Abstract
Chronic kidney disease (CKD) is often associated with a mineral and bone disorder globally described as CKD-Mineral and Bone Disease (MBD), including renal osteodystrophy, the latter ranging from high bone turnover, as in case of secondary hyperparathyroidism (SHPT), to low bone turnover. The present article summarizes the important subjects that were covered during ‘The Parathyroid Day in Chronic Kidney Disease’ CME course organized in Paris in September 2017. It includes the latest insights on parathyroid gland growth, parathyroid hormone (PTH) synthesis, secretion and regulation by the calcium-sensing receptor, vitamin D receptor and fibroblast growth factor 23 (FGF23)–Klotho axis, as well as on parathyroid glands imaging. The skeletal action of PTH in early CKD stages to the steadily increasing activation of the often downregulated PTH receptor type 1 has been critically reviewed, emphasizing that therapeutic strategies to decrease PTH levels at these stages might not be recommended. The effects of PTH on the central nervous system, in particular cognitive functions, and on the cardiovascular system are revised, and the reliability and exchangeability of second- and third-generation PTH immunoassays discussed. The article also reviews the different circulating biomarkers used for the diagnosis and monitoring of CKD-MBD, including PTH and alkaline phosphatases isoforms. Moreover, it presents an update on the control of SHPT by vitamin D compounds, old and new calcimimetics, and parathyroidectomy. Finally, it covers the latest insights on the persistence and de novo occurrence of SHPT in renal transplant recipients.
Keywords: calcium, CKD, hyperparathyroidism, phosphataemia, vitamin D
INTRODUCTION
Chronic kidney disease (CKD) is often associated with a mineral and bone disorder globally described as CKD-Mineral and Bone Disease (MBD), including renal osteodystrophy (ROD), the latter ranging from high-turnover bone diseases such as in secondary hyperparathyroidism (SHPT), with its extreme and severe manifestation osteitis fibrosa, to low-turnover bone diseases such as osteomalacia and adynamic bone disease. Serum parathyroid hormone (PTH) is a recognized marker of bone remodelling in patients with ROD. However, identification of N-terminal truncated PTH fragments, oxidized PTH and new forms of PTH, which interfere with PTH assays and may be responsible for the great variability of PTH values in CKD, gave rise to the wide range of the PTH targets suggested by Kidney Disease: Improving Global Outcomes (KDIGO) [1]. Despite the fact that its importance in CKD has been recognized for decades, insight on PTH measurement, clinical relevance and modes of action still evolve. To provide an update on these most recent developments the ERA-EDTA working group on CKD-MBD organized ‘The Parathyroid Day in Chronic Kidney Disease’. This was a continuous medical education (CME) course organized in Paris in September 2017. In this report, we provide a summary of key aspects with special focus on important novel insights, preceded by a brief review of established knowledge in a historical perspective.
PARATHYROID GLANDS IN CKD—ANATOMY, HISTOLOGY, PHYSIOLOGY AND MOLECULAR BIOLOGY IN CKD
Most frequently, humans have four parathyroid glands. They are variably located on the back of the upper and lower poles of the thyroid gland. However, ∼20% of people have five parathyroid glands, and a few have even six glands, often in ectopic locations. The identification of aberrant parathyroid glands by imaging methods and during neck exploration in case of surgical parathyroidectomy (PTX) often proves extremely difficult. The parathyroid tissue is composed of two main secretory cell types, namely chief cells and oxyphil cells. The synthesis and secretion of PTH are regulated by numerous factors. Besides calcium, which is the most important regulator, calcitriol, phosphate, fibroblast growth factor 23 (FGF23), α-Klotho, PTH itself and PTH-related peptide (PTHrP), as well as their respective receptors, all participate in this process [2]. Additional modulation occurs at the posttranscriptional level via mRNA stabilizing protein AU rich binding factor 1 [3] and differential expression of miRNAs [4]. The physiological control of PTH synthesis and secretion is progressively lost with the progression of CKD, as a consequence of changes in the circulating levels and/or parathyroid tissue expression of these regulators, and also disturbances of hepatic and renal PTH catabolism. In parallel with a chronic stimulation of PTH secretion the parathyroid tissue undergoes an increase in parathyroid cell proliferation (hyperplasia), also due to the above PTH changes in regulators and the recently discovered mechanistic target of rapamycin complex 1 pathway activation [5]. Although a higher apoptosis rate occurs concomitantly, this is unable to compensate for the excessive cell growth [6]. The combined result is the well-known SHPT of CKD. Although initially the hyperplasia is of polyclonal nature and considered to be reversible its growth pattern generally changes from polyclonal to monoclonal or multiclonal proliferation when the SHPT becomes severe [7]. On light microscopy examination, it is characterized by nodular transformation of the parathyroid gland, suggesting the presence of one or several benign tumours. The clonal proliferation is due to loss of tumour growth inhibitory genes and/or gain of tumour growth promoter genes, as reflected by chromosomal gains and losses using comparative genomic hybridization and genome-wide molecular allelotyping techniques [8]. Clinically, clonal parathyroid cell growth probably induces a state of relative resistance to PTH-lowering treatments. Moreover, such benign tumours do not regress after renal transplantation despite the restoration of near normal or at least greatly improved kidney function and give rise to so-called persistent hyperparathyroidism as discussed below.
