Abstract
Background
Autologous stem cell transplantation (ASCT) and novel therapies have improved the prognosis for patients with multiple myeloma (MM). For those who undergo ASCT while on dialysis, a similar survival compared with the overall MM population has been reported. Therefore, for patients achieving remission following ASCT, kidney transplantation is an attractive option, offering an improved quality of life and significant economic advantage.
Method
This case series investigates the outcome of five patients who underwent an ASCT for MM with subsequent kidney transplantation between 2006 and 2012.
Results
Four patients presented with end-stage renal disease (ESRD) and one progressed to ESRD shortly after diagnosis. Induction chemotherapy regimens with novel agents including thalidomide and bortezomib were utilized. Following attainment of very good partial remission or complete remission, high-dose melphalan ASCTs were performed after a median of 10 months. Kidney transplantation (living donor n = 3, deceased donor n = 2) with tacrolimus-based immunosuppression regimens was completed at a median of 27 months after ASCT. Patients 1 and 3 experienced relapse of myeloma at 6 and 16 months after kidney transplantation. Patients 2, 4 and 5 remain alive at 55 months (median) after kidney transplantation with no evidence of relapse.
Conclusion
Forty percent of our cohort experienced a relapse in MM within 2 years of kidney transplantation. Death-censored graft survival and patient survival were 80% at 4 years. Our study adds to the growing literature supporting kidney transplantation following successful ASCT for MM and is useful when counselling patients regarding renal and haematological outcomes.
Keywords: autologous stem cell transplantation, kidney transplantation, multiple myeloma, outcome
INTRODUCTION
Multiple myeloma (MM) is the second most common haematological malignancy, accounting for 1% of all cancers [1]. Induction chemotherapy with novel anti-myeloma agents followed by high-dose melphalan autologous stem cell transplantation (ASCT) remains the gold standard of therapy for younger patients with MM and has led to significantly increased progression-free and overall survival [2].
End-stage renal disease (ESRD) has a substantial impact on morbidity and mortality [3]. Historically, patients with MM and ESRD had a 2.5 times higher relative risk of death relating to a higher tumour burden, lower tolerated chemotherapy doses and higher treatment-related mortality [4–6]. However, since the introduction of novel agents such as bortezomib, thalidomide and lenalidomide, there have been improvements in the rate of response even in patients with renal impairment [7, 8]. Furthermore, after ASCT, a similar survival for patients with ESRD compared with the overall MM population has been reported [1, 9].
Kidney transplantation improves survival compared with remaining on dialysis [5]. Previously, poor outcomes related to infection and disease progression in the context of immunosuppression were reported following kidney transplantation in patients with MM [10]. However, with current treatments and subsequent superior patient survival, successful kidney transplantation in patients with MM in remission is now possible [11].
MATERIALS AND METHODS
This case series reports on the risk of relapse of MM of five patients after kidney transplantation in King’s College and Guys and St Thomas’ Hospitals, London, UK, between 2006 and 2012. The patients were defined as having very good partial remission (VGPR) or complete remission (CR) in concordance with International Myeloma Working Group consensus criteria [12]. Clinical information and data were collected from medical records. Induction immunosuppression following kidney transplantation consisted of with two doses of basiliximab 20 mg on the day of the transplant and four days later. Maintenance immunosuppression included tacrolimus or ciclosporin with mycophenolate mofetil and prednisolone. This study was exempted from approval from an ethics’ board.
RESULTS
Case reports
Three subjects were male and two were female. Median age at diagnosis of MM was 54 years (range 37–64). All patients were Caucasian. Median duration of follow-up from the time of kidney transplantation was 55 months (range 48–56). Results are summarized in Table 1 and Figure 1.
Table 1.
