Abstract
Objective
To clarify the relationship between the amount of mid-thigh subcutaneous adipose tissue (SCAT) and glucose tolerance in men and women.
Methods
Midthigh and abdominal computed tomography (CT) scans were obtained from 63 men and 110 women from the Quebec Family Study. Areas and attenuations of adipose tissue compartments and skeletal muscle measured from CT scans were related to glycemia and insulinemia values from an oral glucose tolerance test (OGTT).
Results
Adjusted for age and fat mass or age and percent fat, negative relationships (all p < 0.05) between the surface area of mid-thigh SCAT and OGTT data (glucose and insulin area under the curve, glycemia and insulin at 120 min) were seen in men (r range –0.22 to –0.37) and women (r range –0.20 to –0.30). Similar but weaker tendencies were observed when correcting for visceral adiposity. Correlations of OGTT variables with ratios of midthigh SCAT to abdominal visceral adipose tissue and to fat mass revealed significant negative relationships in both genders. Tertile analyses showed better glucose handling in subjects with a higher content of mid-thigh SCAT.
Conclusion
These data suggest that the preferential deposition of adipose tissue as mid-thigh SCAT is a strategy to prevent glucose intolerance.
Key Words: Computed tomography, Skeletal muscle, Insulin sensitivity, Abdominal obesity, Human
Introduction
Obesity is associated with numerous health problems, including type 2 diabetes mellitus, hyperlipidemia, hypertension, atherosclerosis, and cardiovascular disease. Furthermore, obesity is a heterogeneous condition characterized by differences in the regional distribution of adipose tissue. Taking these differences into account is important in order to understand how body fatness may affect metabolism and lead to various undesirable conditions [1].
Central abdominal fat accumulation has long been suggested to be a principal promoter of metabolic disorders [2, 3, 4, 5, 6]. This association tends to be stronger with omental fat [7, 8]. It appears that a more peripheral accumulation of adipose tissue has fewer adverse metabolic effects as demonstrated in a study by Snijder et al. [9]. In the latter study, it was shown that low subcutaneous thigh fat is a risk factor for unfavorable glucose and lipid levels independently of abdominal fat.
Abdominal deep subcutaneous [10] and visceral adiposity [7, 11, 12] have both been shown to be correlated with impaired glucose metabolism. However, the association is weaker with gynoid obesity [13, 14], suggesting that peripheral adipose tissue may protect against some of the negative effects of central adiposity. Simple anthropometric measurements have been traditionally used for studies addressing this problem [15]. More specifically, hip and thigh circumferences were associated with a lower risk of type 2 diabetes mellitus independently of body mass index (BMI), age, and waist circumference. However, these measures do not differentiate subcutaneous adipose tissue from visceral depots. It is undoubtedly one of the reasons why we have limited knowledge on the relationship between different thigh adipose tissue compartments (subcutaneous and intra-fascial (deep)) and glucose metabolism, and on the mechanisms underlying such associations if they exist. Even though a previous study [9] found an independent association of subcutaneous thigh adipose tissue with a more favorable glycemia in men, the data were from an aging study where all participants were over 70 years of age. Since data is limited in younger adults, we explored this phenomenon in individuals aged from 20 to 65 years of age. In order to explain why one type of fat distribution is less deleterious than the other, we report here on the relationships between adiposity (from computed tomography (CT)) and indicators of glucose metabolism obtained from oral glucose tolerance tests (OGTTs).
Subjects and Methods
Subjects
This study is based on data obtained from 63 men and 110 women from the Quebec Family Study for whom CT scan and OGTT data were available (table 1). Subjects were Caucasians of French Canadian descent aged from 20 to 65 years, sedentary, and had no prior diagnosis of abnormal glucose tolerance. Subjects displayed a wide range of BMI (men 17–53 kg/m2, women 17–64 kg/m2) and fat mass (FM) (men 3.5–69.8 kg, women 5.6–78.0 kg). Physically active subjects (defined as taking part in leisure or work activities rated over 8 metabolic equivalents (METS)) were excluded based on an analysis of a physical activity diary as previously described [16]. We excluded physically active subjects because of the beneficial effects of activity per se on glucose metabolism, which would introduce an important confounding factor in this study. Subjects diagnosed as having diabetes were also excluded. The study was approved by the Laval University Medical Ethics Committee, and written informed consent was obtained from each participant.
Table 1.