CaSR AND PTH RECEPTOR IN CKD
The extracellular calcium-sensing receptor (CaSR) was first described in the parathyroid gland [9]. It is a 121-kDa protein that plays a crucial role in Ca homeostasis, in particular via a tight control of the extracellular ionized calcium concentration. It belongs to class C of the G-protein-coupled membrane-bound receptor superfamily. At the cell surface, the CaSR is present constitutively in a dimeric configuration. This homodimerized configuration is crucial for its normal function. Recent studies have shown that the CaSR is also expressed in the cardiovascular system but our understanding of its physiological functions in this system remains incomplete [10]. Inactivating CaSR mutations lead to familial hypocalciuric hypercalcaemia or neonatal severe hyperparathyroidism and activating mutations to autosomal dominant hypocalcaemia. In CKD, decreased parathyroid CaSR expression participates in the pathogenesis of SHPT [11]. Several factors are probably involved in this decrease, including uremic toxins. Calcimimetics have been demonstrated to be particularly useful in controlling PTH hypersecretion and concomitantly reducing serum calcium and phosphate levels in dialysis patients. Recent findings also point to the role of CaSR allosteric coactivators as inhibitors of the development of vascular calcification not only by correcting PTH hypersecretion, but also by directly modulating vascular CaSR activity [12].
PTH binds to PTH/PTHrP receptor type 1 (PTH1R), which is expressed in numerous tissues including kidney and bone and activates several intracellular second messengers [13]. Activation of PTH1R reduces serum phosphate by decreasing its renal reabsorption. It also increases calcitriol production by its stimulatory effect on renal 25-hydroxy vitamin D (25OHD)–1α-hydroxylase activity. PTH1R is highly expressed the primary target organs for PTH, kidney and bone, and at relatively lower levels in the vasculature [14]. In addition to PTH1R, a second G protein-coupled PTH receptor, PTH2R, of uncertain physiological significance has been identified [15]. Finally, a receptor with specificity for the C-terminal region of PTH (C-PTHR) has been identified, but not yet fully characterized, in osteoblasts and osteocytes [16]. Available evidence suggests that it acts antagonistically to the PTH/PTH1R system. End-organ hyporesponsiveness to PTH, formally referred to as PTH resistance, has long been recognized in CKD. It is attributed, at least partially, to downregulation or desensitization of PTH1R [17]. Factors such as phosphate, indoxyl sulphate and paracresyl sulphate accumulation may play a role in this phenomenon [18]. Hyporesponsiveness to the skeletal action of PTH in early CKD stages could explain observations of a relatively high prevalence of low bone turnover disease, predominantly adynamic bone disease. An early inhibition of the Wnt pathway with an increase in the expression of sclerosis and other inhibitors of Wnt signalling may be involved. With the progression of CKD to more advanced stages and more severe SHPT, the steadily increasing activation of PTH1R eventually overcomes the skeletal resistance to the action of PTH, and osteitis fibrosa or mixed ROD ensue, if left untreated.
KLOTHO–FGF23–PTH AXIS IN CKD
The physiological role of FGF23, in particular its effect on renal phosphate handling, and its associations with dismal outcomes in CKD is well established and beyond the scope of this report [19].
In parathyroid glands, FGF23 downregulates PTH secretion, principally through the classic Klotho-dependent pathway of mitogen-activated protein kinases (MAPK) activation, or secondarily through the less-explored phospholipase C gamma (PLCγ)-dependent activation of the nuclear factor of activated T-cells (NFAT) cascade [20]. In fact, recent data suggest that in addition to CaSR and vitamin D receptor activation, the FGF23–Klotho endocrine axis suppresses PTH secretion. In parathyroid tissue, FGF23 binds to the cell membrane-located FGF receptor–Klotho complex to elicit activation of the MAPK-pathway, but it can also act in absence of Klotho via a phosphoinositide-specific PLCγ-dependent activation of the NFAT pathway.
Available evidence suggests that a positive phosphate balance occurs even in still normo-phosphataemic CKD patients, secondary to reduced glomerular filtration rate (GFR). These events are thought to trigger both PTH and FGF23 increments to counterbalance phosphate overload [21]. FGF23 resistance, resulting from progressively reduced Klotho expression in kidney and parathyroid gland tissue with the progression of CKD, further enhances FGF23 synthesis and increased circulating FGF23 levels [19, 21].
SHPT is a common consequence of CKD. Initially, this condition may seem counterintuitive, considering that FGF23 inhibits PTH secretion. Currently, the most reasonable explanation for SHPT in CKD patients is resistance of parathyroid tissue to the action of FGF23, particularly in cases with extremely high FGF23 levels. This hypothesis is supported by reduced Klotho and FGF receptor expression observed in surgically resected human hyperplastic parathyroid glands [22].
PTH, CNS AND COGNITIVE FUNCTIONS
In addition to their roles in the periphery, it has been shown that many hormones, which include insulin, leptin, thyroid hormone, sex steroid hormones, glucocorticoids and osteocalcin, can also influence the central nervous system (CNS), regulate brain development, modulate cognitive functions and strongly influence the central regulation of whole-body energy balance [23–31]. Importantly, increasing evidence suggests that changes in their circulating levels may contribute to age-related cognitive decline, as well as to the development of neurodegenerative diseases [32, 33]. While the impact of the hormones on brain functions, both in normal and ageing conditions, is undeniable, the influence of many of them on brain cognitive functions still remains to be explored. A better understanding of the influence of hormonal homeostasis in brain metabolic and cognitive activities may open up new roads for therapeutic intervention to ameliorate disease-related cognitive impairments, and reverse/prevent age-related memory decline.
Interestingly, numerous studies have reported that the age-induced increase in PTH levels is associated with higher risk of cognitive decline and incident dementia [34, 35]. Moreover, observational studies have described impaired cognitive functions such as memory and attention tasks in patients with primary hyperparathyroidism (PHPT) in the presence or even absence of hypercalcaemia. These disturbances can be significantly improved after surgical intervention normalizing PTH levels [36–38]. SHPT, which is generally characterized by high serum PTH levels with normal to low calcium levels as occurs in CKD, is also associated with cognitive impairment, followed by the same postoperative improvement [37, 39–41]. However, little is known about the mechanisms linking PTH and cognition impairments, both in HPT and in ageing populations.
