Abstract
Chronic kidney disease (CKD) expands the prior concept of chronic renal insufficiency by including patients with relatively preserved renal function, as assessed by the estimated glomerular filtration rate (eGFR), as even these early CKD stages are associated with an increased risk for all-cause death and cardiovascular death, CKD progression and acute kidney injury. A decreased eGFR (<60 mL/min/1.73 m2) is by itself diagnostic of CKD when persisting for >3 months. However, when eGFR is ≥60 mL/min/1.73 m2, an additional criterion is required to diagnose CKD. In a recent clinical trial published in The New England Journal of Medicine, all 6190 participants were reported to have CKD: 47% had Stages 1 and 2 CKD and 53% had Stage 3 CKD. This illustrates a widespread misunderstanding of the concept of CKD. Moreover, CKD categories in this study were assigned based on the estimated creatinine clearance. Since both estimated creatinine clearance and creatinine clearance overestimate eGFR, this illustrates another frequent misunderstanding: equating GFR with creatinine clearance. In this commentary, we clarify the concept of CKD and of CKD categories for non-nephrologists. Assigning a diagnosis of CKD to a patient with normal renal function and absence of other evidence of CKD may have negative consequences for the individual (e.g. insurance and others) as well as for the medical community at large by creating confusion about the concept.
Keywords: albuminuria, chronic kidney disease, creatinine clearance, definition, glomerular filtration rate, urate
WHAT IS CHRONIC KIDNEY DISEASE?
The current international consensus definition of chronic kidney disease (CKD) by Kidney Disease: Improving Global Outcomes (KDIGO 2012) states that CKD is defined as abnormalities of kidney structure or function, present for > 3 months, with implications for health [1]. The abnormalities of kidney structure or function may be recognized clinically by different criteria: just one of these criteria is enough to diagnose CKD. Criteria include a decreased glomerular filtration rate (GFR) [<60 mL/min/1.73 m2) or evidence of kidney damage such as albuminuria (albumin excretion rate ≥ 30 mg/24 h; urinary albumin creatinine ratio (UACR) ≥ 30 mg/g], urine sediment abnormalities, electrolyte and other abnormalities due to tubular disorders, abnormalities detected by histology, structural abnormalities detected by imaging or history of kidney transplantation. In clinical practice, this means that in order to diagnose CKD in an individual with normal GFR or with GFR ≥60 mL/min/1.73 m2, a urine analysis or kidney imaging test is required.
WHAT ARE THE IMPLICATIONS FOR HEALTH OF CKD MENTIONED IN THE KDIGO DEFINITION?
A diagnosis of CKD is associated with an increased risk of CKD progression, all-cause death, cardiovascular death and acute kidney injury, among others [1]. These risks have been demonstrated most clearly for pathological albuminuria or decreased GFR [2–4].
HOW IS CKD CATEGORIZED?
CKD categories are recognized based on GFR (G categories G1 through G5) and on albuminuria (A categories A1 through A3). Increasing CKD categories are associated with increasing risks of CKD progression, all-cause death, cardiovascular death and acute kidney injury. G1 (GFR ≥90 mL/min/1.73 m2) and A1 (UACR <30 mg/g) categories represent normal values and, thus, are not diagnostic by themselves of CKD. Patients in category G1A1 are required to fulfill an additional criterion for CKD in order for the physician to diagnose CKD. If this additional criterion were not required to diagnose CKD, then the whole general population would be considered to have CKD, resulting in a CKD prevalence of 100%!
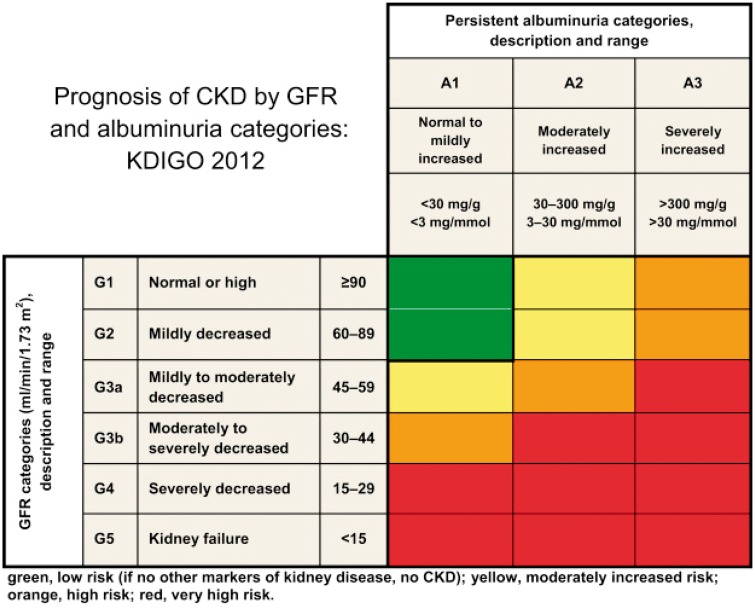
KDIGO recommends that CKD is classified based on cause (C), GFR category (G) and albuminuria (A) category, that is, it recommends using a CGA classification [1]. A heatmap representing risk of CKD progression according to GFR and albuminuria categories has become very popular (Figure 1). Its popularity may be the driving force for two oversimplifications that we have observed in non-nephrologists during routine clinical practice: (i) assuming that CKD followed by a G category and an A category (e.g. CKD G3A2) is in itself a diagnosis, thus forgetting about the key issue of cause, and (ii) assuming that all combinations of categories in the image are, by themselves, diagnostic of CKD, that is, using the term CKD to refer to a patient with G1A1, G1A2, G2A1 or G2A2 categories in the absence of other evidence of CKD, as can be observed in Table 1 from a recent The New England Journal of Medicine (NEJM) clinical trial report [5].
