute indirectly to survival by fuelling repair and recovery post-thaw, mitigating challenges associated with energy drain. Trehalose improved freeze tolerance under more conditions than proline and may therefore be a more potent cryoprotectant. To test potential non-colligative functions and potency of cryoprotectants, future experiments should determine the extent to which each cryoprotectant reduces macromolecule damage accumulation or energy drain in frozen G. veletis. In addition, testing whether cryoprotectant injection improves survival at low temperatures of freeze-intolerant crickets (independent of freezing) could tease apart whether cryoprotectants are important for preventing cold- rather than ice-induced injuries.
We expect that cryoprotectants must be intracellular to protect most macromolecules and therefore hypothesize that cell membrane permeability is important for cryoprotectant function. Our polyol experiments support this hypothesis: glycerol—which is small and highly permeable to cells [19]—improved survival of both freeze-intolerant and freeze-tolerant crickets (and their cells), but the large, cell-impermeable PEG did not. While PEG was at lower concentrations than glycerol (therefore exerting a smaller osmotic effect), the amount of PEG we injected should present a similar quantity of hydroxyl functional groups to glycerol. Trehalose transporters are probably required to facilitate the protective function of trehalose [43,46], and we speculate that upregulation of trehalose transporters in the fat body of freeze-tolerant G. veletis [24] could partially account for trehalose-facilitated survival of freeze-tolerant crickets and their fat body tissue at most of the lethal limits. Conversely, there is no evidence for upregulation of myo-inositol or proline transporters in fat body tissue of freeze-tolerant G. veletis [24], which may limit the function of these cryoprotectants. Additional research is required to determine whether cryoprotectants permeate different cell types of freeze-intolerant and freeze-tolerant insects and how this impacts cell survival in the frozen state.
5. Conclusion
While metabolomics experiments continue to identify a (relatively short) list of cryoprotectants associated with cold tolerance, very few studies have examined the function of these metabolites in vivo. Here, we showed that accumulation of cryoprotectants (myo-inositol, proline and trehalose) is associated with G. veletis freeze tolerance, but no cryoprotectant (alone or in combination) is sufficient to confer freeze tolerance on freeze-intolerant crickets. We manipulated cryoprotectant concentrations to enhance survival of freeze-tolerant G. veletis and their cells at their lethal limits, revealing that cryoprotectants do function in freeze tolerance, but probably in concert with other as-yet-unidentified changes to cellular function. Our functional experiments suggest that cryoprotectants contribute to freeze tolerance largely via non-colligative mechanisms. While our experiments are not comprehensive, this warrants further exploration of how these cryoprotectant roles differ in insect freeze tolerance, with implications for the use of these molecules in cryopreservation for biomedical purposes.
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to many laboratory assistants, for their help with cricket maintenance. In addition, we thank two anonymous reviewers and C. M. Williams for their constructive comments on previous versions of this manuscript, J. F. Staples for the use of equipment, M. Moos and the Laboratory of Analytical Biochemistry at the Czech Academy of Science Biology Centre for assistance with processing metabolomics samples, and A. Kuper-Psenicnik and M. Zhang for assistance with cryoprotectant manipulation experiments.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
J.T. and B.J.S. conceived of the study. J.T. designed, conducted and analysed the metabolomics and cryoprotectant manipulation experiments. V.K. and B.J.S. contributed to experimental design and interpretation of results. J.T. and B.J.S. drafted the manuscript, with contributions from V.K.
Competing interests
The authors declare that they have no completing interests.
Funding
This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) to B.J.S., and by an NSERC Canada Graduate Scholarship, an NSERC Michael Smith Foreign Study Supplement, a Society for Experimental Biology Travel Grant, and an Ontario Graduate Scholarship to J.T.
