Abstract
Objective
We aimed at exploring the expression of neuropeptide Y (NPY), omentin and visfatin in adipose tissues of adults along with clinical parameters and hormones.
Methods
We included 168 adult patients (31 surgical obese patients and 31 surgical controls, 76 non-surgical obese patients, 30 non-surgical controls). We measured plasma NPY (by radioimmunoassay), cortisol (with an electrochemiluminescence immunoassay) and urinary cortisol metabolites (by gas chromatography/mass spectrometry). Expression of NPY, omentin and visfatin in subcutaneous and visceral adipose tissue specimens of the surgical patients was quantified using real-time PCR.
Results
NPY was detectable in adipose tissue specimens and, like plasma NPY concentrations, comparable between groups. Omentin gene expression was higher in visceral than in subcutaneous adipose tissues (p < 0.0001). Visfatin expression was lower in the subcutaneous tissue of obese patients compared with controls (p < 0.05). Cortisol was lower in obese adults compared with controls (136.5 ± 74.1 vs. 162.2 ± 56.1 ng/ml; p < 0.05), cortisol metabolites were comparable between groups.
Conclusion
In our obese adults, plasma NPY levels and the glucocorticoid measures were not elevated. Even though the expression of NPY, omentin and visfatin was comparable between obese individuals and controls, we have to consider differences in the total production rate of adipose tissue-derived factors.
Key Words: Adipose tissue, Obesity, Omentin, Neuropeptide Y, Visfatin
Introduction
Obesity is associated with an increased risk for co-morbidities, principally cardiovascular diseases, type 2 diabetes, degenerative joint disease and also certain cancers [1, 2]. The prevalence of obesity is increasing worldwide and affecting ever younger age groups [3, 4, 5]. Basically, treatment options include diet therapy, physical exercise, lifestyle changes and, if indicated, other strategies such as drug therapy and surgical treatment. Laparoscopic gastric banding (LAGB) is a surgical option for extreme obesity in adults with a BMI ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 together with co-morbidities. With bariatric surgery, weight loss of up to 70% of mean excess weight can be achieved in extremely obese patients [6]. In a former study, we have described the metabolic and endocrine profiles of obese patients treated with LAGB compared with normal-weight controls, focusing on leptin and ghrelin plasma concentration and gene expression in their adipose tissues. We could demonstrate in adipose tissue that the expression of anorexigenic leptin is weight-course dependent, but the expression of orexigenic ghrelin is not [7].
In this study we focus on adipose tissue-derived neuropeptide Y (NPY), omentin and visfatin along with the hormonal profiles of NPY, glucocorticoid measures and clinical parameters in obese patients and controls.
NPY consists of 36 amino acids and is one of the most widely distributed neuropeptides in the central nervous system and peripheral sympathetic nervous system [8, 9]. High concentrations of NPY are found in the brain, especially in the hypothalamus, the nucleus accumbens and the amygdala [10, 11]. It is of interest to know that NPY is also expressed and secreted by adipocytes [12]. NPY is an important orexigenic appetite regulator [12], favoring in particular the intake of carbohydrate-rich foods [13]. In addition to its effect on food intake, NPY displays numerous functions such as vasoconstriction [14] and angiogenesis [15], and exerts neuroendocrine effects on fertility [16]. It has been shown that NPY plasma levels are elevated in patients with primary hypertension [17]. A reduction of NPY labeling, indicating alterations in innervation, is detectable in the ureter tissues of patients with congenital ureteropelvic junction obstruction [18]. Moreover, there is evidence for a critical role for NPY in stress-related exaggeration of abdominal obesity, ‘lipo-remodeling’ and the metabolic syndrome [13]. Basically, NPY release is induced by stress. Its expression and secretion is regulated by e.g. sympathetic stimuli and leptin; glucocorticoids also contribute to its actions [13, 16, 19, 20].
Omentin (or omentin-1) is a 313-amino acids peptide and primarily expressed in visceral adipose tissue [21]. In vitro studies have shown that omentin increases insulin signal transduction and enhances glucose transport in human adipocytes. Omentin increases insulin sensitivity [21]. A homologue of omentin-1 was designated as omentin-2. The two genes, omentin-1 and omentin-2, are localized adjacent to each other at 1q22-q23, a chromosomal region recently linked to type 2 diabetes. Omentin plasma levels are reduced in obese patients, together with a reduced expression of both omentin-1 and omentin-2 in visceral fat tissues, as described in a small cohort of 2 men and 18 women [22].
