Abstract
Background
The importance of vitamin D sufficiency in deficient dialysis patients is uncertain. This study aimed to determine if high-dose cholecalciferol for 1-year affected symptoms, muscle strength, blood pressure (BP), cardiac ischaemia, parathyroid hormone, calcium or phosphate.
Methods
This was a randomized, double-blind, placebo-controlled trial with 1-year follow-up that enrolled dialysis patients with 25-hydroxy-vitamin D [25(OH)D] concentration <50 nmol/L. Consenting patients were randomized to 50 000 U/week oral cholecalciferol or matching placebo. Dosage was adjusted at 3- and 6-month study visits, targeting a 25(OH)D concentration >80 nmol/L. The primary objectives were to assess the effect of supplementation on renal-specific symptoms and on hand-grip strength. Symptoms were assessed using the Kidney Disease Quality of Life Short Form and muscle strength with a hand grip-strength dynamometer. Hypothesis testing was by two-group t-test and Wilcoxon rank-sum on an intention-to-treat basis.
Results
In all, 68 participants were randomized and received study medication. Median 12-month plasma 25(OH)D concentration was 119 nmol/L and 37 nmol/L in the cholecalciferol and placebo groups, respectively. There was no statistical difference in primary outcomes at 12 months. Mean symptom scores at 12 months were two lower in the cholecalciferol group (95% confidence interval −10 to 6) and geometric mean grip-strength was 27 kg in both groups. Symptoms, strength, BP, plasma mineral bone parameters and adverse events were not different between the groups at follow-up.
Conclusions
High-dose cholecalciferol in a deficient dialysis population had no effect on muscle strength or symptoms but appears safe.
Keywords: cholecalciferol, clinical trial, muscle strength, quality of life, renal dialysis
INTRODUCTION
Vitamin D status is determined by the 25-hydroxy-vitamin D [25(OH)D] concentration [1] and levels are often low in dialysis patients [2–4]. In the chronic kidney disease (CKD) population there is an association between higher 25(OH)D levels and improved cardiovascular mortality [2, 5, 6], muscle strength [7] and blood pressure (BP) [8]. However, available randomized trial data are negative for an effect on erythropoietin dose (n = 252) [9], pro-brain naturiuretic peptide (n = 50) [10], muscle strength (n = 45) [11] and depressive symptoms (n = 726) [12]. There are limited non-randomized data in the non-CKD population that supports an effect of vitamin D on strength and body pain [13]. The Kidney Disease Improving Global Outcomes (KDIGO) guideline suggests, with evidence grade C (low), that deficiency in dialysis patients is treated the same as for the non-dialysis population [14]. The 2011 Endocrine Society Guidelines defined vitamin D deficiency as plasma 25(OH)D <50 nmol/L and recommended supplementing to a 25(OH)D >75 nmol/L [15]. This definition of deficiency was adopted for the purposes of the current study.
The vitamin D dose required to reach a particular plasma 25(OH)D level varies [16]. Doses of 70 000 U/week for 4 months appear to be safe in non-CKD populations [17] and such dosing is similar to the estimated dose received from daily whole-body exposure to sunlight [18]. It is accepted that sunlight exposure does not cause vitamin D toxicity [19], despite sometimes resulting in serum concentrations of 250 nmol/L [20]. There is, however, no defined level that denotes vitamin D toxicity.
The primary objectives of this study were to determine if oral cholecalciferol supplementation to achieve a plasma 25(OH)D concentration >80 nmol/L affected symptoms or hand grip muscle strength in a dialysis population with baseline 25(OH)D concentration below 50 nmol/L. Hand grip strength, rather than proximal muscle testing, was selected because it is simple to measure in a chair and because, based on previous experience of the authors in studying the local dialysis population, it was considered likely that many potential study subjects would decline participation if testing was mandated at large joints. Secondary objectives were to determine the effect on BP, cardiac ischaemia, and on plasma parathyroid hormone (PTH), calcium and phosphate. A target 25(OH)D >80 nmol/L was selected to ensure that all subjects in the active group achieved concentrations >75 nmol/L.