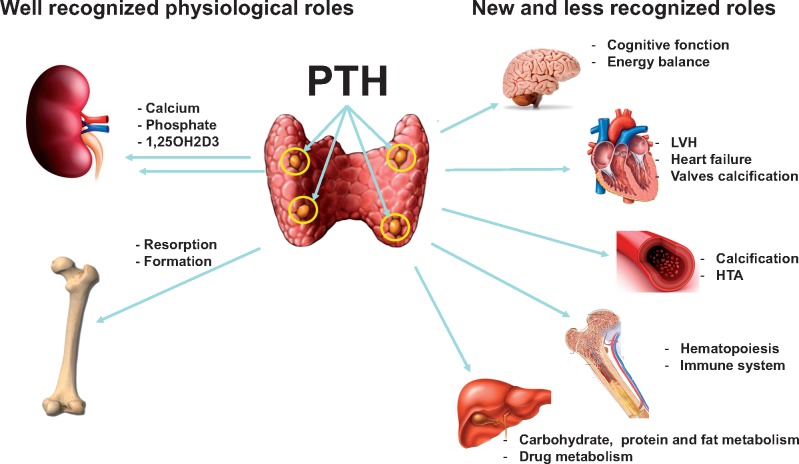
Several hypotheses have been proposed to explain the association between high PTH levels and cognitive deficits. Impaired cognition in primary HPT has been traditionally attributed to hypercalcaemia [42]. High serum calcium levels have been associated with faster decline in cognitive function, neuronal signalling disruption or atrophy in hippocampus and Alzheimer’s disease, frontal-subcortical dementia. Furthermore, PTH also promotes the conversion of vitamin D to its active form (1, 25-dihydroxy vitamin D), which an emerging body of evidence suggests may be neuroprotective. However, based on the fact that PTH crosses the blood–brain barrier [43–45] and that PTH receptors (Figure 1) are highly expressed in human and rodent brains [14, 46–48], it is legitimate to ask whether PTH signalling may also exert direct actions on the CNS. Further neuropsychological investigations of cognitive functions in patients with PHPT or age-related dementia, which include confounding factors such as altered serum calcium, phosphate and vitamin D sterol levels and the examination of a possible direct role of PTH in the CNS, are clearly warranted.
FIGURE 1.
A possible role of PTH signalling in the brain. PTH is a major regulator of the calcium/phosphate balance in the body through its actions on bone and kidney. PTH can bind and activate at least two receptors that belong to the G protein-coupled receptor family, PTH1R and PTH2R; however, its principal functions are associated with the first one. Importantly, these receptors are expressed in the CNS and may also bind paracrine ligands such as PTHrP for PTH1R and Tip 39 for the PTH2R. These findings and the observation that patients with PHPT and SHPT often have memory loss and loss of appetite suggest a direct effect of PTH on the CNS.
PTH MEASUREMENT IN CKD PATIENTS
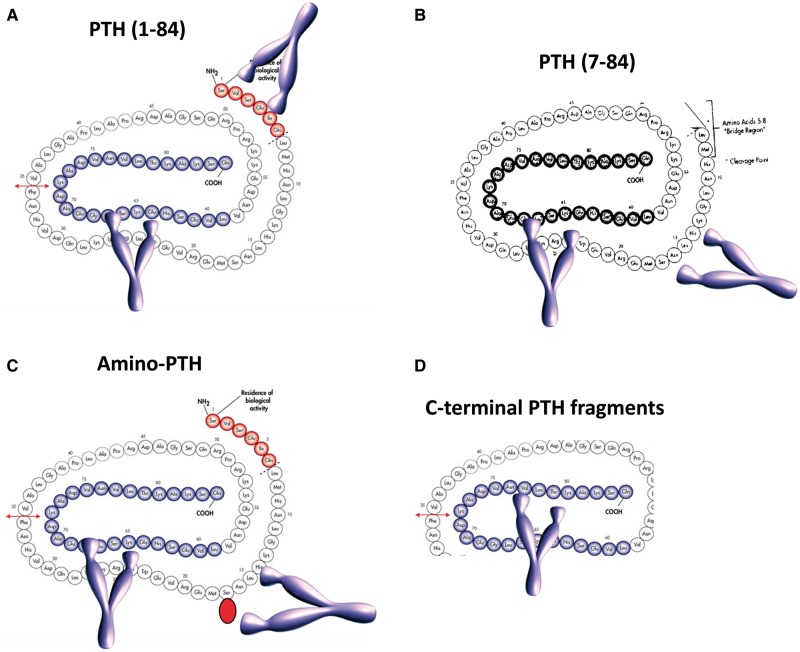
PTH measurement is very important for the follow-up of patients with CKD, in particular more advanced CKD. PTH determination represents the paradigm of quality in laboratory medicine as many variables in the pre-, intra- and post-analytical phases strongly affect the value of the clinical information. PTH circulates as a 1-84 amino acids bioactive peptide, but its analytical determination is difficult because of the presence, in the circulation, of N-terminal truncated fragments [called non-(1-84) PTH] that tend to accumulate in CKD patients [49]. These fragments also have a longer half-life compared with the 1-84 PTH. Basically, two types of assays are present on the market: second-generation (or ‘intact’ PTH: PTH2) and third-generation assays (PTH3). PTH2 recognize the non-(1-84) PTH fragments whereas PTH3 assays do not (Figure 2). PTH determination lacks standardization, despite an International World Health Organization Standard (IS 95/646) being available. Also, no reference method is available yet for PTH measurement. Hence, results provided by the different assays are very different, especially in CKD patients [50]. Since the fragments cross-react differently with the antibodies used in PTH2 assays, a full standardization of PTH will only be possible with PTH3 assays. It has been shown, however, that mathematical factors could help to improve comparability of PTH results [51], but this is not consistent over time and is a lower quality solution. Another problem with PTH is the reference range (RR). Indeed, healthy subjects selected for RR establishment need to be free of SHPT and PHPT [52]. Hence, 25OHD, calcium and creatinine measurements need to be performed prior to enrolment. Unfortunately, most manufacturers have not performed such determinations when establishing the RR, rendering those inaccurate. For instance, up to 18% of PTH values are not comprised within the RR we correctly established with the Roche PTH2 assay and the manufacturer’s RR, and most of these cases actually presented 25OHD values far greater than 30 ng/mL. The KDIGO target range for patients with dialysis-dependent CKD (2–9× upper limit of normal) will also be differently classified, according to a correctly established RR. Manufacturers’ RR and our own studies have shown that using a correctly established RR improves patient classification according to KDIGO guidelines [53]. Besides these issues, PTH can be oxidized on its two methionine amino acids, on Positions 8 and 18. It has been shown that oxidized PTH is biologically inactive. PTH2 and PTH3 assays recognize both oxidized (ox-PTH) and non-oxidized PTH (n-oxPTH). Measurement of n-oxPTH alone needs a pretest capture of ox-PTH by a specific antibody and subsequent measurement of PTH by a PTH2 assay. Since important oxidative stress is present in CKD patients, it has been hypothesized that PTH2 and PTH3 assays do not accurately measure bioactive PTH, but mostly oxidized, inactive PTH. It has indeed been shown that n-oxPTH may reflect more precisely the true hormone status and not the risk of mortality. The increased mortality risk associated with PTH might be reflecting an oxidative stress-related mortality [54] as recently observed by Seiler-Mussler et al. [55] These findings must however be confirmed by further studies.