Descriptive statistics for men and women (data are presented as mean ± SD)
| Variable | Men | Women |
|---|---|---|
| Number | 63 | 110 |
| Age, years | 43 ± 15 | 41 ± 15 |
| BMI, kg/m2 | 27.8 ± 6.1 | 29.6 ± 8.3 |
| FM, kg | 21.4 ± 12.7 | 28.8 ± 15.1 |
| FFM, kg | 63.1 ± 9.0 | 48.0 ± 7.1 |
| Mid-thigh SCAT, cm2 | 92 ± 47 | 187 ± 72 |
| Mid-thigh muscle surface, cm2 | 162 ± 28 | 112 ± 18 |
| Abdominal VAT, cm2 | 147 ± 92 | 119 ± 80 |
| ASAT, cm2 | 249 ± 152 | 382 ± 179 |
ASAT = Abdominal subcutaneous adipose tissue; FFM = fat-free mass; FM = fat mass; SCAT = subcutaneous adipose tissue; VAT = visceral adipose tissue
Computed Tomography
All subjects underwent a CT scan in order to quantify the distribution of body fat. The CT machine was calibrated daily, and cross-sectional measurements of adipose tissue and muscle were obtained for the mid-thigh and abdomen. All scans were performed within a 15-min period following positioning on the scanning bed, and the order of the scans (abdominal first, mid-thigh last) was the same for all subjects. The abdominal slice was taken at the L4–L5 level. Adipose tissue components were calculated by delineating the areas of interest with an attenuation range of –190 to –30 Hounsfield units. The normal range for muscle attenuation was from 35 to 100 Hounsfield units. Muscle registering between 0 and 34 Hounsfield units was considered as having low attenuation and defined as rich in lipids.
Glucose Metabolism
The subject’s response to oral glucose ingestion was measured by OGTT. Subjects drank a 75 g glucose solution, and blood samplings were drawn every 30 min over 2 h. Glucose area under the curve (AUC), glycemia at 120 min, insulin AUC and insulin at 120 min were considered as primary variables. Plasma glucose was measured enzymatically, whereas plasma insulin was measured by radioimmunoassay with polyethylene glycol separation [17, 18].
Body Composition
Body weight was measured to the nearest 0.1 kg using a calibrated balance including a tension gauge (Intertechnology Inc., Don Mills, ON, Canada) and a Digital Panel Indicator (Beckman industrial series 600; Beckman Coulter Canada Inc., Mississauga, ON, Canada). Standing height was measured to the nearest millimeter using a wall stadiometer. Lean body mass and FM were evaluated by the hydrostatic weighing technique as previously described [19].
Statistical Analysis
All statistical analyses were performed using SAS statistical software (version 9.1; SAS Institute Inc., Cary, NC, USA). Data are presented as means ± SD. Student’s t-tests were used for group comparisons. Pearson correlations were used to quantify the relationships between indicators of body composition (including ratios of mid-thigh SCAT to visceral fat and mid-thigh SCAT to FM) and glucose metabolism. Adjustments for percent fat and FM were performed with the GLM procedure in SAS. ANOVAs were used to examine differences across tertiles.
Results
Mid-Thigh Adipose Tissue
In men, significant and positive relationships between the areas of sub-fascial and mid-thigh subcutaneous adipose tissue (SCAT) and markers of glucose tolerance were observed with data adjusted for age (tables 4 and 5). With further adjustment for FM, the relationships reversed for most phenotypes (tables 2, 3, 4, 5), but these became mostly nonsignificant after correction for abdominal visceral adipose tissue (VAT). In women, when data were adjusted for age alone, the relationships were similar to those observed in men: a greater area of thigh adipose tissue related to high blood glucose levels. When the correlations were adjusted for FM and abdominal VAT, the relationship between mid-thigh SCAT surface area and glucose tolerance indicators reversed to become negative, indicating that high thigh lipid content was associated with better glucose tolerance. However, this observation was only found in women and only for this particular compartment of thigh adipose tissue. Improved glucose tolerance in women at a given level of visceral adiposity is thus seen with high levels of subcutaneous thigh adipose tissue (tables 2, 3, 5).
Muscle Attenuation
In men, CT-assessed muscle attenuations were found to correlate with glucose metabolism phenotypes adjusted for age. Low whole muscle (average) attenuation values, indicating a high muscle lipid content, were associated with elevated values of glucose AUC (table 2) and glycemia at 120 min, both indicators of poor glucose tolerance (table 3), and with insulin AUC (table 4) and insulin at 120 min, indicators of insulin resistance (table 5). Moreover, the surface area of fat-rich low-attenuation muscle (the fraction of the whole muscle with an attenuation signal between 0 and 34 Hounsfield units) was negatively correlated with glucose tolerance. These relationships became nonsignificant when corrected for whole-body FM or abdominal VAT. This is concordant with the observation that muscle attenuation and quantity of low attenuation muscle are correlated with whole-body or regional adiposity. In women, weaker relationships between muscle characteristics and glucose metabolism were observed, and the correlations became nonsignificant after adjustment for FM and abdominal VAT.
Table 2.