MATERIALS AND METHODS
Patients receiving peritoneal, satellite or home haemodialysis between 1 May 2012 and 30 April 2015 at the Canberra Hospital Renal Unit or between 1 August 2013 and 30 April 2015 at the John Hunter Hospital Renal Unit were eligible for enrolment.
Inclusion criteria were that patients gave written informed consent to participate, were clinically stable, had been receiving maintenance dialysis for at least 3 months and had a 25(OH)D level <50 nmol/L. Exclusion criteria at enrolment were chronic granulomatous disorders, severe bilateral hand arthritis, plasma albumin corrected calcium >2.55 mmol/L, plasma phosphate >3.0 mmol/L, age <18 years, discharge from an overnight hospital admission within the preceding 4 weeks, life expectancy <12 months, commencement of calciferol within the previous 3 months or unwillingness to avoid commencement of new non-study calciferol for the duration of the study. Patients whose renal replacement therapy was haemodialysis could not receive it >3 times/week or for >18 h/week or receive it less often than twice/week.
All participants provided written informed consent prior to enrolment. The study was approved by the ACT Health Human Research Ethics Committee (ETH.11.9.1004), the Hunter New England Human Research Ethics Committee (12/12/12/4.01) and the NSW Human Research Ethics Committee (HREC/12/HNE/466). It was prospectively registered on the ANZ Clinical Trials Register (ACTRN12611001260910). All persons gave informed consent prior to their inclusion in the study and it was conducted in accordance with the Declaration of Helsinki. The study was part funded by The Canberra Hospital Private Practice Fund and remaining funding was internal.
Study visits were at baseline, and 3, 6 and 12 months. At study visits, each subject’s medical record was reviewed for any plasma cardiac troponin tests that had been performed since the last study visit and subjects were questioned about potential adverse effects, admissions to hospital or cardiac events since the last study visit. All reported events were confirmed by examination of the clinical medical record. Cardiac troponins were anticipated to be performed at different laboratories with no study control over the specific method used. A troponin was considered ‘positive’ if it was recorded as being over the laboratory reference range for the method used. At study visits muscle strength was tested, quality of life assessed and plasma; 25(OH)D, calcium and phosphate were collected. Plasma 25(OH)D was assayed using the LIAISON® total vitamin D chemiluminescence assay (DiaSorin, Saluggia, Italy). Quality of life was assessed using the Kidney Disease Quality of Life Short Form (KDQOL-SF) 1.3 (Rand Corp., Santa Monica, CA, USA) [21]. The KQQOL-SF is divided into domains, including a 12-item renal failure specific symptom domain. All domains in the KDQOL-SF have a possible range of 0–100, with higher scores indicating better quality of life. Muscle strength was assessed using a Smedley hand grip dynamometer (Sportstek, Victoria, Australia). Subjects attempted three maximum contractions on each side, in a seated position with the arm hanging by the side, the forearm flexed to 90% and the wrist in a neutral position. The best recorded force on each side was recorded and primary analysis was based on the recording from the dominant hand.
Randomization
Randomization was by computer-generated random number (http://www.randomizer.org) in balanced blocks of 10, stratified by the presence of diabetes and sex. Drug allocation codes were placed in separate, consecutively numbered, opaque, sealed envelopes that were opened in sequence at the time of randomization.
Intervention, blinding and monitoring
Each participant was randomized to receive one capsule per week containing either 50 000 U of cholecalciferol or placebo. Capsules were identical in appearance and taste and were prepared by Ainslie Chemmart Compounding Pharmacy (Ainsle, ACT, Australia). The content of capsules (active or placebo) was not provided to investigators until after the final participant exited the study. Both participants and study personnel were blinded as to drug allocation.