Demographics, clinical characteristics, MM treatment course and renal transplant outcomes
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | ||||
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age (at time of diagnosis MM, years) | 63 | 54 | 37 | 48 | 64 | |||
| Sex | M | M | M | F | F | |||
| Ethnicity | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | |||
| Timeline | ||||||||
| Date of diagnosis of MM and presentation of renal disease | December 2006 | July 2010 | March 2008 | March 2011 | March 2012 | |||
| Time from MM diagnosis to ASCT (months) | 25 | 10 | 11 | 10 | 10 | |||
| Time from ASCT to kidney transplantation (months) | 42 | 33 | 14 | 27 | 16 | |||
| Time from MM diagnosis to kidney transplantation (months) | 67 | 43 | 25 | 37 | 26 | |||
| Time from kidney transplantation to relapse (months) | 6 | N/A | 16 | N/A | N/A | |||
| Date of death | February 2016 | N/A | April 2014 | N/A | N/A | |||
| Time of follow-up from kidney transplantation (months) | 55 | 53 | 48 | 56 | 55 | |||
| Details of MM | ||||||||
| Stage at diagnosis | Stage III ISS | Stage III ISS | Stage III ISS | Stage III ISS | Stage III ISS | |||
| SFLC at diagnosis (mg/L) | Not available at our hospital in 2006 |
|
|
|
|
|||
| Bone marrow biopsy | 50% plasma cells, IgA Kappa | 90% plasma cells, Lambda Light Chain | 80% plasma cells, IgG Kappa | 5% plasma cells, non-secretory | 90% plasma cells, Lambda Light chain | |||
| Chemotherapy pre-ASCT | TD × 6 |
|
CTD × 6 | TD × 6 |
|
|||
| Achieved VGPR/CR (months after diagnosis MM) | 25 | 8 | 11 | 10 | 10 | |||
| ASCT chemotherapy | HDM 100 mg/m2 | HDM 140 mg/m2 | HDM 140 mg/m2 | HDM 140 mg/m2 | HDM 140 mg/m2 | |||
| Details of relapse of MM | ||||||||
| First relapse of MM post ASCT (months) | 48 | No, remains in CR | 30 | No, remains in CR | No, remains in CR | |||
| First relapse of MM post kidney transplantation (months) | 6 | N/A | 16 | N/A | N/A | |||
| SFLC at first relapse (mg/L) |
|
N/A |
|
N/A | N/A | |||
| Bone marrow biopsy at first relapse | 50% plasma cells | N/A | Not conducted | N/A | N/A | |||
| Chemotherapy for first relapse | VCD × 3 | N/A |
|
N/A | N/A | |||
| Haematological response achieved after treatment of first relapse | CR | N/A | VGPR | N/A | N/A | |||
| Second relapse of MM post kidney transplantation (months) | 38 | N/A | 31 | N/A | N/A | |||
| SFLC at second relapse (mg/L) |
|
N/A |
|
N/A | N/A | |||
| Bone marrow biopsy at second relapse | 70% plasma cells | N/A | Not conducted | N/A | N/A | |||
| Chemotherapy for second relapse | Bendamustine/dexamethasone × 7 | N/A | Melphalan | N/A | N/A | |||
| Adverse impacts of chemotherapy | No | No | Bortezomib – peripheral neuropathy | No | Bortezomib – peripheral neuropathy | |||
| Details of renal disease and transplantation | ||||||||
| Renal involvement at presentation of MM | Yes | Yes | Yes | Yes | Yes | |||
| Renal biopsy results | Cast nephropathy | Cast nephropathy and tubule-interstitial nephritis | Cast nephropathy | Cast nephropathy | Cast nephropathy | |||
| Type of renal allograft | ABO-incompatible, live related | Live unrelated | Live related | Deceased DBD | Deceased DBD | |||
| HLA mismatch | 1-2-1 | 1-2-1 | 1-1-1 | 0-0-0 | 1-1-0 | |||
| Induction immunosuppression |
|
Basiliximab | Basiliximab | Basiliximab | Basiliximab | |||
| Maintenance immunosuppression | Tacrolimus, MMF and prednisolone | Tacrolimus, MMF and prednisolone | Ciclosporin, MMF and prednisolone | Tacrolimus, MMF and prednisolone | Tacrolimus, MMF and prednisolone | |||
| eGFR (mL/min) at: | ||||||||
| 1 year | 78 | 63 | 43 | 15 | 35 | |||
| 2 years | 76 | 61 | 38 | Haemodialysis | 38 | |||
| Last follow-up | 29 | Haemodialysis | 14 | Haemodialysis | 27 | |||
| UPCR (mg/mmol) at: | ||||||||
| Pre-transplant | 13 | 19 | Not tested | Not tested | Not tested | |||
| 1 year | 15 | 13 | 11 | Not tested | Not tested | |||
| 2 years | 48 | 20 | 35 | Not tested | Not tested | |||
DBD, donation after brain death; F, female; M, male; ISS, International Staging System; HDM, high-dose melphalan; N/A, not applicable; MMF, mycophenolate mofetil; TD, thalidomide/dexamethasone.