FIGURE 2.
PTH measurements. PTH circulates as a bioactive 1-84 peptide (A), together with large N-truncated fragments called non-(1-84) PTH (B) and truncated fragments (D). A form called amino-PTH corresponds to a 1-84 PTH containing a phosphorylated serine in Position 17 (C). ‘Intact’ PTH assays recognize the bioactive peptide, but also the non-(1-84) PTH. Third-generation PTH assays use an antibody targeted against the first four amino acids of PTH and recognizes the bioactive peptide, but not the non-(1-84) PTH. PTH can be oxidized on the methionine in Positions 8 and 18 and both third- and second-generation PTH assays recognize it. To measure the non-oxidized PTH, a pretreatment of the sample with an antibody targeted against the oxidized PTH is needed, before determination with a second-generation PTH assay.
BONE METABOLIC MARKERS IN CKD PATIENTS
Bone markers reflect the activities of bone cells. They are divided into two subgroups; humoral factors derived from bone cells, and factors associated with type-I collagen metabolism. Of the bone cell-derived factors, those secreted by osteoclasts are regarded as bone resorption markers, and those originating from osteoblasts are regarded as bone formation markers, except for under-carboxylated osteocalcin, which also reflects vitamin K status.
Bone remodelling is the lifelong process by which mature bone is removed and new bone is formed; it is also the mechanism allowing skeletal growth, bone reshaping and the replacement of injured bone following fractures and micro-damages. Bone remodelling is also crucial for the regulation of circulating calcium and phosphate levels. Dialysis patients display extremely high risk of hip fracture [56–58]. Nevertheless, modern clinical studies repeatedly demonstrated that PTH levels show only a limited association with hip fracture risk [56, 57, 59, 60].
Bone markers roughly and noninvasively estimate activities of bone cells and the degree of bone remodelling. The significance of bone markers may be close to that of PTH action on bone, since PTH is the most crucial determinant of bone remodelling frequency. In fact, PTH itself is regarded as one of the most reliable bone turnover markers in dialysis patients. However, bone metabolism is no longer considered identical to bone cell metabolism in modern bone biology. Bone extracellular material metabolism can be independent from bone cell metabolism, and recent studies revealed that bone material properties, i.e. bone biochemical characteristics rather than bone remodelling frequency, are the critical determinant of bone strength in uraemic condition [61, 62]. Most of the bone markers including PTH do not give information about these features, nor does bone histology examination. Although bone histomorphological analysis is the only way to establish a histological diagnosis, this is also the only information such a bone biopsy can provide. Moreover, estimating bone histology by the level of circulating bone markers remains of limited accuracy.
Total serum alkaline phosphatase (tAP) level showed tight associations with hip fracture risk as well as mortality in dialysis patients [63]. Its level may not totally represent bone remodelling because serum PTH level showed no relation to it. However, this assumption should be taken with caution because the lack of correlation between tAP and PTH may be due to the inaccuracies of PTH determination as above described. Arterial ‘osteonization’, commonly called Mönckeberg-type vascular calcification, in which vascular smooth muscle cells display osteoblast-lineage like phenotype, is frequently observed among dialysis patients. Since serum AP level represents the number of osteoblasts and osteoblast-like cells, it may reflect, at least partially, the development of this disease condition.
DELETERIOUS EFFECTS OF PTH ON THE HEART
PTH—among others—is a prototypic factor linking bone disease to cardiovascular disease, since PTH affects not only this physiological target organ, but also exhibits major ‘off-target’ effects in the cardiovascular system. Noteworthy, numerous cohort studies reported significant and independent associations between serum PTH levels and adverse outcomes including mortality [64]. It is important to mention that the association between PTH and outcome is not linear but J-shaped, suggesting an ‘optimal PTH’ range for these specific outcomes, which could be ∼150–300 pg/mL in dialysis patients [65]. Not only end-stage renal disease (ESRD) patients but also cohorts of primarily cardiology patients confirm the independent association between PTH and clinical outcome [66].