Pearson correlation coefficients (r values) between OGTT glucose area under the curve and measures of mid-thigh soft tissue compositiona
| Mid-thigh CT scan | Men |
Women |
||||
|---|---|---|---|---|---|---|
| age adjusted | adjusted for age and FM | adjusted for age and visceral adiposity | age adjusted | adjusted for age and FM | adjusted for age and visceral adiposity | |
| Muscle | ||||||
| Total muscle | ||||||
| Attenuation | –0.244 | –0.067 | –0.074 | –0.181 | –0.063 | –0.048 |
| Surface area | 0.115 | 0.005 | 0.006 | 0.198 | 0.130 | 0.033 |
| Normal attenuation | ||||||
| Attenuation | –0.100 | –0.209 | –0.223 | –0.005 | –0.043 | –0.131 |
| Surface area | 0.018 | 0.020 | 0.031 | 0.080 | 0.100 | 0.077 |
| Low attenuation (‘fatty’) | ||||||
| Mean value | –0.162 | –0.011 | –0.069 | –0.000 | 0.002 | 0.171 |
| Surface area | 0.198 | –0.036 | –0.056 | 0.179 | 0.055 | –0.089 |
| Adipose tissue area | ||||||
| Total | 0.162 | –0.371 | –0.160 | 0.066 | –0.275 | –0.230 |
| Subcutaneous | 0.156 | –0.353 | –0.162 | 0.056 | –0.284 | –0.234 |
| Subfascial | 0.195 | –0.156 | –0.050 | 0.238 | 0.204 | –0.012 |
Values in italics are statistically significant (p < 0.05).
Table 3.
Pearson correlation coefficients (r values) between OGTT glycemia at 120 min and measures of mid-thigh soft tissue compositiona
| Mid-thigh CT scan | Men |
Women |
||||
|---|---|---|---|---|---|---|
| age adjusted | adjusted for age and FM | adjusted for age and visceral adiposity | age adjusted | adjusted for age and FM | adjusted for age and visceral adiposity | |
| Muscle | ||||||
| Total muscle | ||||||
| Attenuation | –0.267 | –0.146 | –0.140 | –0.177 | –0.070 | –0.048 |
| Surface area | 0.105 | 0.006 | –0.038 | 0.190 | 0.124 | 0.028 |
| Normal attenuation | ||||||
| Attenuation | –0.112 | –0.267 | –0.276 | 0.034 | –0.007 | –0.093 |
| Surface area | 0.003 | 0.002 | –0.024 | 0.057 | 0.078 | 0.055 |
| Low attenuation (‘fatty’) | ||||||
| Mean value | –0.128 | 0.018 | –0.035 | –0.017 | –0.016 | 0.165 |
| Surface area | 0.196 | 0.007 | –0.045 | 0.189 | 0.076 | –0.071 |
| Adipose tissue area | ||||||
| Total | 0.124 | –0.354 | –0.167 | 0.074 | –0.234 | –0.216 |
| Subcutaneous | 0.118 | –0.326 | –0.161 | 0.064 | –0.243 | –0.220 |
| Subfascial | 0.147 | –0.159 | –0.120 | 0.240 | 0.205 | –0.007 |
Values in italics are statistically significant (p < 0.05).
Table 4.
Pearson correlation coefficients (r values) between OGTT insulin area under the curve and measures of mid-thigh soft tissue compositiona
| Mid-thigh CT scan | Men |
Women |
||||
|---|---|---|---|---|---|---|
| age adjusted | adjusted for age and FM | adjusted for age and visceral adiposity | age adjusted | adjusted for age and FM | adjusted for age and visceral adiposity | |
| Muscle | ||||||
| Total muscle | ||||||
| Attenuation | –0.289 | 0.008 | –0.038 | –0.211 | –0.018 | –0.018 |
| Surface area | 0.104 | –0.044 | –0.099 | 0.221 | 0.098 | –0.022 |
| Normal attenuation | ||||||
| Attenuation | –0.011 | –0.264 | –0.201 | 0.167 | 0.098 | 0.010 |
| Surface area | –0.052 | 0.004 | –0.069 | 0.027 | 0.070 | 0.021 |
| Low attenuation (‘fatty’) | ||||||
| Mean value | –0.083 | 0.161 | 0.069 | –0.140 | –0.067 | 0.080 |
| Surface area | 0.296 | –0.120 | –0.095 | 0.251 | –0.026 | –0.136 |
| Adipose tissue area | ||||||
| Total | 0.376 | –0.216 | 0.015 | 0.256 | –0.201 | –0.122 |
| Subcutaneous | 0.372 | –0.201 | 0.018 | 0.245 | –0.213 | –0.125 |
| Subfascial | 0.336 | –0.123 | –0.026 | 0.369 | 0.263 | 0.030 |
Values in italics are statistically significant (p < 0.05).
Table 5.