Like omentin, visfatin is an adipocytokine mainly expressed in visceral adipose tissue [23, 24]. Visfatin, a 52-kDa cytokine, is also known as pre-B-cell colony enhancing factor 1 (PBEF1) or nicotinamide phosphoribosyltransferase (Nampt). It enhances cell proliferation and the biosynthesis of nicotinamide mono- and dinucleotide and may also have hypoglycemic effects [24]. In obesity, plasma levels tend to be elevated [23, 24]. In a study with 101 male and female subjects, a significant positive correlation between the amount of visceral fat mass and visfatin plasma levels was found [23]. It has been demonstrated that visfatin is able to bind to the insulin receptor, but visfatin and insulin apparently do not compete for the binding of the insulin receptor, as they ligate to different binding domains [23]. Although visfatin/Nampt/PBEF may induce glucose uptake in skeletal muscle and adipose tissue under certain circumstances, these data remain controversial [23, 25]. Visfatin may exhibit robust nicotinamide adenine dinucleotide biosynthetic activity, which is essential for beta cell function [25].
It is well-known that glucocorticoids play a major role in determining adipose tissue metabolism and distribution. Cortisol is mainly metabolized by the 11β hydroxysteroid dehydrogenase system (11β HSD): Cortisol is activated by 11β HSD1 (from inactive cortisone) and inactivated by 11β HSD2 (by conversion to cortisone).
The overexpression of 11β HSD1 in murine adipose tissue (comparable to the activity found in fat tissue from obese humans) is accompanied by increased levels of corticosterone [26], a glucocorticoid particularly active in rodents. 11β HSD1 overexpression in murine adipose tissue results in visceral obesity, insulin-resistant diabetes mellitus, hyperlipidemia and hyperphagia despite hyperleptinemia [26]. A tissue-specific deregulation of cortisol metabolism, such as an increased adipocyte 11β HSD1 activity, may therefore be involved in the etiology of visceral obesity and the metabolic syndrome.
This study was designed to investigate the expression of NPY, omentin and visfatin in visceral and subcutaneous adipose tissues of obese adults and controls and their possible correlation with clinical parameters such as BMI and blood pressure (BP). We also aim to determine the correlation between these adipose tissue-derived parameters on the one hand and blood NPY, cortisol and the glucocorticoid bioavailability, as reflected in the urinary (allo-THF + THF)/THE ratio, on the other hand.
Participants and Methods
Participants
Our study was approved by the Ethics Committee of the Friedrich-Alexander-University Erlangen-Nuremberg, Germany. The patients gave informed consent prior to the study. Generally, 168 adult patients were recruited and divided into 4 subgroups (surgical obese patients and surgical controls, non-surgical obese patients and non-surgical controls). In detail, we included 31 obese adults (16 women and 15 men, group A; table 1), who underwent LAGB, and 31 age-matched controls (7 women and 24 men, group B), who underwent laparoscopic fundoplication. In addition to these operative subgroups, we included 2 non-operative subgroups consisting of 76 obese patients (52 women and 24 men, group C) and 30 normal-weight (8 women and 22 men, group D) non-surgical adults of whom we could study merely blood and urine samples.
None of the patients suffered from tumors, infectious or psychiatric diseases. Nine obese patients in group A had type 2 diabetes treated with insulin, metformin or glibenclamide. All subjects with type 2 diabetes had fasting glucose concentrations < 6.9 mmol/l whilst on treatment. All obese subjects had severe obesity (morbid obesity, obesity III) with a BMI > 40 kg/m2 or a BMI > 35 kg/m2 (obesity II) and concomitant type 2 diabetes.
Clinical Parameters
Clinical data were compiled during routine outpatient visits or inpatient treatment. In every patient arterial BP was taken at rest using the Dinamap device (Vital Daten Monitor 1846SX, Critikon, Norderstedt, Germany); weight and height were measured, and BMI calculated as body weight (kg) divided by height (m) squared.
Blood and Urine Samples, Adipose Tissue Specimens
Blood was collected in the fasted state at the same time with early morning urine samples. Samples for NPY analysis were transported on ice. After centrifugation, plasma samples were kept frozen at –20 °C till further analysis, as were urine and serum samples. Adipose tissue specimens of 62 surgical adult patients were investigated (group A, B). 31 obese patients had LAGB (Adjustable Gastric Banding System; BioEnterics Corp., Carpinteria, CA, USA), 31 control subjects received laparoscopic fundoplication because of gastroesophageal reflux disease, chronic gastritis and Barrett’s esophagus, similar to LAGB regarding duration of the anesthetic and operative procedure. Visceral adipose tissue specimens were taken from the region of the perigastric fat tissue, and subcutaneous fat tissue samples were taken from the incision site at the trunk. In our non-surgical cohort (group C, D) we could study blood and urine samples only.
NPY Radioimmunoassay
NPY was measured by a radioimmunoassay which was previously established by our group [27, 28]. Briefly, plasma specimens were extracted after dilution with 1% trifluoroacetetic acid using octadecasilyl-silica cartridges (Sep-Pak, Millipore, Eschborn, Germany), and extracts were prepared and measured by radioimmunoassay as described earlier in detail. This assay has a detection limit of 1.0 pmol/l and reaches 50% binding at 8 pmol/l NPY. Radioactive NPY was labeled with Iodine 125 (GE Healthcare, Munich, Germany); polyclonal rabbit antiserum was used in a final dilution of 1:100,000 as reported elsewhere [27]. The radioactivity in each tube (Sarstedt, Nurnbrecht, Germany) was assessed in a multi-crystal gamma-counter (Berthold, Bad Wildbad, Germany).