Left ventricular hypertrophy (LVH) is among the major contributing factors to morbidity and mortality in CKD and ESRD patients. LVH is driven by several factors among which PTH is a prominent one. A causal link between the two may exist by the stimulatory actions of PTH upon the renin–angiotensin–aldosterone system (RAAS) leading to increased levels of angiotensin II and aldosterone, which in turn cause LV remodelling and arterial hypertension [67]. Data from a meta-analysis of PTX effects upon LVH support the hypothesis of a causal link between PTH and LVH since LVH regressed in most patients with PHPT after surgery [68]. Underlying mechanisms may be (i) PTH-induced specific vascular pathology such as endothelial dysfunction and atherosclerosis; or (ii) direct detrimental myocardial effects by PTH that could lead to increased myocardial susceptibility to ischaemia or an increased risk for heart failure of non-ischaemic origin. However, proof of a causal role for PTH in cardiovascular disease is still lacking. Actually, lowering of PTH might in parallel induce lowering of other factors (i.e. FGF23), which are by themselves propelling cardiovascular morbidity and mortality [69]. Some experimental research data even point towards protection by PTH: application of PTH in rodent myocardial infarction models led to reduction in infarction size and improved cardiac function parameters [70]. Better recruitment of stem cells via PTH-induced mobilization might serve as an explanation for these remarkable observations [71].
CONTROL OF SHPT BY OLD AND NEW VITAMIN D COMPOUNDS
True deficiency, or relative insufficiency, of 25OHD is highly prevalent among patients with CKD or ESRD and is a critical component in the pathogenesis of SHPT. Accordingly, current guidelines suggest that in the setting of CKD G3a–G5D, 25OHD levels might be measured, and repeated testing depending on baseline values and therapeutic interventions. They also suggest that vitamin D deficiency and insufficiency be corrected using treatment strategies recommended for the general population [1, 72].
Whereas nutritional vitamin D replacement may restore 25OHD concentration to near, or even above ‘normal’, the real target of treating vitamin D insufficiency is and will remain the successful treatment of SHPT, which is largely unaffected by nutritional vitamin D.
Ranged against that is softer evidence for the usefulness of using vitamin D to treat ‘renal bone disease’ which has been clinical custom and practice now for nearly six decades. The practice has been in the main to use high doses of synthetic vitamin D compounds, not naturally occurring ones. It is disappointingly true to say that even in 2018 there is paucity of evidence concerning the clinical benefits of vitamin D supplementation to treat vitamin D insufficiency in patients with Stages 3b–5 CKD [73].
While there are a number of studies that report the impact of vitamin D supplementation on serum vitamin D concentrations and some variable evidence of serum PTH concentration suppression, there has been much less focus on hard endpoint analysis (e.g. fractures, hospitalizations and overall mortality). In 2018, with the practice pattern changes of widespread clinical use of vitamin D and widespread supplementation of cholecalciferol or ergocalciferol by patients, it is now next to impossible to run a placebo-controlled trial over a decent period of time to explore relevant clinical outcomes. In this challenging situation, we need to ask what it is we are trying to achieve here for our patients, and how best to balance potential benefits with potential harm [74].
Extended-release calcifediol (ERC) 30 µg capsules were recently approved by the United States Food and Drug Administration for the treatment of SHPT in adults with Stages 3–4 CKD and vitamin D insufficiency (serum total 25OHD <75 nmol/L) [75]. Calcifediol is 25-hydroxyvitamin D3, a prohormone of the active calcitriol (1, 25-dihydroxyvitamin D3). ERC capsules have a lipophilic fill, which gradually releases calcifediol, corrects vitamin D insufficiency and increases serum calcitriol, and thereby suppresses production of PTH in CKD patients without perturbing normal vitamin D and mineral metabolism. Randomized clinical trials (RCTs) have demonstrated that non-modified nutritional vitamin D is ineffective for treating SHPT (when used in conventional doses, up to the equivalent of around 4000 IU/day) whereas vitamin D receptor activators can very easily and significantly correct elevated PTH concentrations, but with increased risk of hypercalcaemia and hyperphosphataemia [74]. ERC offers healthcare professionals a new treatment option that has been demonstrated in RCTs to be safe and effective for controlling SHPT without meaningfully increasing serum concentrations of calcium or phosphorus [75, 76].
OLD AND NEW CALCIMIMETICS
Calcimimetics are established treatments for SHPT. They increase the sensitivity of CaSR leading to the decrease of the set-point for systemic calcium homeostasis. This enables a decrease in plasma PTH levels, and consequently of calcium levels. Cinacalcet was the first calcimimetic approved for clinical use and effectively reduces PTH and improves biochemical control of mineral and bone disorders in dialysis patients [77]. Three randomized controlled trials analysed the effects of treatment with cinacalcet on vascular calcification, bone histology and cardiovascular mortality and morbidity [78–80]. The Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) trial [81] was a RCT enrolling 3883 haemodialysis patients with moderate to severe SHPT assigned to receive cinacalcet or placebo. All patients could simultaneously receive conventional treatment including phosphate binders and vitamin D sterols. The primary composite endpoint was time until death, myocardial infarction, hospitalization for unstable angina, heart failure or a peripheral vascular event. Regrettably, this large RCT could not demonstrate improvement of hard outcomes with cinacalcet based on intention-to-treat analysis. However, the interpretation of a negative study outcome has been considered inconclusive rather than definitive, due to significant cross-over of placebo-treated cases to the active treatment arm.