Pearson correlation coefficients (r values) between OGTT insulinemia at 120 min and measures of mid-thigh soft tissue compositiona
| Mid-thigh CT scan | Men |
Women |
||||
|---|---|---|---|---|---|---|
| age adjusted | adjusted for age and FM | adjusted for age and visceral adiposity | age adjusted | adjusted for age and FM | adjusted for age and visceral adiposity | |
| Muscle | ||||||
| Total muscle | ||||||
| Attenuation | –0.297 | –0.050 | –0.052 | –0.179 | –0.019 | 0.011 |
| Surface area | 0.173 | –0.038 | –0.078 | 0.199 | 0.106 | –0.039 |
| Normal attenuation | ||||||
| Attenuation | 0.014 | –0.315 | –0.232 | 0.144 | 0.087 | –0.021 |
| Surface area | 0.004 | –0.004 | –0.053 | 0.041 | 0.079 | 0.041 |
| Low attenuation (‘fatty’) | ||||||
| Mean value | –0.124 | 0.114 | 0.035 | –0.127 | –0.081 | 0.113 |
| Surface area | 0.331 | –0.083 | –0.072 | 0.195 | –0.031 | –0.207 |
| Adipose tissue area | ||||||
| Total | 0.330 | –0.260 | –0.078 | 0.178 | –0.214 | –0.218 |
| Subcutaneous | 0.320 | –0.240 | –0.076 | 0.167 | –0.226 | –0.220 |
| Subfascial | 0.337 | –0.116 | –0.051 | 0.319 | 0.273 | –0.030 |
Values in italics are statistically significant (p < 0.05).
Tertile Analyses
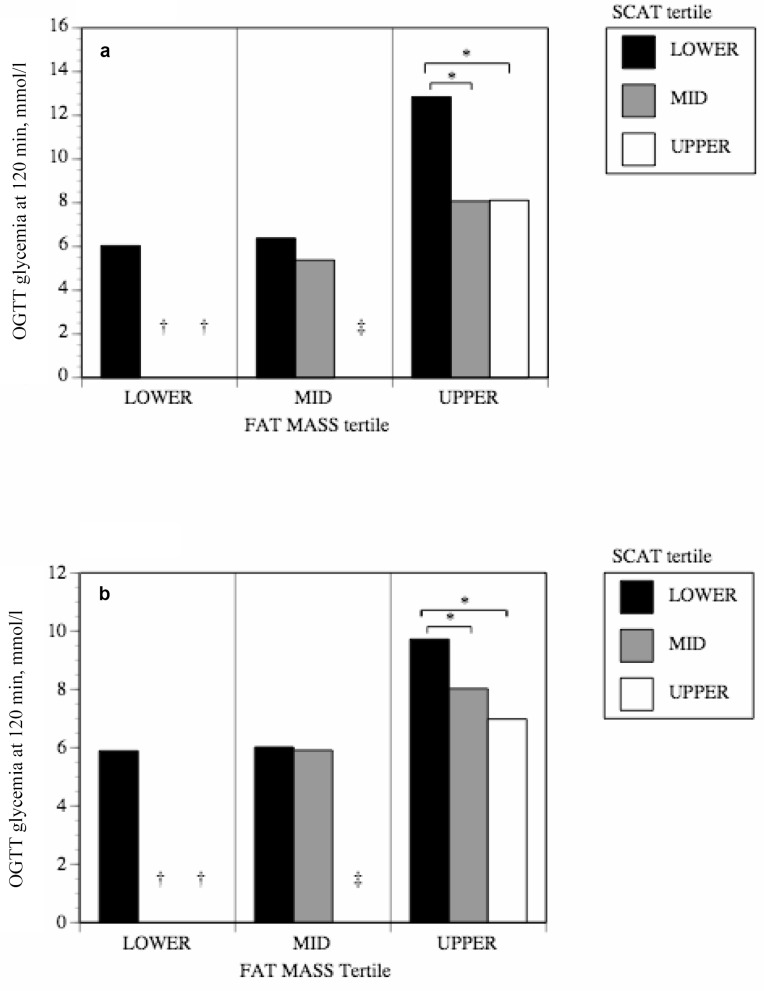
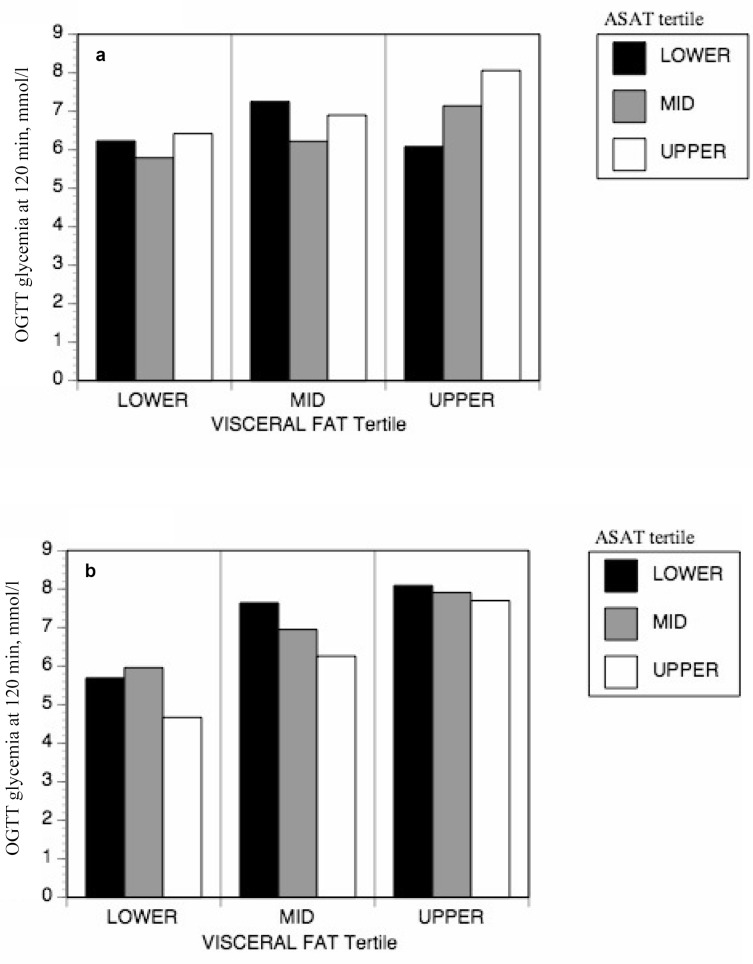
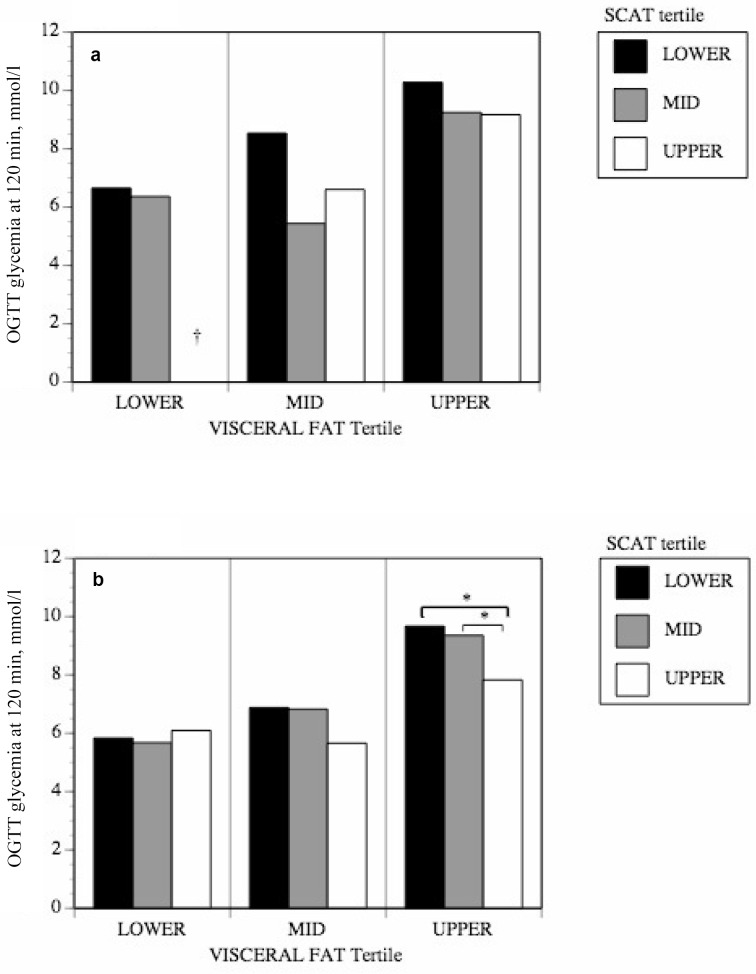
Tertile analyses were performed to evaluate differences in glucose tolerance among people with the same level of overall or visceral adiposity but with different levels of subcutaneous mid-thigh adipose tissue. Generally, men and women who had more mid-thigh SCAT for a given level of overall body fatness or visceral adiposity were more glucose tolerant (fig. 1, 2). The positive effects of mid-thigh SCAT were most obvious and reached statistical