FIGURE 1.
Heatmap representing the risk for CKD progression according to GFR and albuminuria categories [1]. Similar heatmaps represent the risk for all-cause death and cardiovascular death.
CAN CKD BE DIAGNOSED IN INDIVIDUALS WITH NORMAL GFR?
Yes indeed. CKD can be diagnosed when GFR is normal (i.e. ≥90 mL/min/1.73 m2) if there is other evidence of kidney damage such as albuminuria (albumin excretion rate ≥30 mg/24 h; UACR ≥30 mg/g), urine sediment abnormalities, electrolyte and other abnormalities due to tubular disorders, abnormalities detected by histology, structural abnormalities detected by imaging or history of kidney transplantation. However, if GFR is normal, then CKD cannot be diagnosed in the absence of at least one positive study exploring these items.
CAN CKD BE DIAGNOSED IN INDIVIDUALS WITH NORMAL GFR AND NORMAL ALBUMINURIA?
Yes indeed. CKD can be diagnosed when GFR is normal (i.e. ≥90 mL/min/1.73 m2) and albuminuria is normal (<30 mg/g) if there is other evidence of kidney damage persisting longer than 3 months, such as urine sediment abnormalities, electrolyte and other abnormalities due to tubular disorders, abnormalities detected by histology, structural abnormalities detected by imaging or history of kidney transplantation. However, when both GFR and albuminuria are normal, CKD cannot be diagnosed in the absence of at least one positive study exploring these items. As an example, an individual with autosomal dominant polycystic kidney disease having the kidney replaced by numerous large cysts that increase the kidney size may be diagnosed with CKD despite normal GFR and albuminuria. This patient will have CKD G1A1 caused by polycystic kidney disease. In another example, it may be argued that all classical Fabry males be considered to have CKD G1A1 caused by Fabry disease when GFR and albuminuria values are normal, since the disease is known to be characterized by glycolipid deposits in podocytes and endothelial and other kidney cells that are already evident in childhood, that is, it is known to be associated with histological evidence of CKD [6].
HOW SHOULD GFR BE ASSESSED?
KDIGO recommends using serum creatinine and a GFR estimating equation for initial assessment of GFR. It additionally recommends that clinicians understand clinical settings in which estimated GFR (eGFR) creatinine is less accurate (e.g. muscle mass, that is creatinine generation, much lower or much higher than expected for age and sex). In this regard, for the review of evidence on GFR estimating equations, KDIGO only considered equations that were developed using assays that were traceable to reference methods and study populations in which serum creatinine concentration was measured using traceable assays, that is, the Modification of Diet in Renal Disease (MDRD) Study equation, Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation and modifications of these. The Cockcroft and Gault formula is explicitly indicated not to be based on traceable assays. Furthermore, Cockcroft and Gault estimates creatinine clearance, which usually overestimates eGFR [7]. Thus, the use of the Cockcroft and Gault formula may result in assigning individual patients to different G categories than using the CKD-EPI or MDRD equations, as discussed extensively in reference [7]. Measuring GFR is more accurate, and iohexol-based methods allow clinical GFR measurement in patients for whom a precise assessment of GFR is clinically relevant or in whom creatinine-based equations are not reliable [8, 9].
WHAT ARE THE ISSUES WITH THE RECENT NEJM PUBLICATION?
A recent 2018 publication in NEJM reported the results of a randomized clinical trial comparing the safety of allopurinol and febuxostat treatment to decrease serum urate levels [5, 10]. Table 1 in this manuscript describes the baseline characteristics of the participants (Figure 2). Apparently, 100% of the 6190 patients had CKD since they are represented as either having Stage 1 or 2 CKD (47% of the patients) or Stage 3 CKD (53% of the patients). This would be very unusual, even allowing for the median age of 65 years and the presence of cardiovascular disease and hyperuricaemia. Indeed, a careful review of the manuscript, the supplementary data and the trial protocol did not disclose what additional criterion, on top of a normal or near normal GFR, was used to diagnose CKD. In a later report, the authors indicate that albuminuria was indeed not assessed [11]. It is likely that the authors (together with reviewers and editors) missed the clinical implications of the concept of CKD and used the term Stage 1 or 2 CKD to indicate that the estimated creatinine clearance (since they used the Cockcroft and Gault formula, which, in fact, estimates creatinine clearance, not GFR) is ≥90 mL/min or between 60 and 89 mL/min, even if the patient does not fulfill diagnostic criteria for CKD. In our opinion, this is a dangerous use of the term CKD. Given the prestige of NEJM, readers may imitate the journal and diagnose CKD in individuals with normal renal function and in the absence of any other criteria to diagnose CKD. This may have negative consequences for the individual from the point of view of a wrong diagnosis and potential insurance issues, among others. Furthermore, this may have negative consequences for the medical community at large by creating confusion about the CKD concept and assuming that 100% of the population has CKD or, conversely, that a diagnosis of CKD G1A1 does not in fact reflect the real existence of CKD, thus depriving the term of its meaning and consequences when applied to someone that does have CKD G1A1 (e.g. an individual with autosomal dominant polycystic kidney disease and normal GFR and albuminuria values).