Analysis of Serum Cortisol and Urinary Steroid Profile
Morning cortisol levels in human serum were measured using a cortisol reagent kit in conjunction with a Roche Cobas e 411 analyzer (Roche Diagnostics, Mannheim, Germany). Briefly, this assay is a competitive electrochemiluminescence immunoassay with a within-run and between-run imprecision of 1.6–2.4% [30]. For the detection of urinary steroid profiles, we performed gas chromatography/mass spectrometry [31]. In detail, we extracted THE, THF and allo-THF in urine samples by the use of C18 SPE columns (Machery-Nagel, Düren, Germany) and elution with methanol [31]. The eluates were dried and hydrolyzed with β-glucuronidase/arylsulfatase (Roche, Penzberg, Germany) in sodium acetate buffer. Following addition of the internal standards androstandiol, coprostane and cortisol-d4, methyloxime-trimethylsilyl ether derivatives were produced by the use of 2% methoxyamine hydrochloride in pyridine and N-methyl-N-trimethylsilyltrifluoracetamide, 1-trimethylsilylimidazole and trimethylchlorsilane. The derivatives were analyzed on a Shimadzu QP5050 gas chromatograph (Shimadzu, Kyoto, Japan) with an integrated mass selective detector and a ZB-5ms column (Phenomenex, Aschaffenburg, Germany). For single ion monitoring we chose the following masses (qualifier ions): m/z 398.5 (488.7, 578.7) THE, m/z 382.5 (652.7, 472.7, 562.7) THF and allo-THF. The interassay coefficients of variation of quality control samples were 10% for THE (mean concentration 2.86 mg/ml), 11% for THF (mean concentration 1.92 mg/ml) and allo-THF (mean concentration 2.11 mg/ml). We here assessed the overall activity of 11β-HSD using the ratio of (allo-THF + THF)/THE.
Analysis of Gene Expression of NPY, Omentin and Visfatin in Adipose Tissues
Adipose tissue specimens (mean weight 0.5 g) were transported in liquid nitrogen and frozen at -80 °C until RNA was isolated. Then we performed reverse transcription of 1 µg of total RNA. In order to monitor gene expression of NPY, omentin and visfatin together with the housekeeping gene β-actin, we used quantitative real-time reverse-transcription PCR [18]. Subsequently, we normalized the quantities of NPY, omentin and visfatin transcripts to the mRNA levels of β-actin. Primer sequences were: NPY (GenBank accession: K01911): 5’-CGG AGG ACA TGG CCA GAT ACT-3’ (sense), 5’-TCC ATA TCT CTG CCT GGT GAT G-3’ (antisense), fluorogenic probe 5’(FAM)-CGG CGC TGC GAC ACT ACA TCA ACC-(TAMRA)3’ (sense); omentin (GenBank accession: AY549722): 5’-AAC GCC TTG TGT GCT GGA AT-3’ (sense), 5’-GTA TCC TCC TCC ACC AAT GCA-3’ (antisense), fluorogenic probe 5’(FAM)- TCA CCG GAT GTA ACA CTG AGC ACC A-(TAMRA)-3’ (sense); visfatin (GenBank accession: NM_021524): 5’-GGC CTT GGG ATT AAC GTCTTC-3’ (sense), 5’-AAT CGG CCC TTT TTG GAC C-3’ (antisense), fluorogenic probe 5’(FAM)-AGG ACC CAG TTG CTG ATC CCA ACA AA-(TAMRA)3’ (sense); β-actin (GenBank accession: M10277): 5’-CCG CGA GAA GAT GAC CCA G-3’ (sense), 5’-CCA GTG GTA CGG CCA GAG G-3’ (antisense), fluorogenic probe 5’(FAM)- CCA GCC ATG TAC GTT GCT ATC CAG GC-(TAMRA)3’ (sense). Each fluorogenic probe was marked with a reporter dye, FAM (6-carboxy-fluorescein), and a quenching dye, TAMRA (6-carboxy-tetram-ethyl-rhodamine). Further analytical details are reported elsewhere [29].
Statistical Analysis
To analyze our data, we used GraphPad Prism software 4.0 (San Diego, CA, USA). If not otherwise stated, values are given as mean ± standard deviation (SD). We calculated Spearman’s correlation coefficient and linear regression with a 95% confidence interval, if applicable. In order to assess differences or similarities, we used the Mann Whitney test (nonparametric t-test). A p value < 0.05 was considered significant.
Results
Clinical Parameters
Basically, in obese patients, not only BMI was significantly higher compared with controls (p < 0.0001) but also systolic (p < 0.0001) and diastolic (p < 0.05) BP (table 1). Moreover, we found a significant correlation between BMI and BP. In detail, BMI was positively correlated with systolic BP (r = 0.3296, p < 0.001) and diastolic BP (r = 0.3183, p < 0.01) in the entire cohort.