Etelcalcetide is a new, second-generation calcimimetic administered intravenously. Its pharmacokinetic profile allows thrice-weekly dosing at the time of haemodialysis. It was recently approved in Europe. A double-bind, double-dummy active RCT was conducted comparing intravenous etelcalcetide with oral placebo versus oral cinacalcet with intravenous placebo in 683 haemodialysis patients with PTH higher than 500 pg/mL [82]. The primary efficacy endpoint was non-inferiority of etelcalcetide at achieving more than a 30% reduction from baseline in mean pre-dialysis PTH concentration. Secondary endpoints included superiority in achieving biochemical endpoints (>50% and >30% reduction in PTH) and self-reported nausea or vomiting. Etelcalcetide was not inferior to cinacalcet in reducing PTH concentration and also met superiority criteria. The proportion of patients who achieved a greater than 30% and 50% PTH reduction was greater for etelcalcetide compared with cinacalcet treatment [83]. Hypocalcaemia was more frequent in the etelcalcetide group. Etelcalcetide did not prove to have fewer gastrointestinal symptoms despite intravenous administration. Overall safety and tolerability between etelcalcetide and cinacalcet were similar. Etelcalcetide treatment yielded more pronounced reduction in FGF23 levels than cinacalcet. Of note, in the EVOLVE trial (using oral cinacalcet) a 30% reduction of FGF23 levels was associated with significant reduction of primary composite endpoint, heart failure and death [69]. This promising finding of calcimimetics raises the possibility of a more pronounced impact in cardiovascular outcomes, mediated by FGF23 reductions.
IS THERE ANY INDICATION LEFT FOR PTX IN 2018?
The 2017 update of the KDIGO guideline indicates the need for PTX ‘in patients with CKD G3a–G5D with severe SHPT who fail to respond to medical or pharmacological therapy’. The strength of this recommendation, unchanged from the previous edition, is graded 2B (‘we suggest’ with ‘moderate evidence’) [1, 72]. Thus, PTX continues to be the last therapeutic step to control SHPT in CKD. Since in recent years the number of drugs available for SHPT increased, the need for PTX should progressively decrease. Indeed, data from the dialysis outcomes and practice patterns study [84] demonstrated that from 1996 to 2008 there has been a decrease in PTX rates, accompanied by an increased prescription of vitamin D and cinacalcet. Notably, this change of therapeutic strategy was associated with increasing PTH values in Europe, Australia-New Zealand and North America, but not in Japan (where PTH values were stable and the proportion of patients at target increased). However, a more recent study from the United States Renal Data System registry, which included a huge number of PTX cases (32 971) recorded between 2002 and 2011, showed that the rates of PTX have not declined in recent years. It is interesting to notice, in this study, that the nadir was reached in 2005, concomitant with cinacalcet commercialization, but then increased and remained stable at roughly 5/1000 dialysis patients by 2006 [85].
Thus, new more powerful drugs do not seem to reduce the incidence rate of PTX for biochemical control of SHPT. Unfortunately, available data indicate that after PTX, the biochemical control of SHPT sometimes does not improve [86] or might even worsen in the medium-long term, due to a high prevalence of low PTH values [87]. Hyperparathyroidism after PTX is of concern because of the assumed risk of low turnover bone, even though bone biopsies before and after PTX are rarely performed. A very recent prospective study, which included only 19 patients, demonstrated that 1 year after PTX almost all patients developed adynamic bone disease, and this was associated with a significant increment of coronary calcium content [88]. This negative finding is partly compensated by another recent study, showing lower hip fracture rates in patients who received PTX as compared with a control group [89]. Finally, Ishani et al. [90] reported increased hospitalization rates in the 30 days and 1-year periods following PTX, which would contribute to discourage this surgery. However, in contrast to these findings, the vast majority of observational studies evaluating hard clinical outcomes in the medium-long term after PTX indicate improvement as compared with control cases. A large recent observational study [91] including 4428 PTX cases and 4428 propensity-score matched controls from a Japanese registry showed a statistically significantly lower all-cause and cardiovascular mortality in the PTX group, in the 12 months following surgery. Despite the use of adequate methodology to adjust for confounding, selection bias can never be excluded in observational data analysis, which would favour the outcome for those allowed for surgery. The need for a prospective randomized trial persists for the optimal treatment of SHPT [92].
PREOPERATIVE PARATHYROID IMAGING
Whatever the surgical procedure performed, that is, subtotal PTX or total PTX with auto-transplantation, identification by the surgeon of all parathyroid glands is required, as outlined above. In one recent series, 12.8% of patients had fewer than four parathyroid glands identified at surgery and this was associated with the risk of persistent SHPT [93]. This is consistent with a large retrospective analysis showing that in ∼10% of operated patients, serum PTH levels at 1 month remained elevated (≥897 pg/mL) [86]. Difficulty in identifying all parathyroid glands is also associated with a higher risk of surgical complications, such as bleeding, haematoma, infection and injury to the recurrent laryngeal nerve [94].
Ultrasound is a useful and widely used preoperative imaging technique. However, even in experienced hands, ultrasound has limitations, mainly due to its inability to detect ectopic parathyroid glands, such as retro-tracheal, retro-oesophageal or mediastinal glands [95].
99mTc-sestamibi scintigraphy has higher sensitivity at detecting ectopic parathyroid glands than ultrasound [96, 97]. Moreover, hybrid gamma cameras now allow delineating the precise anatomical position of ectopic glands by fusing three-dimensional 99mTc-sestamibi images with computerized tomography (CT) cross-sectional images (single photon emission computerized tomography (SPECT)/CT) [98]. In the neck area, various protocols have been proposed to differentiate parathyroid lesions from the thyroid gland. The highest sensitivity is obtained through dual-tracer imaging using 99mTc-sestamibi + 123-iodine, with image subtraction [98, 99].