significance for glucose levels at 120 min of the OGTT (fig. 1). As shown in figure 1, those individuals in the lower tertile of mid-thigh SCAT and in the upper tertile of FM were actually over the diagnostic threshold for diabetes even though they had not been clinically diagnosed as such. Those in the lower tertile of mid-thigh SCAT and in the upper tertile of FM in figure 1b had glucose levels indicative of glucose intolerance, as were those in the lower or mid tertile of mid-thigh SCAT and in the upper tertile of VAT in figure 2b. In order to verify whether SCAT from all depots was equally protective, tertile analyses were also performed using abdominal subcutaneous adipose tissue (ASAT) instead of mid-thigh SCAT. No significant differences were observed in glucose tolerance among groups with varying levels of ASAT (fig. 3).
Fig. 1.
The impact of mid-thigh subcutaneous adipose tissue area on the relationship between FM and glucose tolerance in a men and b women. Data were corrected for age.
*p < 0.05.
†No subjects in the mid and upper SCAT tertiles were found in the lower and mid FM tertiles.
‡No subjects in the upper SCAT tertile were found in the mid FM tertiles.
Fig. 3.
The impact of ASAT area on the relationship between abdominal VAT area and glucose tolerance in a men and b women.
*p < 0.05.
Adipose Tissue Distribution and Glucose Handling
In both men and women, significant negative correlations were observed between glucose and insulin variables measured during OGTT and ratios of mid-thigh SCAT to VAT and to FM (table 6). The strength of the relationships was similar between sexes, except for glycemia at 120 min and the AUC for glycemia which show somewhat weaker correlations with the ratio of mid-thigh SCAT to FM in women (table 6).