Table 1.
Distribution of patients’ sex, age, BMI and blood pressure (BP) for the surgical and nonsurgical cohorts
| Group A operative obese patients | Group B operative controls | Group C non-operative obese patients | Group D non-operative controls |
|
|---|---|---|---|---|
| Number of patients | 31 | 31 | 76 | 30 |
| Sex female/male (number) | 16/15 | 7/24 | 52/24 | 8/22 |
| Age, years (mean ± SD) | 40.8 ± 10.1 | 45.0 ± 14.3 (n.s.) | 39.1 ± 10.4** | 48.1 ± 15.4 |
| BMI, kg/m2 (mean ± SD) | 48.2 ± 6.4**** | 27.7 ± 3.5 | 49.1 ± 8.8**** | 26.7 ± 4.1 |
| Systolic BP, mm Hg (mean ± SD) | 142.9 ± 15.3**** | 128.1 ± 16.8 | 138.9 ± 15.6** | 128.7 ± 17.0 |
| Diastolic BP, mm Hg (mean ± SD) | 88.9 ± 9.6*** | 80.0 ± 9.3 | 86.6 ± 9.3* | 80.9 ± 8.3 |
| Visceral adipose tissue samples (number) | 31 | 29 | N/A | N/A |
| Subcutaneous adipose tissue samples (number) | 31 | 30 | vN/A | N/A |
N/A = Not available; n.s. = not significant.
a Data are given as mean ± SD.
p < 0.05,
p < 0.01,
p < 0.001,
p < 0.0001 compared with controls.
Plasma NPY Concentrations
In the entire cohort, NPY plasma concentrations ranged from ≤1.0–13.3 pg/ml. In detail, mean NPY plasma concentrations (± SD) were 3.8 ± 2.3 pg/ml for obese adults (range ≤1.0–12.5 pg/ml) versus 3.9 ± 3.5 pg/ml for controls (range ≤1.0–13.3 pg/ml).
To our surprise, there was no significant correlation between plasma NPY concentrations and systolic or diastolic BP, serum cortisol levels, urinary cortisol metabolites or BMI in either group (data not shown).
Serum Cortisol Levels and Urinary Cortisol Metabolites
Circulating cortisol concentrations were somewhat lower in obese than in non-obese patients. In detail, in obese patients, morning serum cortisol levels were 137 ± 74 ng/ml (range 40–375 ng/ml) versus 162 ± 56 for controls (range 70–264 ng/ml), p < 0.05.
Urinary cortisol metabolites were not significantly different between groups. Next, we calculated the urinary (allo-THF + THF)/THE ratio, which reflects the activity of the enzyme 11β HSD.
For obese patients, the (allo-THF + THF)/THE ratio was 1.13 ± 0.58 (range 0.65–3.90) compared to 1.16 ± 0.35 for controls (range 0.60–2.00). This finding suggests that the bioavailability of cortisol within tissues (renal) was not increased in the group of obese patients compared with controls.
Gene Expression of NPY, Omentin and Visfatin in Visceral and Subcutaneous Adipose Tissues and Correlation with Other Parameters
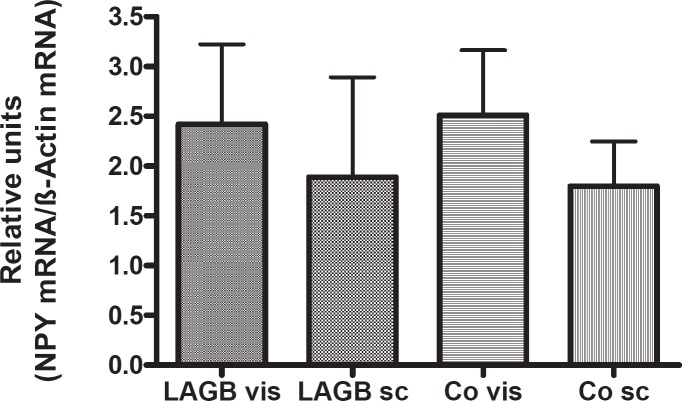
NPY gene expression was found in visceral and subcutaneous adipose tissues of obese or normal-weight adults in comparable quantities (fig. 1). Moreover, it was similar for male and female patients (data not shown). NPY gene expression did not correlate with either systolic/diastolic BP or BMI in our patients. In this study NPY gene expression in adipose tissue did not correlate with NPY plasma concentrations.
Fig. 1.
NPY gene expression normalized to β-actin for reference in visceral (vis) and subcutaneous (sc) adipose tissue specimens of adult patients (LAGB = gastric banding group (n = 31); Co = controls (n = 31)); no significant differences between groups.