FIGURE 1.
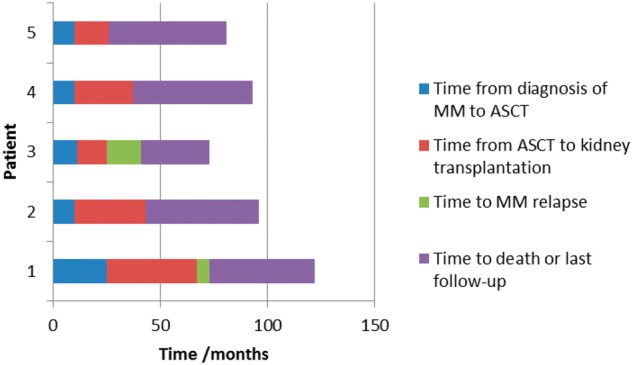
Timeline of diagnosis of MM to last follow-up.
Patient 1
A 63-year-old male presented with ESRD related to cast nephropathy and immunoglobulin A (IgA) Kappa-related MM and was treated with thalidomide and dexamethasone inducing a VGPR followed by ASCT 25 months after diagnosis of MM. An ABO-incompatible living-related donor kidney transplant was performed 42 months later. Pre-transplant treatment with double filtration plasmapheresis and a single dose of rituximab 375/m2 was administered to achieve anti-A1 titres of 1:8. MM relapse occurred 6 months after kidney transplantation and was treated with bortezomib, cyclophosphamide and dexamethasone (VCD) to CR. A second relapse, 32 months after the first relapse, was treated with bendamustine and dexamethasone. After the seventh cycle, he developed neutropenic sepsis related to H1N1 infection and chest infection with acute kidney injury requiring haemodialysis. Renal biopsy undertaken at this time demonstrated acute tubular necrosis with no evidence of myeloma-related kidney disease or rejection (no donor-specific antibody was detected, C4d stains were negative). His kidney function recovered to an estimated glomerular filtration rate (eGFR) of 29 mL/min but unfortunately, he died from pneumonia at 55 months after kidney transplantation.
Patient 2
A 54-year-old male presented with ESRD related to lambda light chain myeloma and was treated with cyclophosphamide, thalidomide and dexamethasone (CTD) followed by VCD achieving CR. An ASCT was performed 10 months after CR and 33 months later he received a living donor kidney transplant. Two years after kidney transplantation, his eGFR was 61 mL/min. During a routine clinic visit, 28 months after transplantation, a reduction in eGFR to 40 mL/min was detected. Renal biopsy confirmed cellular rejection (Banff Type IIA [13]) and chronic antibody-mediated rejection [donor-specific antibody present to HLA A1 (MFI 1999), B8 (MFI 4071), B38 (MFI 1822), DQB1*06:03 (MFI 5619), C4d stains positive]. Despite treatment with intravenous methylprednisolone, his renal function continued to decline, and he started dialysis 53 months after transplantation. He remains in CR and is currently being assessed for re-transplantation.
Patient 3
A 37-year-old male presented IgG kappa myeloma and ESRD as a consequence of cast nephropathy. He was treated with CTD achieving VGPR followed by ASCT 11 months after diagnosis. He underwent living donor kidney transplantation 14 months after ASCT.