Table 6.
Pearson correlation coefficients (r values) of ratios of mid-thigh SCAT to abdominal VAT and FM with glucose and insulin markers during OGTTa
| SAT/VAT men | women | SAT/FM men |
women | |
|---|---|---|---|---|
| Glycemia 120 min | –0.50 | –0.52 | –0.56 | –0.39 |
| Glycemia AUC | –0.55 | –0.54 | –0.61 | –0.38 |
| Insulin 120 min | –0.46 | –0.48 | –0.47 | –0.46 |
| Insulin AUC | –0.44 | –0.40 | –0.45 | –0.41 |
All are statistically significant with p < 0.0001.
Discussion
This study was performed to examine the association between mid-thigh SCAT and glucose tolerance. Our results are consistent with those of others [9, 15] in suggesting a protective role for mid-thigh SCAT and add to the existing literature by establishing the existence of such an effect in a population aged between 20 and 65 years. Even though overall fatness or perhaps more specifically abdominal adiposity may have many adverse effects on whole-body glucose metabolism [7, 12], our results suggest that mid-thigh SCAT has a specific protective effect.
Our first set of correlational analyses uncovered a favorable association between mid-thigh SCAT and glucose tolerance. With data adjusted only for age, higher mid-thigh adiposity at first appears related to poorer glucose handling. However, upon adjustment for whole-body adiposity, positive relationships between mid-thigh SCAT and glucose tolerance were revealed in both men and women. This was also true when correcting for visceral adiposity in women, but the effect was much less pronounced in men.
The correlational observations were corroborated by tertile analyses in which men and women in the upper tertile of FM or women in the upper tertile of VAT who were also in the mid or upper tertile of mid-thigh SCAT actually showed improved glucose tolerance.
Our results suggest a sex dimorphism in the relationships between SCAT and glucose metabolism, the effects being much more pronounced in women. This is likely explained by important quantitative differences in fat depots between sexes. In men, a greater proportion of the mid-thigh is occupied by skeletal muscle and they generally have a lower total volume of SCAT compared to women (table 1). It is thus not surprising that in men the protective effect of the mid-thigh SCAT on glucose metabolism is less pronounced, notably in those men with elevated visceral adiposity. In fact, our results suggest that another tissue may be partially compensating in men for lesser SCAT; the area of muscle with normal attenuation values, representative of muscle that is not fat-laden, appears to be a key variable [20]. A greater area of normal attenuation muscle tissue was associated with better glucose tolerance in men, even after adjustment for visceral adiposity or general adiposity. These results agree with previous work showing that higher muscle attenuation values are associated with better glucose uptake, possibly through reduced levels of intramyocellular lipids (IMCLs) or their derivatives that are known to impact on insulin sensitivity [21]. Interestingly, after adjustment for age and adiposity, relationships between the fat content of normal attenuation muscle and glucose tolerance (that fraction of the whole muscle which has an attenuation signal between 35 and 100 Hounsfield units) are revealed, showing that having more ‘fat-rich’ normal muscle can contribute to lower glucose tolerance (tables 2, 3, 4, 5).
Correlations between markers of glucose metabolism (glycemia at 120 min, glycemia AUC, insulin at 120 min and insulin AUC) and ratios of mid-thigh SCAT/abdominal VAT and mid-thigh SCAT/FM revealed statistically significant negative correlations, indicating that higher levels of mid-thigh SCAT are associated with better glucose handling. These results speak to the importance of fat distribution in addition to total FM. The latter seems less predictive of glucose metabolism than distribution in women where correlations are markedly lower for glucose markers correlated to mid-thigh SCAT/FM. In men, a larger part of the fat accumulation is visceral which explains why mid-thigh SCAT ratio with abdominal VAT or FM yields the same results. These correlations emphasize the beneficial effect of having an elevated mid-thigh SCAT / abdominal VAT ratio, in agreement with the tertile analyses.
As noted above, the ratio of visceral to subcutaneous adiposity seems to contribute to the observed differences between men and women, notably with respect to the apparent lack of a protective effect of the upper tertile of visceral adiposity in men. For a given level of adiposity, men have a proportionally higher level of visceral adiposity. Simply put, men may not have enough subcutaneous peripheral adipose tissue to act as an efficient sink for insulin-stimulated glucose disposal. For example, in our study, the average level of mid-thigh SCAT for women in the upper tertile was 266.5 cm2, whereas it was only 145.7 cm2 in men. It is also possible that upon reaching a certain level of visceral adiposity, no amount of mid-thigh SCAT can compensate for its negative effects on glucose tolerance.