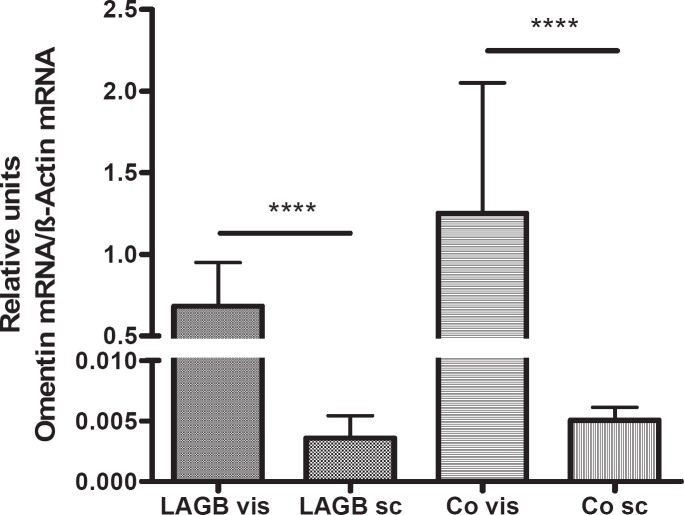
Omentin gene expression was much higher in visceral than in subcutaneous adipose tissues of obese and normal-weight adults (p < 0.0001) (fig. 2). Its expression was slightly but not significantly decreased in visceral adipose tissues of obese adults compared with controls. Omentin gene expression did not correlate with BP values. In obese males, but not in females, we found a negative correlation between visceral omentin gene expression and BMI (r = –0.5516, p < 0.05). Omentin gene expression correlated with visfatin gene expression in visceral adipose tissue (r = 0.3945, p < 0.01) and, to a lesser extent, in subcutaneous adipose tissue samples (r = 0.5050, p < 0.001). There was a positive correlation between subcutaneous omentin gene expression and subcutaneous visfatin gene expression in all obese subjects (r = 0.5050, p < 0.001), particularly in obese females (r = 0.8872, p = 0.0001).
Fig. 2.
Omentin gene expression normalized to β-actin for reference in visceral (vis) and subcutaneous (sc) adipose tissue specimens of adult patients (LAGB = gastric banding group (n = 31); Co = controls (n = 31)). ****p < 0.0001 for the omentin gene expression in visceral compared with subcutaneous adipose tissues. Omentin gene expression was slightly, but not significantly decreased in visceral adipose tissues of obese patients compared with controls.
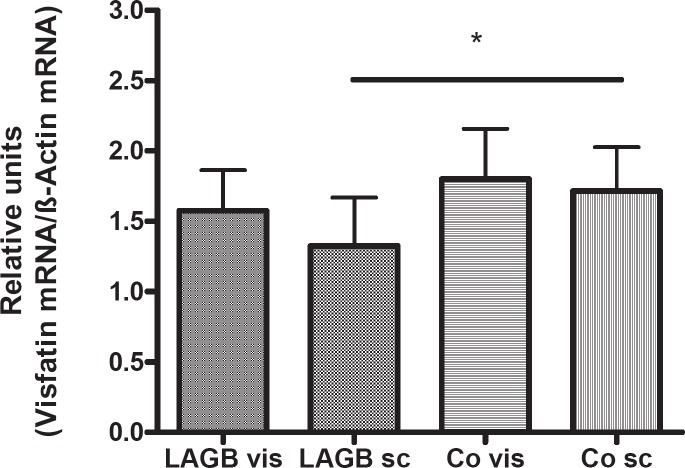
Visfatin expression in adipose tissues was slightly higher in controls (fig. 3), with no significant difference between female and male subjects (data not shown). Visfatin gene expression in subcutaneous adipose tissue of normal-weight controls was significantly higher than in samples of obese adults (p < 0.05), (fig. 3). Visceral visfatin gene expression was negatively correlated with systolic blood pressure (r = –0.7164, p < 0.01) in obese male patients. We found a positive correlation between NPY and visfatin gene expression in visceral adipose tissue in obese female (r = 0.6727, p < 0.05) and male patients (r = 0.7846, p < 0.001). There was a positive correlation between NPY and visfatin gene expression in subcutaneous fat tissue for female controls (r = 0.89, p < 0.05) and as a trend for male controls (r = 0.4361, p = 0.055).
Fig. 3.
Visfatin gene expression normalized to β-actin for reference in visceral (vis) and subcutaneous (sc) adipose tissue specimens of adult patients (LAGB = gastric banding group (n = 31); Co = controls (n = 31)). Visfatin gene expression was decreased in subcutaneous adipose tissues of obese patients compared with controls (*p < 0.05).
Discussion
The purpose of our study was to investigate the expression of NPY, omentin and visfatin in visceral and subcutaneous adipose tissues in humans and to analyze a possible correlation with clinical parameters and hormonal profiles in obese patients and controls.