References
- 1.Sinclair BJ, Coello Alvarado LE, Ferguson LV. 2015. An invitation to measure insect cold tolerance: methods, approaches, and workflow. J. Therm. Biol. 53, 180–197. ( 10.1016/j.jtherbio.2015.11.003) [DOI] [PubMed] [Google Scholar]
- 2.Lee RE. 2010. A primer on insect cold-tolerance. In Low temperature biology of insects (eds Denlinger DL, Lee RE), pp. 3–34. New York, NY: Cambridge University Press. [Google Scholar]
- 3.Toxopeus J, Sinclair BJ. 2018. Mechanisms underlying insect freeze tolerance. Biol. Rev. 93, 1891–1914. ( 10.1111/brv.12425) [DOI] [PubMed] [Google Scholar]
- 4.Duman JG. 2015. Animal ice-binding (antifreeze) proteins and glycolipids: an overview with emphasis on physiological function. J. Exp. Biol. 218, 1846–1855. ( 10.1242/jeb.116905) [DOI] [PubMed] [Google Scholar]
- 5.Purać J, Kojić D, Petri E, Popović ŽD, Grubor-Lajšić G, Blagojević DP. 2016. Cold adaptation responses in insects and other arthropods: an ‘omics’ approach. In Short views on insect genomics and proteomics (eds Raman C, Goldsmith MR, Agunbiade TA), pp. 89–112. New York, NY: Springer. [Google Scholar]
- 6.Koštál V, Zahradníčková H, Šimek P. 2011. Hyperprolinemic larvae of the drosophilid fly, Chymomyza costata, survive cryopreservation in liquid nitrogen. Proc. Natl Acad. Sci. USA 108, 13 041–13 046. ( 10.1073/pnas.1107060108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramløv H, Bedford JJ, Leader JP. 1992. Freezing tolerance of the New Zealand alpine weta, Hemideina maori Hutton [Orthoptera; Stenopelmatidae]. J. Therm. Biol. 17, 51–54. ( 10.1016/0306-4565(92)90019-C) [DOI] [Google Scholar]
- 8.Toxopeus J, Lebenzon JE, McKinnon AH, Sinclair BJ. 2016. Freeze tolerance of Cyphoderris monstrosa (Orthoptera: Prophalangopsidae). Can. Entomol. 148, 668–672. ( 10.4039/tce.2016.21) [DOI] [Google Scholar]
- 9.Koštál V, Šimek P, Zahradníčková H, Cimlová J, Štětina T. 2012. Conversion of the chill susceptible fruit fly larva (Drosophila melanogaster) to a freeze tolerant organism. Proc. Natl Acad. Sci. USA 109, 3270–3274. ( 10.1073/pnas.1119986109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benoit JB, Lopez-Martinez G, Elnitsky MA, Lee RE, Denlinger DL. 2009. Dehydration-induced cross tolerance of Belgica antarctica larvae to cold and heat is facilitated by trehalose accumulation. Comp. Biochem. Physiol. A 152, 518–523. ( 10.1016/j.cbpa.2008.12.009) [DOI] [PubMed] [Google Scholar]
- 11.Mazur P. 2010. A biologist's view of the relevance of thermodynamics and physical chemistry to cryobiology. Cryobiology 60, 4–10. ( 10.1016/j.cryobiol.2009.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pegg DE. 2010. The relevance of ice crystal formation for the cryopreservation of tissues and organs. Cryobiology 60, S36–S44. ( 10.1016/j.cryobiol.2010.02.003) [DOI] [PubMed] [Google Scholar]
- 13.Sinclair BJ, Klok CJ, Chown SL. 2004. Metabolism of the sub-Antarctic caterpillar Pringleophaga marioni during cooling, freezing and thawing. J. Exp. Biol. 207, 1287–1294. ( 10.1242/jeb.00880) [DOI] [PubMed] [Google Scholar]
- 14.Layne JR, Blakeley DL. 2002. Effect of freeze temperature on ice formation and long-term survival of the woolly bear caterpillar (Pyrrharctia isabella). J. Insect Physiol. 48, 1133–1137. ( 10.1016/S0022-1910(02)00206-8) [DOI] [PubMed] [Google Scholar]
- 15.Rudolph AS, Crowe JH. 1985. Membrane stabilization during freezing: the role of two natural cryoprotectants, trehalose and proline. Cryobiology 22, 367–377. ( 10.1016/0011-2240(85)90184-1) [DOI] [PubMed] [Google Scholar]