Besides depicting ectopic and supernumerary glands, 99mTc-sestamibi scanning can offer functional information in SHPT and tertiary hyperparathyroidism that can be helpful to select the remnant parathyroid tissue with the least autonomy, thus reducing the risk of recurrence [96, 100, 101].
Recurrent SHPT can be due to hyperplasia of remnant tissue after subtotally resected parathyroid gland or autograft, a supernumerary parathyroid gland, or both [102]. Reoperation for persistent or recurrent SHPT generally is a difficult procedure with a higher risk of complications than initial surgery. A combination of imaging techniques (ultrasound, 99mTc-sestamibi/iodine-123 subtraction, CT, magnetic resonance imaging (MRI)) is necessary to localize the offending lesion with enough certainty.
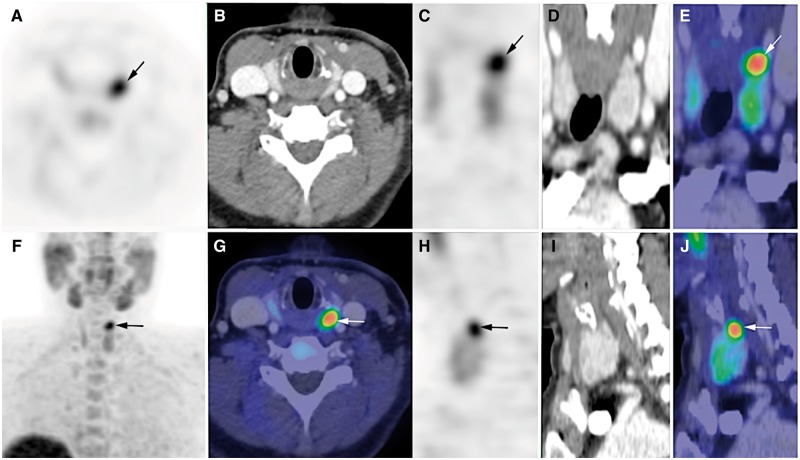
The clinical utility of radiopharmaceuticals labelled with isotopes and used in positron-emitting tomography (PET), such as the amino acid 11C-methionine or the lipid tracer 18F-fluorocholine, is also being investigated [103, 104]. Large series comparing 18F-fluorocholine PET/CT (or PET/MR) with state-of-the-art 99mTc-sestamibi/iodine-123 subtraction scintigraphy in SHPT are warranted (Figure 3).
FIGURE 3.
18F-fluorocholine PET/CT of parathyroid glands. 18F-fluorocholine PET/CT (contrast-enhanced CT) in a patient with PHPT and doubtful results on 99mTc-sestamibi scintigraphy. PET, CT and PET/CT images are displayed in axial (A, B and G), coronal (C, D and E) and sagittal (H, I and J) views, as well as PET maximum-intensity projection (F). Choline-avid hyperfunctioning parathyroid gland is seen at upper pole of left thyroid lobe (arrow). Neck ultrasound confirmed presence of hypoechoic 10 × 5 × 12 mm nodule behind left upper pole of thyroid. Adapted from Hindié et al. [102].
PERSISTENT HYPERPARATHYROIDISM AFTER RENAL TRANSPLANTATION
Successful kidney transplantation corrects the metabolic abnormalities responsible for SHPT. PTH levels show a biphasic decline after successful renal transplantation: an initial rapid drop (by ∼50%) during the first 3–6 months, attributed to a reduction of the parathyroid functional mass, followed by a more gradual decline. The long lifespan of parathyroid cells (approximately 20 years) contributes to the very slow involution of the hyperplastic parathyroid glands after renal transplantation [105]. Parathyroid hyporesponsiveness (resistance), conversely, rapidly wanes after renal transplantation, parallel to the recovery of renal function, giving rise to the frequently encountered hypophosphataemia after kidney transplantation [13].
There is wide variation in the reported prevalence of post-transplant HPT, ranging between 10% and 66%. This huge variation may be explained at least partly by differences in diagnostic criteria, differences in study era and differences in interval since transplantation. A long dialysis vintage and severe SHPT at the time of transplantation reflected by high levels of PTH, calcium, phosphorus, and/or tAP or need for calcimimetic therapy confer an increased risk for persistent HPT [105]. After transplantation, suboptimal graft function, immunosuppressive drug therapy, metabolic acidosis, hyperphosphatoninism and low vitamin D levels may also increase PTH secretion and contribute to post-transplant HPT [105].
It should be emphasized that post-transplant HPT is the composite of truly persistent HPT and de novo SHPT. Estimating the relative contribution of these two components to high PTH levels observed in the individual patient may prove difficult. In general, persistent HPT prevails in the early post-transplant period while de novo SHPT will become more prominent as kidney transplant function declines. While persistent HPT by definition is maladaptive and may contribute to specific post-transplant complications such as hypercalcaemia [106], hypophosphataemia [107, 108], (cortical) bone loss [109], fractures [110, 111] and nephrocalcinosis [112], de novo SHPT may be appropriate to the declining transplant function. Consequently, patients with a (predominant) persistent HPT phenotype may be hypothesized to benefit most from PTH suppressive therapy. Calcimimetics, nutritional and active vitamin D (analogues) and PTX may all be considered in these patients. Calcimimetics effectively controlled hypercalcaemia in patients with post-transplant HPT but, opposite to PTX [113] and paricalcitol [114], failed to demonstrate a beneficial impact on bone mineral density [115]. Bone biomarkers [116], imaging techniques and ultimately bone histomorphometry [117] may help identifying patients that will benefit most from PTH-lowering therapy.