Basically, we found positive correlations between BMI and systolic and diastolic BP, indicative of an increased cardiovascular risk in obese patients [32, 33]. However, in our obese patients, peripheral plasma NPY concentrations were not significantly different from those of the controls. The central expression of NPY is inhibited by leptin; it is supposed that the suppression of NPY results in a reduction of food intake, an increase of energy expenditure and in a change of peripheral metabolic status [34]. However, we can only speculate about the NPY levels in the nervous system and whether our findings may be in line with central leptin resistance as this is beyond the scope of our study. NPY gene expression in adipose tissues was comparable in obese patients and controls and also in men and women. Considering the huge differences in total body fat, total NPY production and tissue concentrations may be higher in obese patients and associated with increased sympathetic nervous activity and increased total peripheral vascular resistance [35]. In our study, NPY plasma concentrations did not differ between groups. One possible reason for this finding could be that our obese patients were otherwise apparently healthy; only 9 obese patients had type 2 diabetes and were treated with antidiabetic drugs. In addition, we did not focus on NPY gene polymorphisms or catecholamine concentrations which could provide us with further information regarding, e.g., sympathetic nerve activity.
Omentin is mainly expressed in visceral adipose tissue [21, 36]. However, we were able to demonstrate omentin gene expression in subcutaneous adipose tissue, too, although at much lower amounts compared with visceral fat. De Souza Batista et al. [22] described a negative correlation between omentin gene expression and BMI. We also found a negative correlation between visceral omentin gene expression and BMI, particularly in obese males. As omentin plasma concentrations are reduced in obese patients [22], a down-regulation of omentin-1 may contribute to the insulin-resistant state in obese individuals, particularly in males with visceral obesity.
Next, we confirmed that visfatin/PBEF/Nampt is expressed in adipose tissue of obese and normal-weight patients. Basically, visfatin is proposed to exert insulin-mimicking effects and to attenuate insulin resistance. It is particularly important for the biosynthesis of nicotinamide adenine dinucleotide [37] and acts as pre-B cell colony enhancing factor [38], but there are contradictory data regarding the mechanisms underlying its putative beneficial effects on insulin sensitivity [25, 39–41]. Whereas Fukuhara et al. [23] reported in 2006 that visfatin had an insulin-mimetic effect, binding to the insulin receptor and leading to the reduction of plasma glucose concentrations, Revollo et al. [25] reported in 2007 that visfatin does not have insulin-mimetic effects neither in vitro nor in vivo, but regulates the biosynthesis of NAD. As a possible explanation for the diverse findings, a very recent study investigated the possibility that single nucleotide polymorphisms (SNPs) in the visfatin gene may be associated with either obesity or type 2 diabetes [42]. Blakemore et al. [42] described one rare SNP, rs10487818, located in intron 4 of the visfatin gene which was associated with severe obesity and had lower allele frequency in controls.
At last, we focused on cortisol metabolites. It has been demonstrated that an increased activity of 11β HSD1, which regenerates cortisol from cortisone, causes hyperphagia along with visceral obesity and metabolic complications in mice [26]. However, it is of interest to show that morning serum cortisol levels were significantly lower in our obese patients than in controls. A previous study has also demonstrated that serum cortisol concentrations may be lower in obese than in normal-weight adults [43]. Excess cortisol, therefore, is not a consistent finding in human obesity, but cortisol production rates may be altered in the course of the disorder. Along these lines a recent study demonstrates that increased 11β HSD-1 gene expression in subcutaneous fat is a consequence rather than cause of obesity, particularly in male patients [44].
Our study corroborates that visceral and subcutaneous adipose tissues produce NPY, omentin and visfatin with only subtle alterations in obese subjects.
As obesity in humans is a complex and multifactorial disease with a huge spectrum, parameters such as adipocy-tokines, NPY or cortisol must be considered within the frame of a large variety of other biomarkers [45]. Further investigations involving a systemic biology approach, combining cell culture techniques (including primary adipocytes) with genomics, proteomics and metabolomics, will clarify links between adipose tissue and metabolic disease.
Disclosure
The authors declare that there is no conflict of interests
Acknowledgements
The authors are deeply grateful to all patients for their participation. We acknowledge the staff of the Department of Surgery, University of Erlangen-Nuremberg, for their important contribution in collecting the samples. We wish to thank Tina Vogler for excellent technical assistance and Patricia Schmid for valuable help and stimulating discussion. The research described here has been supported by the APE ‘Klaus Kruse Grant’ to I.K.