He suffered a relapse of MM 16 months after kidney transplantation and was treated with VCD chemotherapy, which was limited by peripheral neuropathy. Serum-free light chains (SFLC) concentrations decreased but the response was short-lived, and he then received lenalidomide and dexamethasone. This was stopped after 2 weeks despite reduction in SFLC due to renal graft dysfunction. A biopsy confirmed cellular rejection (Banff Type IIB [13]) with features of acute antibody-mediated rejection although C4d stain was negative and no donor-specific antibody was detected. He was treated with intravenous methylprednisolone and Ig and plasma exchange. Renal function improved (eGFR 27 mL/min). He received bendamustine and bortezomib and then CTD to a VGPR. He subsequently experienced a second relapse, 15 months after the first relapse in association with a decline in eGFR to 17 mL/min. Melphalan (25 mg/m2) and dexamethasone were commenced but not tolerated. Unfortunately, the patient experienced a myocardial infarction and cardiorespiratory arrest resulting in death at 48 months after kidney transplantation with a eGFR of 14 mL/min.
Patient 4
A 48-year-old female was diagnosed with a non-secretory Kappa light chain myeloma and cast nephropathy. Her eGFR was 15 mL/min, and she received thalidomide and dexamethasone to CR, followed by ASCT 10 months later. She developed ESRD 1 month after presentation with MM and underwent deceased donor kidney transplantation 27 months after ASCT. Her transplant function remained suboptimal and a biopsy confirmed cellular rejection (Banff Type IIA [13]), which was treated with intravenous methylprednisolone. A subsequent biopsy showed ongoing cellular rejection. A second course of intravenous methylprednisolone was administered and a further biopsy showed resolution of the rejection but evidence of calcineurin-inhibitor toxicity. Her eGFR at 1 year after transplantation was 15 mL/min, and she recommenced haemodialysis 24 months after transplantation. She is currently listed for further renal transplantation but remains in CR 56 months after kidney transplantation.
Patient 5
A 64-year-old female presented with Lambda light chain myeloma and cast nephropathy requiring dialysis. She was treated with bortezomib, adriamycin and dexamethasone (PAD), cyclophosphamide, bortezomib and thalidomide (CVTD) followed by CTD as she developed bortezomib-related peripheral neuropathy. She achieved a VGPR and underwent ASCT 10 months after diagnosis of MM and then deceased donor kidney transplantation 166 months after ACST. Her eGFR is 27 mL/min at 55 months after kidney transplantation and she remains in CR.
DISCUSSION
This study describes the outcome of patients who underwent kidney transplantation following ASCT for MM. ASCTs for MM were performed after a median of 10 months (range 10–25) following diagnosis of MM. Kidney transplantation was performed at a median of 27 months after ASCT (range 16–42). Three patients did not experience relapse of MM with median follow-up period of 55 months (range 53–56) after kidney transplantation. Two patients experienced relapse of myeloma at a median of 11 months after kidney transplantation. These two patients died at a median of 52 months after kidney transplantation. Patient 1 received an ABO-incompatible transplant and therefore received additional immunosuppressive treatment with plasmapheresis and rituximab. It is conceivable that this contributed to the early relapse of MM. However, this relapse was successfully treated to CR and the patient developed a second relapse 38 months after kidney transplantation. The first relapse for Patient 3 was also treated to VGPR and he remained in remission for a further 15 months. Both patients died with a functioning renal transplant at a median of 52 months after transplantation.
For the two of the three patients who remain in CR, the transplant failed at a median of 39 months after transplantation. Graft loss in both cases was attributed to rejection. Of note, treatment of the rejection episodes was not de-escalated due to the history of MM.
This case series report contributes to the small number of case series that have previously been reported in patients with MM treated with contemporary chemotherapeutic regimens with successful outcomes following kidney transplantation (Table 2). Lum et al. reported on two patients who received bortezimib-based treatments and continued with fortnightly bortezomib after kidney transplantation [10]. Induction immunosuppression for kidney transplantation consisted of basiliximab, similar to our patient cohort. At 25 and 13 months, both patients remain in remission with serum creatinine of 1–2 mg/dL. Hassoun et al. reported on two patients treated with thalidomide, dexamethasone, melphalan and doxorubicin followed by ASCT and kidney transplantation 14.1 and 45.7 months after achieving CR [14]. At 21.8 and 24.1 months, respectively, both patients remain in remission with functioning renal allografts. Sánchez Quintana et al. reported on two patients treated with lenalidomide followed by ASCT and then kidney transplantation [15]. At 48 and 36 months, both patients remain in remission with functioning renal allografts. Le et al. reported on four patients treated with bortezomib, lenalidomide, cyclophosphamide and thalidomide followed by ASCT and then kidney transplantation at between 20 and 66 months after remission [16]. At between 16 and 58 months of follow-up, the patients have an eGFR of 59–73 mL/min. Two patients continued with maintenance therapy of lenalidomide or bortezomib and one patient relapsed but was treated successfully with carfilzomib, cyclophosphamide and dexamethasone. It is interesting to note that the patient who relapsed received antithymocyte induction in the context of ABO-incompatible transplantation. In summary for these case series, the median time to transplant from remission was 39 months and median follow-up after transplantation was 31 months. Only one patient suffered with a relapse but that was treated successfully to CR. Patient and kidney transplant survival was 100% with no episodes of rejection reported. One patient developed BK viraemia necessitating a reduction in immunosuppression.