How can mid-thigh SCAT exert such a positive influence on glucose metabolism? It has been suggested that the thigh subcutaneous fat depot is an insulin-sensitive metabolic sink which has an important capacity for glucose uptake [22], and could thus compensate for insulin resistance in other tissues although in lean subjects the response to insulin is comparable among various adipose tissue compartments. Even though glucose uptake is significantly higher in the omental depot, it becomes progressively resistant to insulin action in subjects with a central distribution of adiposity [23]. Glucose uptake in adipose tissue will increase in absolute quantity but will decrease relatively to total fat in subjects with central obesity [24]. Individuals with a greater peripheral adiposity distribution do not appear to exhibit this pattern and would thus be relatively protected against glucose intolerance.
Furthermore, the quantity of mid-thigh SCAT that is available for glucose disposal from the circulation can be phenomenal (on average 65% more transversal surface than muscle), notably in women. Even if it is metabolizing less glucose per unit of tissue than muscle, it could compensate by sheer volume and thus dispose of enough glucose to normalize glycemia even in the presence of important visceral adiposity and insulin resistance. This is suggested by our findings in the group of women in the upper tertile of visceral adiposity (fig. 2b). For a given level of visceral adiposity, higher levels of mid-thigh SCAT can make the difference between normal glucose tolerance (average glycemia below 7.8 mmol/l at 120 min of the OGTT) or glucose intolerance (glycemia averaging 9.4 mmol/l at 120 min of the OGTT). The lack of any such protective effect when performing a similar analysis with ASAT (fig. 3) implies that it is specific to the mid-thigh compartment and that the ASAT depot does not contribute similarly to glucose metabolism. In support of this idea, recent work by other groups showed a similar protective effect of mid-thigh SCAT against various metabolic abnormalities resulting from excess whole body fat, and further show that this is especially true in women. Koska et al. [25] showed that insulin resistance in Pima Indians was greater in women presenting lower mid-thigh SCAT tissue and higher ASAT, an effect that was not noted in men. Also, Jun et al. [26] showed that a higher level of mid-thigh SCAT is independently associated with a lower incidence of non-alcoholic fatty liver disease for a given level of BMI in women, with no impact noted in men. Furthermore, they show that mid-thigh SCAT is negatively correlated to fasting blood sugar and homeostasis model assessment indices of insulin resistance (HOMA-IR), again most strongly in women. However, our data do not allow to test the role of other SCAT depots although we suspect that they may not all provide the same level of protection with respect to glucose metabolism. Further studies are required to elucidate the mechanisms underlying the specific effects reported here.
Fig. 2.
The impact of mid-thigh SCAT area on the relationship between abdominal VAT area and glucose tolerance in a men and b women. Data were corrected for age.
*p < 0.05.
†No subjects in the upper SCAT tertile were found in the lower visceral fat tertile.
Conclusion
This study demonstrates that a higher level of mid-thigh SCAT is associated with better glucose tolerance at a given level of whole-body or visceral adiposity, most notably in women. One potential explanation for the apparent sex difference of this effect could be that men do not often accumulate sufficient quantities of mid-thigh SCAT for this specific effect to arise. In conjunction with this finding, another variable of importance seems to be the ratio of mid-thigh SCAT / abdominal VAT. Our findings suggest that accumulating fat specifically in the mid-thigh SCAT compartment may be a strategy of choice to protect against glucose intolerance when fat accumulation is inevitable. Indeed, this appears to be one of the pathways through which the glitazones exert their favorable metabolic effects [27, 28]. However, further studies will be required to clearly elucidate the role of other adipose tissue depots on glucose tolerance.
Disclosure
The authors declared no conflict of interest.
Acknowledgements
The authors express their gratitude to the participants for their excellent collaboration and the staff of the Physical Activity Sciences Laboratory for their contribution to this study. We especially thank G. Thériault, I. Lemieux, G. Fournier, M. Chagnon, L. Allard, and C. Leblanc for their help in the collection and analysis of the data. This work was funded by a grant from the Medical Research Council of Canada.