At each study visit, adherence to study medication dosing was assessed by means of a pill count on study medication bottles for home dialysis participants. Adherence for nurse administered medication was by review of the hospital medication chart, and plasma 25(OH)D, calcium, phosphate and PTH concentrations were measured. To minimize the risk of inadvertent un-blinding, data entry was based on a unique study ID and the actual plasma concentration of 25(OH)D was not visible to investigators within the database. An albumin adjusted plasma calcium (mmol/L) was calculated from the total plasma calcium (Ca) and plasma albumin (g/L) according the formula:
At the 3- and 6-month study visits the dose of study medication was adjusted to between zero and three capsules per week, based on the plasma 25(OH)D concentration (Table 1). All plasma calcium, phosphate and PTH results were also monitored by participants’ usual physicians. Prescription of phosphate binders, calcitriol, dialysate calcium and cinacalcet were adjusted according to the instructions of the treating physician. The dose of calcium carbonate phosphate binders varied from 500 to 600 mg elemental calcium per tablet. Many patients purchased these without use of a written prescription and were usually uncertain which strength calcium carbonate they were taking. For the purposes of reporting, all calcium containing phosphate binders were assumed to contain 550 mg elemental calcium.
Table 1.
Study medication dose adjustment algorithm at 3- and 6-month visits
| 25(OH)D concentration | Study medication dose adjustment |
|---|---|
| ≥250 nmol/L | Dosing ceased until next study visit |
| 200 to <250 nmol/L | Dosing withheld for 4 weeks and then resumed at quadruple the previous dosing interval |
| 175 to <200 nmol/L | Dosing interval doubled |
| 80 to <175 nmol/L | Dosing continued at current frequency or, if previous 25(OH)D level ≥250 nmol/L, then recommence dosing at one capsule every 4 weeks |
| <80 nmol/L | Double the weekly dose (to a maximum of three capsules per week) or halve the dosing interval if current dosing interval is longer than weekly. If previous 25(OH)D level ≥250 nmol/L, then recommence dosing at one capsule every 2 weeks |
Statistics
Data are presented as mean ± SD or median with interquartile range (IQR), as appropriate. Hypothesis testing for categorical outcomes was by Fisher’s exact test and for continuous outcomes was by t-test, analysis of variance or Wilcoxon rank-sum, as appropriate. Analysis of secondary endpoint was considered exploratory. Statistical analysis was performed using Stata14 (StataCorp, College Station, TX, USA). Analysis was by intention-to-treat (randomized group) with all P-values two sided. The null hypothesis was rejected at a significance level of <0.05.
Sample size and power analysis
A target sample size of 88 was calculated to achieve at least 80% power, with two-tailed alpha 5%, to detect a minimum clinically significant change in both primary outcomes. This calculation was based on the following assumptions:
The distribution of KDQOL-SF symptom subscale scores in haemodialysis patients would be normally distributed, with mean of 71 and SD of 16.6 [21]. A score difference of 10 was considered to be clinically significant and a sample size of 88 was calculated to provide 80% power to detect a change of at least this magnitude, with a two-sided alpha of 5%.
Mean hand grip strength in males on peritoneal dialysis, when measured using a Smedley hand dynamometer (Sportstek) is 24.8 kg with SD of 10.3 kg [22]. Based on data from a similar device showing 33% lower hand-grip strength in malnourished (compared with well-nourished) men with severe CKD [23], the same proportionate difference using the Smedley device (8.3 kg) was considered clinically significant. A sample size of 50 subjects was calculated to provide 80% power, with two-sided alpha of 5% to detect an 8.3 kg strength difference.