- 16.Arakawa T, Timasheff SN. 1982. Stabilization of protein structure by sugars. Biochemistry 21, 6536–6544. ( 10.1021/bi00268a033) [DOI] [PubMed] [Google Scholar]
- 17.Arakawa T, Timasheff SN. 1983. Preferential interactions of proteins with solvent components in aqueous amino acid solutions. Arch. Biochem. Biophys. 224, 169–177. ( 10.1016/0003-9861(83)90201-1) [DOI] [PubMed] [Google Scholar]
- 18.Gekko K, Timasheff SN. 1981. Mechanism of protein stabilization by glycerol: preferential hydration in glycerol-water mixtures. Biochemistry 20, 4667–4676. ( 10.1021/bi00519a023) [DOI] [PubMed] [Google Scholar]
- 19.Izumi Y, Sonoda S, Yoshida H, Danks HV, Tsumuki H. 2006. Role of membrane transport of water and glycerol in the freeze tolerance of the rice stem borer, Chilo suppressalis Walker (Lepidoptera: Pyralidae). J. Insect Physiol. 52, 215–220. ( 10.1016/j.jinsphys.2005.11.001) [DOI] [PubMed] [Google Scholar]
- 20.Yi S-X, Lee RE. 2016. Cold-hardening during long-term acclimation in a freeze-tolerant woolly bear caterpillar, Pyrrharctia isabella. J. Exp. Biol. 219, 17–25. ( 10.1242/jeb.124875) [DOI] [PubMed] [Google Scholar]
- 21.Baust JG, Lee RE. 1981. Divergent mechanisms of frost-hardiness in two populations of the gall fly, Eurosta solidaginsis. J. Insect Physiol. 27, 485–490. ( 10.1016/0022-1910(81)90100-1) [DOI] [Google Scholar]
- 22.Neufeld DS, Leader JP. 1998. Freezing survival by isolated Malpighian tubules of the New Zealand alpine weta Hemideina maori. J. Exp. Biol. 201, 227–236. [DOI] [PubMed] [Google Scholar]
- 23.Yancey PH. 2005. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 208, 2819–2830. ( 10.1242/jeb.01730) [DOI] [PubMed] [Google Scholar]
- 24.Toxopeus J, Des Marteaux LE, Sinclair BJ.. 2019. How crickets become freeze-tolerant: the transcriptomic underpinnings of acclimation in Gryllus veletis. Comp. Biochem. Physiol. D 29, 55–66. ( 10.1016/j.cbd.2018.10.007) [DOI] [PubMed] [Google Scholar]
- 25.Toxopeus J, McKinnon AH, Štětina T, Turnbull KF, Sinclair BJ. 2019. Laboratory acclimation to autumn-like conditions induces freeze tolerance in the spring field cricket Gryllus veletis (Orthoptera: Gryllidae). J. Insect Physiol. 113, 9–16. ( 10.1016/j.jinsphys.2018.12.007) [DOI] [PubMed] [Google Scholar]
- 26.Li Y, et al. 2015. Shifts in metabolomic profiles of the parasitoid Nasonia vitripennis associated with elevated cold tolerance induced by the parasitoid's diapause, host diapause and host diet augmented with proline. Insect. Biochem. Mol. Biol. 63, 34–46. ( 10.1016/j.ibmb.2015.05.012) [DOI] [PubMed] [Google Scholar]
- 27.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 28.Venables WN, Ripley BD. 2002. Modern applied statistics with S. New York, NY: Springer. [Google Scholar]
- 29.Zachariassen KE, Kristiansen E, Pedersen SA, Hammel HT. 2004. Ice nucleation in solutions and freeze-avoiding insects: homogeneous or heterogeneous? Cryobiology 48, 309–321. ( 10.1016/j.cryobiol.2004.02.005) [DOI] [PubMed] [Google Scholar]
- 30.Rozsypal J, Koštál V. 2018. Supercooling and freezing as eco-physiological alternatives rather than mutually exclusive strategies: a case study in Pyrrhocoris apterus. J. Insect Physiol. 111, 53–62. ( 10.1016/j.jinsphys.2018.10.006) [DOI] [PubMed] [Google Scholar]
- 31.Rozsypal J, Moos M, Šimek P, Koštál V. 2018. Thermal analysis of ice and glass transitions in insects that do and do not survive freezing. J. Exp. Biol. 221, jeb.170464 ( 10.1242/jeb.170464) [DOI] [PubMed] [Google Scholar]
- 32.Ramløv H. 1999. Microclimate and variations in haemolymph composition in the freezing-tolerant New Zealand alpine weta Hemideina maori Hutton (Orthoptera: Stenopelmatidae). J. Comp. Physiol. B 169, 224–235. ( 10.1007/s003600050215) [DOI] [Google Scholar]