Table 2.
Published case reports of renal transplantation in patients treated with autologous stem cell transplantation for multiple myeloma
| Reference | Patient demographics | Native kidney biopsy | MM treatment | Time to kidney transplant after remission (months) | Type of kidney transplant and immunosuppression | Last follow-up after kidney transplant (months) | Haematological response at last follow-up | eGFR at last follow-up (mL/min) |
|---|---|---|---|---|---|---|---|---|
| Lum et al. [10] | 67-year-old male | No biopsy but renal disease thought to be hypertensive nephrosclerosis. | Dexamethasone/bortezomib; bortezomib maintenance | 12 | Living unrelated transplant with basiliximab induction and maintenance with tacrolimus, mycophenolic acid and prednisolone and then ciclosporin and prednisolone (due to BK viraemia) | 25 | CR | 34 |
| 62-year-old female | Cast nephropathy | Plasmapheresis; dexamethasone/bortezomib; bortezomib maintenance | 24 | Living unrelated transplant with basiliximab induction and maintenance with tacrolimus and prednisolone | 13 | CR | 60 | |
| Hassoun et al. [13] | 42-year-old male | LCDD | Thalidomide/dexamethasone; dexamethasone; melphalan/dexamethasone/ doxorubicin/dexamethasone; cyclophosphamide mobilization; melphalan conditioning; ASCT | 14 | No details given | 22 | CR | Normal |
| 51-year-old female | LCDD | Thalidomide/dexamethasone; dexamethasone; melphalan/dexamethasone/ doxorubicin/dexamethasone; cyclophosphamide mobilization; melphalan conditioning; ASCT | 46 | No details given | 24 | CR | Normal | |
| Sánchez Quintana et al. [14] | 38-year-old male | LCDD | Dexamethasone; ASCT; lenalidomide maintenance | 48 | Deceased donor transplantation (DBD); no induction details given; maintenance with tacrolimus and prednisolone | 48 | CR | Not given |
| 44-year-old female | No biopsy | Vincristine/adriamycin/dexamethasone; ASCT; maintenance with thalidomide then lenalidomide | 48 | Deceased donor transplantation (DBD); no induction details given; maintenance with tacrolimus and prednisolone | 36 | VGPR | Not given | |
| Le et al. [15] | 52-year-old male | LCDD with cryoglobul-inaemic GN | Plasmapheresis, thalidomide/dexamethasone; vincristine/doxil/dexamethasone; cyclophosphamide mobilization; melphalan conditioning then ASCT | 66 | No details given | 58 | CR | 73 |
| 50-year-old male | No biopsy | Bortezomib/dexamethasone; lenalidomide/ doxorubicin/cyclophosphamide/dexamthasone; melphalan conditioning then ASCT lenalidamide followed by bortezomib maintenance; lenalidomide/dexamethasone (progression); carfilzomid/cyclophosphamide/dexamethasone; pomalidomide/cyclophosphamide/dexamethasone | 20 | ABO-incompatible kidney transplant with antithymocyte globulin induction | 48 | SD | 59 | |
| 50-year-old male | LCDD | Bortezomib/dexamethasone/lenalidomide; melphalan conditioning then ASCT; lenalidomide, then bortezomib maintenance | 32 | No transplant details given; no induction details given; maintenance with tacrolimus, mycophenolic acid and prednisolone | 43 | CR | 59 | |
| 47-year-old male | No biopsy | Bortezomib/dexamethasone/lenalidomide; cyclophosphamide mobilization; melphalan conditioning then ASCT; lenalidamide maintenance | 53 | No transplant details given with basiliximab induction and maintenance with tacrolimus and mycophenolic acid | 16 | CR | 60 |
SD, stable disease; LCDD, light chain deposition disease; DBD, donation after brain death; GN, glomerulonephropathy.