RESULTS
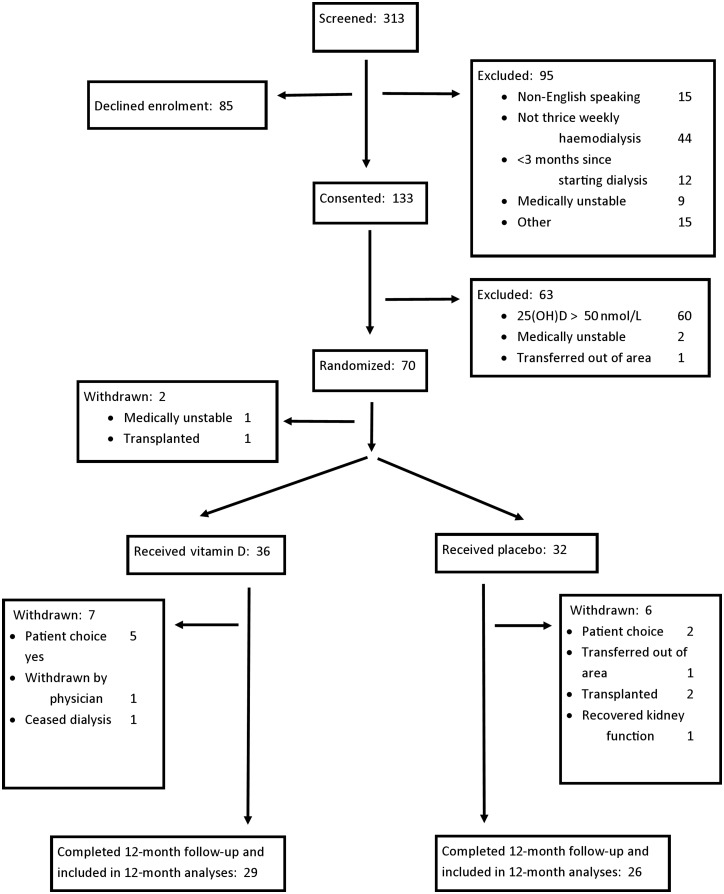
Enrolment commenced in May 2012 and the last patient was enrolled in April 2015. Participant flow through the study is shown in Figure 1. In all, 70 participants were randomized to receive study medication. Two participants were withdrawn post-randomization but before receiving study medication and these withdrawals were not included in subsequent analyses. Participants that withdrew from the study were included in analyses until the date of withdrawal only. The only exception to this was for the single participant that was withdrawn after deciding to cease all active medical treatment (including dialysis). That patient died soon after withdrawal and was counted as a death during the study.
FIGURE 1:
Study participant flow.
Participants were well matched at baseline. Mean age at enrolment was 59.5 ± 15.6 years (cholecalciferol group) and 63.8 ± 14.2 years (placebo group)—P-value for difference 0.25. A total of 32% of participants were female and half were diabetic. There were no significant differences in KDQOL-SF domain scores. Baseline data are summarized in Table 2. Mean baseline 25(OH)D levels were similar between groups and did not vary according to season of enrolment (P = 0.31). Adherence was excellent; however, the introduction of a new electronic medical record during the study period resulted in incorrect transcribing of some nurse-administered medications. This affected four participants, with another five missing doses due to changing their dialysis location or delays in posted medication reaching them. In total, 31 study medication doses were not received by participants during the study (representing 1% of the prescribed doses). Adherence in three home dialysis participants was uncertain as they failed to return study medication bottles for a pill count.
Table 2.
Baseline data
| Variable | Cholecalciferol (n = 36) | Placebo (n = 32) | P-value |
|---|---|---|---|
| Age (years) | 59.5 ± 15.6 | 63.8 ± 14.2 | 0.25 |
| Female sex | 13 | 9 | 0.61 |
| Peritoneal dialysis | 3 | 3 | 1.0 |
| Mean systolic BP (mmHg) ± SD | 142.1 ± 18.8 | 142.2 ± 19.4 | 0.98 |
| Mean diastolic BP (mmHg) ± SD | 74.9 ± 13.3 | 71.6 ± 11.2 | 0.26 |
| Peritoneal dialysis | 3 | 3 | 1 |
| Median dialysis vintage in months (IQR) | 21.7 (5.3–54.9) | 7.6 (3.7–43.1) | 0.27 |
| Diabetic | 18 | 16 | 1.0 |
| Median adjusted plasma calcium in mmol/L (IQR) | 2.3 (2.11–2.4) | 2.33 (2.25–2.41) | 0.25 |
| Median plasma phosphate in mmol/L (IQR) | 1.59 (1.22–2.15) | 1.64 (1.41–2.14) | 0.51 |
| Median plasma PTH in pmol/L (IQR) | 37 (15.3–62.6) | 33 (13.9–57.4) | 0.45 |
| Mean 25(OH)D (nmol/L) ± SD | 33.9 ± 9.0 | 33.8 ± 11.2 | 0.96 |
| Median dominant hand strength in kg (IQR) | 27.5 (22–37.5) | 24 (20–35.8) | 0.46a |
| Mean KDQOL-SF symptom score ± SD | 70.4 ± 13 | 73.2 ± 13.8 | 0.39 |
t-test of log transformed data.