- 33.Tanaka K, Tanaka S. 1997. Winter survival and freeze tolerance in a northern cockroach, Periplaneta japonica (Blattidae: Dictyoptera). Zool. Sci. 14, 849–853. ( 10.2108/zsj.14.849) [DOI] [Google Scholar]
- 34.Olsson T, MacMillan HA, Nyberg N, Staerk D, Malmendal A, Overgaard J. 2016. Hemolymph metabolites and osmolality are tightly linked to cold tolerance of Drosophila species: a comparative study. J. Exp. Biol. 219, 2504–2513. ( 10.1242/jeb.140152) [DOI] [PubMed] [Google Scholar]
- 35.Vesala L, Salminen TS, Koštál V, Zahradníčková H, Hoikkala A. 2012. Myo-inositol as a main metabolite in overwintering flies: seasonal metabolomic profiles and cold stress tolerance in a northern drosophilid fly. J. Exp. Biol. 215, 2891–2897. ( 10.1242/jeb.069948) [DOI] [PubMed] [Google Scholar]
- 36.Koštál V, Korbelová J, Poupardin R, Moos M, Šimek P. 2016. Arginine and proline applied as food additives stimulate high freeze tolerance in larvae of Drosophila melanogaster. J. Exp. Biol. 219, 2358–2367. ( 10.1242/jeb.142158) [DOI] [PubMed] [Google Scholar]
- 37.Des Marteaux LE, Štětina T, Koštál V. 2018. Insect fat body cell morphology and response to cold stress is modulated by acclimation. J. Exp. Biol. 221, jeb.189647 ( 10.1242/jeb.189647) [DOI] [PubMed] [Google Scholar]
- 38.Des Marteaux LE, Stinziano JR, Sinclair BJ.. 2018. Effects of cold acclimation on rectal macromorphology, ultrastructure, and cytoskeletal stability in Gryllus pennsylvanicus crickets. J. Insect Physiol. 104, 15–24. ( 10.1016/j.jinsphys.2017.11.004) [DOI] [PubMed] [Google Scholar]
- 39.Koštál V, Urban T, Řimnáčová L, Berková P, Šimek P. 2013. Seasonal changes in minor membrane phospholipid classes, sterols and tocopherols in overwintering insect, Pyrrhocoris apterus. J. Insect Physiol. 59, 934–941. ( 10.1016/j.jinsphys.2013.06.008) [DOI] [PubMed] [Google Scholar]
- 40.Storey KB, Storey JM. 1988. Freeze tolerance in animals. Physiol. Rev. 68, 27–84. ( 10.1152/physrev.1988.68.1.27) [DOI] [PubMed] [Google Scholar]
- 41.Bäumer A, Duman JG, Havenith M. 2016. Ice nucleation of an insect lipoprotein ice nucleator (LPIN) correlates with retardation of the hydrogen bond dynamics at the myo-inositol ring. Phys. Chem. Chem. Phys. 18, 19 318–19 323. ( 10.1039/C6CP02399A) [DOI] [PubMed] [Google Scholar]
- 42.Crowe JH, Crowe LM, Wolkers WF, Oliver AE, Auh JH, Tang M, Norris J, Tablin F. 2005. Stabilization of dry mammlian cells: lessons from nature. Comp. Physiol. 45, 810–820. [DOI] [PubMed] [Google Scholar]
- 43.Sakurai M, et al. 2008. Vitrification is essential for anhydrobiosis in an African chironomid, Polypedilum vanderplanki. Proc. Natl Acad. Sci. USA 105, 5093–5098. ( 10.1073/pnas.0706197105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson SN. 2003. Trehalose: the insect ‘blood' sugar. In Advances in insect physiology (eds Simpson SJ.), pp. 205–287. Oxford, UK: Elsevier. [Google Scholar]
- 45.Weeda E, Koopmanschap AB, de Kort CAD, Beenakkers AMT. 1980. Proline synthesis in fat body of Leptinotarsa decemlineata. Insect Biochem. 10, 631–636. ( 10.1016/0020-1790(80)90052-9) [DOI] [Google Scholar]
- 46.Kikawada T, Saito A, Kanamori Y, Nakahara Y, Iwata K-I, Tanaka D, Watanabe M, Okuda T. 2007. Trehalose transporter 1, a facilitated and high-capacity trehalose transporter, allows exogenous trehalose uptake into cells. Proc. Natl Acad. Sci. USA 104, 11 585–11 590. ( 10.1073/pnas.0702538104) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.