In comparison with the published case series, our patients were transplanted 12 months earlier after ASCT and we have follow-up data for a further 21 months. In this study, all the patients had renal disease attributable to cast nephropathy and received an ASCT. The only patient that experienced relapse of MM also received intensive induction immunosuppression for an ABO-incompatible transplant. In our case series, the relapse rate was increased with inferior patient and graft survival compared with previous cases. Our 4-year death-censored graft survival was 80% and 4-year patient survival after transplantation was 80%. Of note, our patients did not receive maintenance chemotherapy after kidney transplantation, and our patients had longer follow-up, and these factors may account for the differences observed.
Treatment of relapsed myeloma remains challenging. In our series, both patients were treated effectively for the first relapse resulting in disease-free interval of 24 months (median). However, treatment of the second relapse was not successful. Patient 3 was treated with lenalidomide, which can precipitate kidney transplant rejection [17, 18], and therefore perhaps these agents should be avoided. However, others have reported maintenance as well as treatment for relapse with lenalidomide without adverse impact to the transplant kidney (Table 2). In addition, sepsis is the second most common cause of death following kidney transplantation [19]. Relapse of myeloma confers an additional risk of sepsis related to immunoparesis and chemotherapy. Careful consideration of immunosuppression regimens and immunological risk of the transplant, to avoid sepsis and minimize the risk of kidney transplant rejection, is imperative.
The main limitation of our study is the small number of patients and therefore caution must be applied when considering the relapse rate and graft outcome data. However, the strength is the length of follow-up. Our study supports kidney transplantation as the preferred treatment for ESRD following successful ASCT for MM and is useful when counselling patients regarding outcomes following kidney transplantation after MM. Furthermore, there are emerging novel agents that are suitable to be employed in ESRD which may improve the depth of response prior to transplant and can be employed to treat relapse following transplantation.
European Best Practice Guidelines advise a waiting period of 2 years between successful induction treatment and renal transplantation [20]. In the future, it may be possible to risk-stratify and select a subgroup of patients with myeloma who are predicted to have a deep response following ASCT [12], and these patients with a better prognosis could be considered for earlier kidney transplantation. However, further evidence is needed to support this [21]. Uncertainty remains around the role of continuing chemotherapy after kidney transplantation to prevent relapse and the optimal treatment of relapsed MM.
We have presented a case series of five patients submitted for renal transplantation after ASCT and CR of MM and demonstrated that 40% of our cohort experienced a relapse in MM within 2 years of kidney transplantation. Death-censored graft survival and patient survival was 80% at 4 years. From our experience, we suggest avoiding transplantation from donors, which would require intensive immunosuppression and immunomodulatory chemotherapy agents to reduce the risk of MM relapse and renal rejection.