At the 12-month visit all patients in the active group and 13% of patients in the placebo group had a plasma 25(OH)D concentration over 80 nmol/L. The median plasma 25(OH)D was 82 nmol/L higher in the cholecalciferol group (119 nmol/L; IQR 101–157 nmol/L) than the in the placebo group (37 nmol/L; IQR 28–57 nmol/L). The highest 25(OH)D recorded at any time during the study in the active group was 185 nmol/L. The median dose of active and placebo tablets at 12 months was one and three capsules, respectively.
Three participants completed 12 months in the study but did not provide 12-month primary outcome data and 12-month BP data (one received a cadaveric renal transplant on the day of the 12-month study visit at a different hospital, one was unconscious in the intensive care unit and another declined). Geometric mean dominant hand strength was 27 kg in both the cholecalciferol and placebo study groups at 12 months and the median strength was 26 kg (IQR 22–38 kg) and 27 kg (IQR 19–39 kg), respectively. The P-value for the comparison of medians was 0.81. The data were not suitable for hypothesis testing by t-test (as had been planned prior to commencing the study). There was no significant difference at the 12-month visit for the other primary outcome, with no difference in KDQOL-SF symptom scores between the active (score 73 ± 15) and the placebo group (score 75 ± 14), with P-value 0.66. The 95% confidence interval for the difference in symptom scores between study groups was −10 to 6. Re-analysis restricted to those with more severe baseline vitamin D deficiency [those with 25(OH)D plasma concentration <27.5 nmol/L] did not alter these findings. No significant differences were seen between study groups in other KDQOL-SF domains.
There was no difference between study groups at 12 months in BP or in adjusted plasma calcium, phosphate or PTH. Cardiac ischaemia was assessed as either the occurrence of a myocardial infarction (as diagnosed by the treating physicians), or as a participant recording a positive troponin I (no positive troponin T measurements were recorded). Since troponins were often performed serially in the same participant, only the first positive for a given participant was analysed. Two participants suffered a myocardial infarction, both in the placebo group (P = 0.22). A positive troponin was recorded in eight participants from the placebo group and in four participants from the cholecalciferol group (P = 0.34 by Fisher's exact test). Examination of the clinical record indicated that, apart from participants with diagnosed myocardial infarction, the raised troponins were of doubtful clinical significance. Other secondary endpoint data are shown in Table 3. Exploratory analysis of within cholecalciferol group changes showed that the mean adjusted plasma calcium was 0.05 mmol/L higher at 12 months [95% confidence interval (CI) −0.04 to 0.15], the mean plasma phosphate was 0.12 mmol/L higher (95% CI −0.11 to 0.36) and the median PTH was 13 pmol/L lower (P = 0.73). None of these within group changes was statistically significant.
Table 3.
Secondary outcomes at 12 months
| Outcome | Cholecalciferol (n = 29) | Placebo (n = 26) | P-value |
|---|---|---|---|
| Mean systolic BP (mmHg)a ± SD | 142 ± 21 | 143 ± 19 | 0.78b |
| Mean diastolic BP (mmHg) ± SD | 73 ± 15 | 73 ± 15 | 0.86b |
| Median adjusted plasma calcium in mmol/L (IQR) | 2.35 (2.23–2.46) | 2.38 (2.32–2.45) | 0.49 |
| Median plasma phosphate in mmol/L (IQR) | 1.78 (1.51–2.20) | 1.49 (1.32–1.9) | 0.11 |
| Median plasma PTH in pmol/La (IQR) | 24 (11–55) | 34 (21–63) | 0.26 |
Data missing in two cholecalciferol and one placebo participants for BP outcome and in one placebo participant for PTH outcome.
t-test of log transformed data.