References
- 1.Bessesen DH. Update on obesity. J Clin Endocrinol Metab. 2008;93:2027–2034. doi: 10.1210/jc.2008-0520. [DOI] [PubMed] [Google Scholar]
- 2.Alastair JJ, Wood MD. Obesity. N Engl J Med. 2002;346:591–602. doi: 10.1056/NEJMra012586. [DOI] [PubMed] [Google Scholar]
- 3.Sorof J, Daniels S. Obesity Hypertension in children. Hypertension. 2002;40:441–447. doi: 10.1161/01.hyp.0000032940.33466.12. [DOI] [PubMed] [Google Scholar]
- 4.Meigen CKeller A, Gausche R, Kromeyer-Hauschild K, Blüher S, Kiess W, Keller E. Secular trends in body mass index in German children and adolescents: a cross-sectional data analysis via CrescNet between 1999 and 2006. Metabolism. 2008;57:934–939. doi: 10.1016/j.metabol.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Rudolf MCJSahota P, Barth JH, Walker J. Increasing prevalence of obesity in primary school children: cohort study. BMJ. 2001;322:1094–1095. doi: 10.1136/bmj.322.7294.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchwald HAvidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 7.Knerr IHerzog D, Rauh M, Rascher W, Horbach T. Leptin and ghrelin expression in adipose tissues and serum levels in gastric banding patients. Eur J Clin Invest. 2006;36:389–394. doi: 10.1111/j.1365-2362.2006.01642.x. [DOI] [PubMed] [Google Scholar]
- 8.Baltatzi MHatzitolios A, Tziomalos K, Iliadis F, Zamboulis CH. Neuropeptide Y and alpha-melanocyte-stimulating hormone: interaction in obesity and possible role in the development of hypertension. Int J Clin Pract. 2008;62:1432–1440. doi: 10.1111/j.1742-1241.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 9.Sulyok E, Tulassay T. Natriuresis of fasting: the possible role of leptin-neuropeptide Y system. Med Hypotheses. 2001;56:629–633. doi: 10.1054/mehy.2000.1176. [DOI] [PubMed] [Google Scholar]
- 10.Chronwall BM. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides. 1985;6((suppl 2)):1–11. doi: 10.1016/0196-9781(85)90128-7. [DOI] [PubMed] [Google Scholar]
- 11.O’Donohue TLChronwall BM, Pruss RM, Mezey E, Kiss JZ, Eiden LE, Massari VJ, Tessel RE, Pickel VM, DiMaggio DA, et al. Neuropeptide Y and peptide YY neuronal and endocrine system. Peptides. 1985;6:755–768. doi: 10.1016/0196-9781(85)90180-9. [DOI] [PubMed] [Google Scholar]
- 12.Kos K, Harte A, James S, Snead D, O’Hare J, McTernan P, Kumar S. Secretion of neuropeptide Y in human adipose tissue and its role in maintenance of adipose tissue mass. Am J Physiol Endocrinol Metab. 2007;293:E1335–E1340. doi: 10.1152/ajpendo.00333.2007. [DOI] [PubMed] [Google Scholar]
- 13.Kuo LKitlinska JTilan J, Li L, Baker S, Johnson M, Lee E, Burnett M, Fricke S, Kvetnansky R, Herzog H, Zukowska Z. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 14.Hanko JHTornebrandt K, Hardebo JE, Kalstrom J, Nobin A, Owman CH. Neuropeptide Y induces and modulates vasoconstriction in intracranial and peripheral vessels of animals and men. J Auton Pharmacol. 1986;52:175–179. doi: 10.1111/j.1474-8673.1986.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 15.AbeK, Tilan JU, Zukowska Z. NPY and NPY receptors in vascular remodeling. Curr Top Med Chem. 2007;7:1704–1709. doi: 10.2174/156802607782340948. [DOI] [PubMed] [Google Scholar]
- 16.Crown AClifton DK, Steiner RA. Neuropeptide signaling in the integration of metabolism and reproduction. Neuroendocrinology. 2007;86:175–182. doi: 10.1159/000109095. [DOI] [PubMed] [Google Scholar]
- 17.Lettgen BWagner S, Hänze J, Lang RE, Rascher W. Elevated plasma concentration of neuropeptide Y in adolescents with primary hypertension. J Hum Hypertens. 1994;8:345–349. [PubMed] [Google Scholar]
- 18.Knerr IDittrich K, Miller J, Kummer W, Rösch W, Weidner W, Rascher W. Alteration of neuronal and endothelial nitric oxide synthase and neuropeptide Y in congenital ureteropelvic junction obstruction. Urol Res. 2001;29:134–140. doi: 10.1007/s002400000165. [DOI] [PubMed] [Google Scholar]
- 19.Halaas JLGajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 20.Smith FJCampfield LA, Moschera JA, Ballon PS, Burn P. Feeding inhibition by neuropeptide Y. Nature. 1996:382–307. doi: 10.1038/382307a0. [DOI] [PubMed] [Google Scholar]