FIGURE 2.
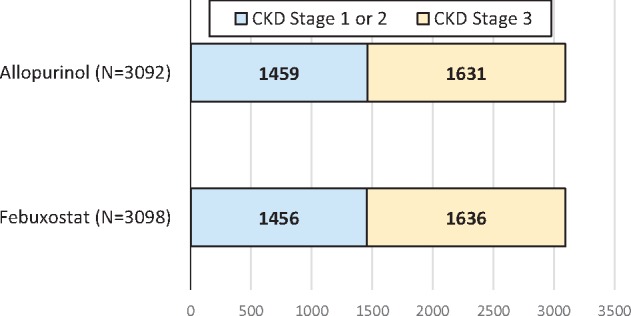
Data presented in Table 1 of the recent 2018 NEJM manuscript by White et al. [5]. All 6190 participants were reported to have CKD. Patients were randomized to either febuxostat (N = 3098) or allopurinol (N = 3092). The table legend stated that ‘estimated creatinine clearance was calculated with the use of the Cockcroft–Gault formula and was corrected for ideal body weight. A value of 60 mL per minute or more indicated Stage 1 or 2 chronic kidney disease, and a value of at least 30 but less than 60 mL per minute indicated Stage 3 chronic kidney disease’. However, no other evidence of CKD (e.g. such as pathological albuminuria in those with creatinine clearance >60 mL/min) was provided. Indeed, albuminuria data were not available [11].
CONCLUSIONS
The medical community should be especially careful with language and make an effort to use appropriately terms implying specific diseases or that have implications for health. CKD is a case in point, confusion being generated by using the terms CKD Stage 1 or 2 or CKD categories G1A1 or G2A1 to refer to individuals who do not have CKD or have no evidence of CKD.
FUNDING
This research was supported by FIS PI16/02057, ISCIII-RETIC REDinREN RD016/0009 FEDER funds, Sociedad Española de Nefrología, Fundacion Renal Iñigo Álvarez de Toledo (FRIAT), ISCIII Rio Hortega (MVPG) and Comunidad de Madrid Biomedicina B2017/BMD-3686 CIFRA2-CM. No financial conflict of interest exists.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 2. Ortiz A, Fernandez-Fernandez B.. Humble kidneys predict mighty heart troubles. Lancet Diabetes Endocrinol 2015; 3: 489–491 [DOI] [PubMed] [Google Scholar]
- 3. Matsushita K, Coresh J, Sang Y. et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 2015; 3: 514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ortiz A, Covic A, Fliser D. et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 2014; 383: 1831–1843 [DOI] [PubMed] [Google Scholar]
- 5. White WB, Saag KG, Becker MA. et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 2018; 378: 1200–1210 [DOI] [PubMed] [Google Scholar]
- 6. Ortiz A., Oliveira JP, Waldek S, Warnock DG. et al. Nephropathy in males and females with Fabry disease: cross-sectional description of patients before treatment with enzyme replacement therapy. Nephrol Dial Transplant 2008; 23: 1600–1607 [DOI] [PubMed] [Google Scholar]
- 7. Fernandez-Prado R, Castillo-Rodriguez E, Velez-Arribas FJ. et al. Creatinine clearance is not equal to glomerular filtration rate and Cockcroft-Gault equation is not equal to CKD-EPI collaboration equation. Am J Med 2016; 129: 1259–1263 [DOI] [PubMed] [Google Scholar]
- 8. Delanaye P, Melsom T, Ebert N. et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 2: Why to measure glomerular filtration rate with iohexol? Clin Kidney J 2016; 9: 700–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delanaye P, Ebert N, Melsom T. et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 1: How to measure glomerular filtration rate with iohexol? Clin Kidney J 2016; 9: 682–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perez-Gomez MV, Bartsch LA, Ortiz A. Cardiovascular safety of febuxostat. N Engl J Med 2018; 379: 1583. [DOI] [PubMed] [Google Scholar]
- 11. White WB, Gunawardhana L. Cardiovascular safety of febuxostat. N Engl J Med 2018; 379: 1584. [DOI] [PubMed] [Google Scholar]