Government funding for cinacalcet ceased in August 2015. At the time of funding withdrawal, eight participants were receiving study drug and three of these had been prescribed cinacalcet at some stage during the trial. No surgical parathyroidectomies were performed on study participants after August 2015. Elemental calcium dose, calcitriol dose and cinacalcet usage were similar in both study groups at 12 months.
Adverse events are summarized in Table 4. Possible adverse events were reported by four participants and none of these events (rash or cramps) was considered related to study medication by the treating physician. There was one death during the study (in a participant that had withdrawn from all active treatment). One further death occurred in a participant 1 day after their 12-month follow-up visit. This participant had been in the cholecalciferol group. Reanalysis of study data with inclusion of this out-of-study death did not materially alter the statistically insignificant P-value shown in Table 4.
Table 4.
Adverse events
| Event | Cholecalciferol (n = 36) | Placebo (n = 32) | P-value |
|---|---|---|---|
| Mild rash (%) | 3 (8) | 0 (0) | 0.24 |
| Cramps (%) | 0 (0) | 1 (3) | 0.47 |
| Death (%) | 1 (3) | 0 (0) | 0.54 |
| Hypercalcaemia (%) | 9 (25) | 6 (19) | 0.57 |
| Parathyroidectomy (%) | 0 (0) | 1 (3) | 0.47 |
| Bacteraemia (%) | 0 (0) | 1 (3) | 0.48 |
| Hospital admissionsa (%) | 22 (61) | 17 (53) | 0.63 |
Overnight hospital admissions, excluding admission for creation or revision of vascular accesses.
DISCUSSION
This randomized, double blind, placebo-controlled trial aimed to determine if oral cholecalciferol targeting a 25(OH)D >80 nmol/L affected symptoms or hand grip muscle strength in a deficient dialysis population. There was no difference between groups in either symptoms or hand grip strength after 12 months of follow-up. Treatment was safe, with no apparent differences in hypercalcaemia, hospital admissions or other adverse events between the groups and no participants recording 25(OH)D concentrations higher than those reported from sunlight exposure [20].
The effect of cholecalciferol on symptoms or strength in dialysis patients were studied in two previous randomized placebo-controlled studies. One study looked at depression scores in 726 subjects over one year [12]. That study found no difference in outcome between study groups, however most subjects enrolled had baseline plasma 25(OH)D concentrations >50 nmol/L, which may have reduced study power. The other study looked at 45 subjects and found no difference in muscle strength in the hand, shoulder, elbow and knee joints after 6 months’ follow-up and no difference in symptom scores [11]. However, study power in that study may also have been reduced by enrolment of participants with higher baseline 25(OH)D (<60 nmol/L) and failure to achieve 25(OH)D target in some of the active group. The most likely reason for our not finding a difference in strength or symptoms is that vitamin D status, as assessed by 25(OH)D concentration, has no impact on muscle strength or on common symptoms in renal failure. An alternative explanation for the lack of effect is that 25(OH)D concentrations at baseline were not low enough to induce muscle weakness or symptoms. Evidence against this alternate explanation is that results were unchanged when our primary outcome analysis was restricted to those participants with more severe vitamin D deficiency.
A secondary objective in this study was to assess the effect of supplementation on cardiac ischaemia. There were only two episodes of diagnosed acute myocardial infarction during the study, both in the placebo group, and twice as many recorded a positive troponin I. These results were not statistically significant, and the troponin result is of doubtful clinical relevance since, aside from the two participants suffering diagnosed myocardial infarction, most positive troponins were only slightly above the laboratory reference range and not associated with clinically likely acute coronary events. A conclusion therefore cannot be drawn regarding the effect of cholecalciferol on coronary ischaemia. No difference was seen for the other secondary endpoints of BP, and plasma PTH, calcium or phosphate, which is congruent with the literature [9].