CONFLICT OF INTEREST STATEMENT
No disclosures or conflicts of interest for all authors. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Baraldi O, Grandinetti V, Donati G. et al. Hematopoietic cell and renal transplantation in plasma cell dyscrasia patients. Cell Trans 2015; 25: 995–1005 [DOI] [PubMed] [Google Scholar]
- 2. Jurczyszyn A, Nahi H, Avivi I. et al. Characteristics and outcomes of patients with multiple myeloma aged 21–40 years versus 41–60 years: a multi-institutional case-control study. Br J Haematol 2016; 175: 884–891 [DOI] [PubMed] [Google Scholar]
- 3. Augustson BM, Begum G, Dunn JA. et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002 - Medical Research Council Adult Leukaemia Working Party. J Clin Oncol 2005; 23: 9219–9226 [DOI] [PubMed] [Google Scholar]
- 4. Abbott KC, Agodoa LY.. Multiple myeloma and light chain-associated nephropathy at end-stage renal disease in the United States: patient characteristics and survival. Clin Nephrol 2001; 56: 207–210 [PubMed] [Google Scholar]
- 5. Tsakiris DJ, Stel VS, Finne P. et al. Incidence and outcome of patients starting renal replacement therapy for end-stage renal disease due to multiple myeloma or light-chain deposit disease: an ERA-EDTA Registry study. Nephrol Dial Transplant 2009; 25: 1200–1206 [DOI] [PubMed] [Google Scholar]
- 6. Korbet SM, Schwartz MM.. Multiple myeloma. J Am Soc Nephrol 2006; 17: 2533–2545 [DOI] [PubMed] [Google Scholar]
- 7. Ludwig H, Adam Z, Greil R.. Reversal of acute renal impairment by bortezomib-doxorubicin-dexamethasone in multiple myeloma. Results from a phase II study. Haematologica 2009; 94 (Suppl): 154, Abstr 38519118377 [Google Scholar]
- 8. Tosi P, Zamagni E, Cellini C. et al. Thalidomide alone or in combination with dexamethasone in patients with advanced, relapsed or refractory multiple myeloma and renal failure. Eur J Haematol 2004; 73: 98–103 [DOI] [PubMed] [Google Scholar]
- 9. Floro L, Lazana I, Streetly M. et al. Retrospective analysis of the impact of kidney function on the outcomes of first autografts for de novo myeloma patients in the era of novel agents. Clin Lymphoma Myeloma Leuk 2015; 15: e160–e161 [Google Scholar]
- 10. Lum EL, Kogut N, Pham T. et al. Kidney transplantation in patients with active multiple myeloma: case reports. Transplant Direct 2017; 3: e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.End-Stage Renal Disease in the United States. U.S. Renal Data System (USRDS) Annual Report 2012. https://www.usrds.org/2012/pdf/v2_ch7_12.pdf (4 January 2019, date last accessed)
- 12. Chng WJ, Dispenzieri A, Chim C-S. et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia 2014; 28: 269–277 [DOI] [PubMed] [Google Scholar]
- 13. Haas M, Loupy A, Lefaucheur C. et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 2018; 18: 293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hassoun H, Flombaum C, D'Agati VD. et al. High-dose melphalan and auto-SCT in patients with monoclonal Ig deposition disease. Bone Marrow Transplant 2008; 42: 405–412 [DOI] [PubMed] [Google Scholar]
- 15. Sánchez Quintana A, Rull PR, Atienza JB. et al. Renal transplant in plasma cell dyscrasias with lenalidomide treatment after autologous stem cell transplantation. Nephrology 2013; 18: 641–643 [DOI] [PubMed] [Google Scholar]
- 16. Le TX, Wolf JL, Peralta CA. et al. Kidney transplantation for kidney failure due to multiple myeloma: case reports. Am J Kidney Dis 2017; 69: 858–862 [DOI] [PubMed] [Google Scholar]
- 17. Lum EL, Huang E, Bunnapradist S. et al. Acute kidney allograft rejection precipitated by lenalidomide treatment for multiple myeloma. Am J Kidney Dis 2017; 69: 701–704 [DOI] [PubMed] [Google Scholar]
- 18. Walavalkar V, Adey DB, Laszik ZG. et al. Severe renal allograft rejection resulting from lenalidomide therapy for multiple myeloma: case report. Transplant Proc 2018; 50: 873–876 [DOI] [PubMed] [Google Scholar]
- 19. Yalci A, Celebi ZK, Ozbas B. et al. Evaluation of infectious complications in the first year after kidney transplantation. Transplant Proc 2015; 47: 1429–1432 [DOI] [PubMed] [Google Scholar]
- 20.EBPG (European Expert Group on Renal Transplantation); European Renal Association (ERA-EDTA); European Society for Organ Transplantation (ESOT). European Best Practice Guidelines for Renal Transplantation (Part 1). Nephrol Dial Transplant 2000; 15 (Suppl 7): 1–85 [PubMed] [Google Scholar]
- 21. Bansal T, Garg A, Snowden JA. et al. Defining the role of renal transplantation in the modern management of multiple myeloma and other plasma cell dyscrasias. Nephron Clin Pract 2012; 120: c228–c235 [DOI] [PubMed] [Google Scholar]