References
- 1.Bouchard C, Despres JP, Mauriege P. Genetic and nongenetic determinants of regional fat distribution. Endocr Rev. 1993;14:72–93. doi: 10.1210/edrv-14-1-72. [DOI] [PubMed] [Google Scholar]
- 2.Despres JP, Allard C, Tremblay A, Talbot J, Bouchard C. Evidence for a regional component of body fatness in the association with serum lipids in men and women. Metabolism. 1985;34:967–973. doi: 10.1016/0026-0495(85)90147-7. [DOI] [PubMed] [Google Scholar]
- 3.Donahue RP, Abbott RD. Central obesity and coronary heart disease in men. Lancet. 1987;ii:1215. doi: 10.1016/s0140-6736(87)91357-2. [DOI] [PubMed] [Google Scholar]
- 4.Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, Adams PW. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54:254–260. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 5.Larsson B, Svardsudd K, Welin L, Wilhelmsen L, Bjorntorp P, Tibblin G. Abdominal adipose tissue distributionobesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 1984;288:1401–1404. doi: 10.1136/bmj.288.6428.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohlson LO, Larsson B, Svardsudd K, Welin L, Eriksson H, Wilhelmsen L, Bjorntorp P, Tibblin G, The influence of body fat distribution on the incidence of diabetes mellitus 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34:1055–1058. doi: 10.2337/diab.34.10.1055. [DOI] [PubMed] [Google Scholar]
- 7.Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36:54–59. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- 8.Shuman WP, Morris LL, Leonetti DL, Wahl PW, Moceri VM, Moss AA, Fujimoto WY. Abnormal body fat distribution detected by computed tomography in diabetic men. Invest Radiol. 1986;21:483–487. doi: 10.1097/00004424-198606000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, De Rekeneire N, Kanaya AM, Newman AB, Tylavsky FA, Seidell JC. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levelsindependently of high abdominal fat. The health abc study. Diabetologia. 2005;48:301–308. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 10.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–E948. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 11.Park KS, Rhee BD, Lee KU, Kim SY, Lee HK, Koh CS, Min HK. Intra-abdominal fat is associated with decreased insulin sensitivity in healthy young men. Metabolism. 1991;40:600–603. doi: 10.1016/0026-0495(91)90050-7. [DOI] [PubMed] [Google Scholar]
- 12.Pouliot MC, Despres JP, Nadeau A, Moorjani S, Prud’Homme D, Lupien PJ, Tremblay A, Bouchard C. Visceral obesity in men. Associations with glucose toleranceplasma insulin and lipoprotein levels. Diabetes. 1992;41:826–834. doi: 10.2337/diab.41.7.826. [DOI] [PubMed] [Google Scholar]
- 13.Lew EA, Garfinkel L. Variations in mortality by weight among 750,000 men and women. J Chronic Dis. 1979;32:563–576. doi: 10.1016/0021-9681(79)90119-x. [DOI] [PubMed] [Google Scholar]
- 14.Van Itallie TB. Obesity: adverse effects on health and longevity. Am J Clin Nutr. 1979;32:2723–2733. doi: 10.1093/ajcn/32.12.2723. [DOI] [PubMed] [Google Scholar]
- 15.Snijder MB, Dekker JM, Visser M, Yudkin JS, Stehouwer CD, Bouter LM, Heine RJ, Nijpels G, Seidell JC. Larger thigh and hip circumferences are associated with better glucose tolerance: The hoorn study. Obes Res. 2003;11:104–111. doi: 10.1038/oby.2003.18. [DOI] [PubMed] [Google Scholar]
- 16.Simonen RL, Perusse L, Rankinen T, Rice T, Rao DC, Bouchard C. Familial aggregation of physical activity levels in the Quebec Family Study. Med Sci Sports Exerc. 2002;34:1137–1142. doi: 10.1097/00005768-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Desbuquois B, Aurbach GD. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971;33:732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- 18.Kvist H, Sjostrom L, Tylen U. Adipose tissue volume determinations in women by computed tomography: Technical considerations. Int J Obes. 1986;10:53–67. [PubMed] [Google Scholar]
- 19.Bouchard C, Leon AS, Rao DC, Skinner JS, Wilmore JH, Gagnon J. The heritage family study. Aimsdesign, and measurement protocol. Med Sci Sports Exerc. 1995;27:721–729. [PubMed] [Google Scholar]
- 20.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 21.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89:104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 22.Lemieux I. Energy partitioning in gluteal-femoral fat: does the metabolic fate of triglycerides affect coronary heart disease risk? Arterioscler Thromb Vasc Biol. 2004;24:795–797. doi: 10.1161/01.ATV.0000126485.80373.33. [DOI] [PubMed] [Google Scholar]
- 23.Stolic M, Russell A, Hutley L, Fielding G, Hay J, MacDonald G, Whitehead J, Prins J. Glucose uptake and insulin action in human adipose tissue – influence of BMIanatomical depot and body fat distribution. Int J Obes Relat Metab Disord. 2002;26:17–23. doi: 10.1038/sj.ijo.0801850. [DOI] [PubMed] [Google Scholar]
- 24.Virtanen KA, Lonnroth P, Parkkola R, Peltoniemi P, Asola M, Viljanen T, Tolvanen T, Knuuti J, Ronnemaa T, Huupponen R, Nuutila P. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metab. 2002;87:3902–3910. doi: 10.1210/jcem.87.8.8761. [DOI] [PubMed] [Google Scholar]
- 25.Koska J, Stefan N, Votruba SB, Smith SR, Krakoff J, Bunt JC. Distribution of subcutaneous fat predicts insulin action in obesity in sex-specific manner. Obesity (Silver Spring) 2008;16:2003–2009. doi: 10.1038/oby.2008.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jun DW, Han JH, Kim SH, Jang EC, Kim NI, Lee JS, Song MH, Kim SH, Jo YJ, Park YS. Association between low thigh fat and non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2008;23:888–893. doi: 10.1111/j.1440-1746.2008.05330.x. [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87:2784–2791. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- 28.Mori Y, Murakawa Y, Okada K, Horikoshi H, Yokoyama J, Tajima N, Ikeda Y. Effect of troglitazone on body fat distribution in type 2 diabetic patients. Diabetes Care. 1999;22:908–912. doi: 10.2337/diacare.22.6.908. [DOI] [PubMed] [Google Scholar]