Associations between vitamin D status and clinical events are prone to confounding in observational studies because adequate vitamin D relies on sunlight exposure [24], which tends to be lower in sicker patients. Extrapolating positive findings in non-dialysis populations [13] to a dialysis population is illogical since dialysis patients are likely to lack renal 25(OH)D 1-alpha hydroxylation activity [25]. Indeed, our study suggests that dialysis patient do behave differently to non-dialysis patients and that, in this group, 25(OH)D may not be a suitable measure of vitamin D status.
Our study has some limitations. It was underpowered for the 12-month symptom score outcome, which limits confidence in our finding of no difference between groups in symptom scores. This power calculation was robust since the experimental data in our study had similar mean and distribution to that assumed in the pre-study power calculation. In fact, a post hoc power calculation based on the distribution of symptom scores at study end would suggest that a sample size 64 should have 80% power to detect a difference of 10 for this outcome. Hand grip strength study power was adequate, according to our pre-study power calculation, but this power calculation was not robust since we lacked interventional data for muscle strength response and because the outcome was not normally distributed. Non-normal distribution violated a key power calculation assumption, making this calculation unreliable. Our results apply only to the population studied and may be different in those with more severe vitamin D deficiency. We did not assess vascular calcification and so cannot comment on this surrogate outcome. However, animal data suggest vascular calcification from vitamin D toxicity occurs concomitantly with hypercalcaemia [26] and hypercalcaemia was not more frequent in the cholecalciferol group.
In conclusion, high-dose weekly oral cholecalciferol compared with placebo in a deficient dialysis population does not affect muscle strength or symptoms. Such treatment appears to be safe, with no significant difference in the frequency of hypercalcaemia observed between groups. No differences were seen in the secondary endpoints examined.
ACKNOWLEDGEMENTS
The authors acknowledge Patricia Johnson (Renal unit, Canberra Hospital), Susan Sheehan and Leanne Garvey (Renal clinical trial unit, John Hunter Hospital) for assistance with recruitment and data collection. We thank The Canberra Hospital Private Practice Fund for funding the study.
FUNDING
This study received a grant of A$38267 from The Canberra Hospital Private Practice Fund to conduct this study.
CONFLICT OF INTEREST STATEMENT
None declared. The results have not been published previously in whole or in part.
REFERENCES
- 1.Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academies Press, 1997 [PubMed] [Google Scholar]
- 2. Drechsler C, Verduijn M, Pilz S. et al. Vitamin D status and clinical outcomes in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant 2010; 26: 1024–1032 [DOI] [PubMed] [Google Scholar]
- 3. Clayton P, Singer R.. 25-hydroxyvitamin D levels in prevalent Australian dialysis patients. Nephrology 2009; 14: 554–559 [DOI] [PubMed] [Google Scholar]
- 4. Wolf M, Shah A, Gutierrez O. et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 2007; 72: 1004–1013 [DOI] [PubMed] [Google Scholar]
- 5. Wang AY-M, Lam CW-K, Sanderson JE. et al. Serum 25-hydroxyvitamin D status and cardiovascular outcomes in chronic peritoneal dialysis patients: a 3-y prospective cohort study. Am J Clin Nutr 2008; 87: 1631–1638 [DOI] [PubMed] [Google Scholar]
- 6. Pilz S, Tomaschitz A, Friedl C. et al. Vitamin D status and mortality in chronic kidney disease. Nephrol Dial Transplant 2011; 26: 3603–3609 [DOI] [PubMed] [Google Scholar]