- 21.Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, Shuldiner AR, Fried SK, McLenithan JC, Gong DW. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–E1261. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 22.De Souza Batista CMYang RZLee MJ, Glynn NM, Yu DZ, Pray J, Ndubuizu K, Patil S, Schwartz A, Kligman M, Fried SK, Gong DW, Shuldiner AR, Pollin TI, McLenithan JC. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–1661. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 23.Fukuhara AMatsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 24.Gualillo OGonzález-Juanatey JR, Lago F. The emerging role of adipokines as mediators of cardiovascular function: physiologic and clinical perspectives. Trends Cardiovasc Med. 2007;17:275–283. doi: 10.1016/j.tcm.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Revollo JRKörner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masuzaki HPaterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 27.Rascher WKremens B, Wagner S, Feth F, Hunneman DH, Lang RE. Serial measurements of neuropeptide Y in plasma for monitoring neuroblastoma in children. J Pediatr. 1993;122:914–916. doi: 10.1016/s0022-3476(09)90018-x. [DOI] [PubMed] [Google Scholar]
- 28.Bald MGerigk M, Rascher W. Elevated plasma concentrations of neuropeptide Y in children and adults with chronic and terminal renal failure. Am J Kidney Dis. 1997;30:23–27. doi: 10.1016/s0272-6386(97)90560-6. [DOI] [PubMed] [Google Scholar]
- 29.Knerr I, Dachert C, Beinder E, Metzler M, Dötsch J, Repp R, Rascher W. Adrenomedullin calcitonin gene-related peptide and their receptors: evidence for a decreased placental mRNA content in preeclampsia and HELLP syndrome. Eur J Obstet Gynecol Reprod Biol. 2002;101:47–53. doi: 10.1016/s0301-2115(01)00519-x. [DOI] [PubMed] [Google Scholar]
- 30.Yu V, Tudor Y, Hale C, Plant M, Kim KW, Wang M, Nguyen Y, Miguel TS, Chen M, Nybo R, Baumgartner J, Kurzeja RJ, Powers D. High capacity homogeneous non-radioactive cortisol detection assays for human 11beta-hydro teroid dehydrogenase type 1. Assay Drug Dev Technol. 2007;5:105–115. doi: 10.1089/adt.2006.047. [DOI] [PubMed] [Google Scholar]
- 31.Plank CMeißner U, Rauh M, Wollmann H, Dörr HG, Rascher W, Dötsch J. Cortisol-cortisone ratios in small for gestational age (SGA) children without postnatal catch-up growth. Clin Endocrinology. 2007;67:304–309. doi: 10.1111/j.1365-2265.2007.02884.x. [DOI] [PubMed] [Google Scholar]
- 32.National Task Force on the Prevention and Treatment of Obesity Overweight obesity, and health risk. Arch Intern Med. 2000;160:898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- 33.Montague CT, O’Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49:883–888. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- 34.Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A, et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377:530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- 35.Walker PHGrouzmann E, Burnier M, Wheber B. The role of neuropeptide Y in cardiovascular regulation. Trends Pharmacol Sci. 1991;12:111–115. doi: 10.1016/0165-6147(91)90518-w. [DOI] [PubMed] [Google Scholar]
- 36.Fain JNSacks HS, Buehrer B, Bahouth SW, Garrett E, Wolf RY, Carter RA, Tichansky DS, Madan AK. Identification of omentin mRNA in human epicardial adipose tissue: comparison to omentin in subcutaneousinternal mammary artery periadventitial and visceral abdominal depots. Int J Obes (Lond) 2008;32:810–815. doi: 10.1038/sj.ijo.0803790. [DOI] [PubMed] [Google Scholar]
- 37.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 38.Samal BSun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arner P. Visfatin – a true or false trail to type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:28–30. doi: 10.1210/jc.2005-2391. [DOI] [PubMed] [Google Scholar]
- 40.Stephens JM, Vidal-Puig AJ. An update on visfatin/pre-B cell colony-enhancing factor an ubiquitously expressed illusive cytokine that is regulated in obesity. Curr Opin Lipidol. 2006;17:128–131. doi: 10.1097/01.mol.0000217893.77746.4b. [DOI] [PubMed] [Google Scholar]
- 41.Sethi JK. Is PBEF/visfatin/Nampt an authentic adipokine relevant to the metabolic syndrome? Curr Hypertens Rep. 2007;9:33–38. doi: 10.1007/s11906-007-0007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blakemore AIMeyre D, Delplanque J, Vatin V, Lecoeur C, Marre M, Tichet J, Balkau B, Froguel P, Walley AJ. Rare variant in the visfatin gene (NAMPT/PBEF1) is associated with protection from obesity. Obesity. 2009;17:1549–1553. doi: 10.1038/oby.2009.75. [DOI] [PubMed] [Google Scholar]
- 43.Travison TGO’Donnell AB, Araujo AB, Matsumoto AM, McKinlay JB. Cortisol levels and measures of body composition in middle-aged and older men. Clin Endocrinol. 2007;67:71–77. doi: 10.1111/j.1365-2265.2007.02837.x. [DOI] [PubMed] [Google Scholar]
- 44.Purnell JQ, Kahn SE, Samuels MH, Brandon D, Loriaux DL, Brunzell JD. Enhanced cortisol production ratesfree cortisol and 11beta-HSD-1 expression correlate with visceral fat and insulin resistance in men: effect of weight loss. Am J Physiol Endocrinol Metab. 2009;296:E351–E357. doi: 10.1152/ajpendo.90769.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eyzaguirre F, Mericq V. Insulin resistance markers in children. Horm Res. 2009;71:65–74. doi: 10.1159/000183894. [DOI] [PubMed] [Google Scholar]