- 7. Visser M, Deeg DJH, Lips P.. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the longitudinal aging study Amsterdam. J Clin Endocrinol Metabol 2003; 88: 5766–5772 [DOI] [PubMed] [Google Scholar]
- 8. Argiles A, Lorho R, Servel M-F. et al. Blood pressure is correlated with vitamin D(3) serum levels in dialysis patients. Blood Purif 2002; 20: 370–375 [DOI] [PubMed] [Google Scholar]
- 9. Miskulin DC, Majchrzak K, Tighiouart H. et al. Ergocalciferol supplementation in hemodialysis patients with vitamin d deficiency: a randomized clinical trial. J Am Soc Nephrol 2016; 27: 1801–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mose FH, Vase H, Larsen T. et al. Cardiovascular effects of cholecalciferol treatment in dialysis patients–a randomized controlled trial. BMC Nephrol 2014; 15: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hewitt NA, O'Connor AA, O'Shaughnessy DV. et al. Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. Clin J Am Soc Nephrol 2013; 8: 1143–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Liu Y, Lian Y. et al. Efficacy of high-dose supplementation with oral vitamin D3 on depressive symptoms in dialysis patients with vitamin D3 insufficiency: a prospective, randomized, double-blind study. J Clin Psychopharmacol 2016; 36: 229–235 [DOI] [PubMed] [Google Scholar]
- 13. Glerup H, Mikkelsen K, Poulsen L. et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int 2000; 66: 419–424 [DOI] [PubMed] [Google Scholar]
- 14. KDIGO 2017 Clinical Practice Guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2017; 7: 1–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holick MF, Binkley NC, Bischoff-Ferrari HA. et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96: 1911–1930 [DOI] [PubMed] [Google Scholar]
- 16. Trang HM, Cole DE, Rubin LA. et al. Evidence that vitamin D3 increases serum 25-hydroxyvitaminD more efficiently than does vitamin D2. Am J Clin Nutr 1998; 68: 854–858 [DOI] [PubMed] [Google Scholar]
- 17. Heaney RP, Davies KM, Chen TC. et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 2003; 77: 204–210 [DOI] [PubMed] [Google Scholar]
- 18. Vieth R. Vitamin D supplementation, 25-hydroxy D concentrations and safety. Am J Clin Nutr 1999; 69: 842–856 [DOI] [PubMed] [Google Scholar]
- 19.National Institutes of Health Office of Dietary Supplements. Vitamin D Fact Sheet for Health Professionals https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/#h8 (17 April 2018, data last accessed)
- 20. Haddock L CJ, Vazquez MC.. 25(OH) D serum levels in the normal Puerto Rican population and in subjects with tropical sprue and parathyroid disease. P R Health Sci J 1982; 1: 85–91 [Google Scholar]
- 21. Hays RD KJ, Mapes DL, Coons SJ. et al. Kidney Disease Quality of Life Short Form (KDQOL-SF), Version 1.3: A Manual for Use and Scoring. Santa Monica, CA: RAND, 1997 [Google Scholar]
- 22. Wang AY-M, Sea MM-M, Ho ZS-Y. et al. Evaluation of handgrip strength as a nutritional marker and prognostic indicator in peritoneal dialysis patients. Am J Clin Nutr 2005; 81: 79–86 [DOI] [PubMed] [Google Scholar]
- 23. Heimbürger O, Qureshi AR, Blaner WS. et al. Hand-grip muscle strength, lean body mass and plasma proteins as markers of nutritional status in patients with chronic renal failure close to start of dialysis therapy. Am J Kidney Dis 2000; 36: 1213–1225 [DOI] [PubMed] [Google Scholar]
- 24.US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americanshttps://health.gov/dietaryguidelines/2015/guidelines/chapter-2/a-closer-look-at-current-intakes-and-recommended-shifts/ (17 April 2018, data last accessed)
- 25. Fraser DR, Kodicek E.. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature 1970; 228: 764–766 [DOI] [PubMed] [Google Scholar]
- 26. Price PA, June HH, Buckley JR. et al. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arter Thromb Vasc Biol 2001; 21: 1610. [DOI] [PubMed] [Google Scholar]