CONCLUSIONS AND PERSPECTIVES
A broad range of aspects related to PTH biology and pathology in CKD was covered during the PTH day, where many different specialties gathered. As outlined above, exciting, and even more important, highly clinically relevant new insights about PTH biology, its measurement, classical and off-target effects, continuously emerge from ongoing basic and clinical research, even today. Recent data suggest that the skeletal action of PTH in early CKD stages to the steadily increasing activation of PTH1R may be a key one, and the therapeutic strategies to decrease PTH levels at these stages might not be recommended. Of high relevance to patients with advanced CKD stages, complicated by SHPT, ongoing efforts to improve and fine-tune treatment, indeed broadened the therapeutic armamentarium. This is exemplified by the discovery, and subsequent targeting by drugs, of the CaSR, which are now developed into the recently released second-generation calcimimetics. Insights into the regulatory role of the FGF23 and Klotho on PTH release may also eventually be applied in clinical practice, as pharmaco-interventions on this recently discovered integrated system are at their dawn. Even the first available medical treatment for SHPT, vitamin D therapy, is about to undergo a revival, as the kinetic profile of a metabolic form of vitamin D for oral use has been modified by ERC, with promising preliminary efficacy and safety. For a long time, surgical treatment has been a more or less definite solution for unremitting SHPT. A major drawback of both subtotal PTX and total PTX with auto-transplantation has been, besides early surgical complications, the difficulty in estimating the amount of parathyroid tissue to remove, frequently leading to persistent HPT or iatrogenic HPT inducing adynamic bone disease. During the PTH day the most recent imaging and functional techniques, combining the use of dedicated tracers and high-resolution imaging, were summarized. These techniques hold promise to future better ‘dosing’ of surgical treatment of SHPT.
In addition to the advances above, patient-tailored approaches may eventually become feasible. This may in the future be achieved by deciphering in an individual patient to what extent SHPT contributes not only to bone disease, but also to cardiac dysfunction and even cognitive function, as recent data suggests a role for PTH in these morbidities, which highly impact on quality of life.
All in all, the comprehensive update on PTH in CKD as presented in Paris, September 2017 during the ERA-EDTA CKD-MBD Working Group meeting, reinforced the importance of this hormonal system, revived interest and holds promise to further improvements in care for our patients.
ACKNOWLEDGEMENTS
The ‘PTH Day in CKD’ CME course organized in Paris in September 2017 was sponsored by Vifor Pharma Fresenius Medical Care and Amgen, Inc. The abstracts have not been presented elsewhere.
CONFLICT OF INTEREST STATEMENT
A.C. reports personal fees from FMC during the conduct of the study. P.A.U.-T. reports grants from Vifor Pharma Fresenius Medical Care, grants from Amgen during the conduct of the study. All other authors declare no conflicts of interest.
REFERENCES
- 1. Ketteler M, Block GA, Evenepoel P. et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney Int 2017; 92: 26–36 [DOI] [PubMed] [Google Scholar]
- 2. Drueke TB. Hyperparathyroidism in chronic kidney disease. In: De Groot LJ, Chrousos G, Dungan K et al. (eds). Endotext South Dartmouth, MA: MDText.com, Inc., 2000 [Google Scholar]
- 3. Sela-Brown A, Naveh-Many T, Silver J.. Transcriptional and post-transcriptional regulation of PTH gene expression by vitamin D, calcium and phosphate. Miner Electrolyte Metab 1999; 25: 342–344 [DOI] [PubMed] [Google Scholar]
- 4. Shilo V, Mor-Yosef Levi I, Abel R. et al. Let-7 and MicroRNA-148 regulate parathyroid hormone levels in secondary hyperparathyroidism. J Am Soc Nephrol 2017; 28: 2353–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Volovelsky O, Cohen G, Kenig A. et al. Phosphorylation of ribosomal protein S6 mediates mammalian target of rapamycin complex 1-induced parathyroid cell proliferation in secondary hyperparathyroidism. J Am Soc Nephrol 2016; 27: 1091–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang P, Duchambon P, Gogusev J. et al. Apoptosis in parathyroid hyperplasia of patients with primary or secondary uremic hyperparathyroidism. Kidney Int 2000; 57: 437–445 [DOI] [PubMed] [Google Scholar]
- 7. Arnold A, Brown MF, Ureña P. et al. Monoclonality of parathyroid tumors in chronic renal failure and in primary parathyroid hyperplasia. J Clin Invest 1995; 95: 2047–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imanishi Y, Tahara H, Palanisamy N. et al. Clonal chromosomal defects in the molecular pathogenesis of refractory hyperparathyroidism of uremia. J Am Soc Nephrol 2002; 13: 1490–1498 [DOI] [PubMed] [Google Scholar]
- 9. Brown EM, Gamba G, Riccardi D. et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 1993; 366: 575–580 [DOI] [PubMed] [Google Scholar]
- 10. Massy ZA, Hénaut L, Larsson TE. et al. Calcium-sensing receptor activation in chronic kidney disease: effects beyond parathyroid hormone control. Semin Nephrol 2014; 34: 648–659 [DOI] [PubMed] [Google Scholar]
- 11. Gogusev J, Duchambon P, Hory B. et al. Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int 1997; 51: 328–336 [DOI] [PubMed] [Google Scholar]
- 12. Hénaut L, Boudot C, Massy ZA. et al. Calcimimetics increase CaSR expression and reduce mineralization in vascular smooth muscle cells: mechanisms of action. Cardiovasc Res 2014; 101: 256–265 [DOI] [PubMed] [Google Scholar]
- 13. Evenepoel P, Bover J, Urena Torres P.. Parathyroid hormone metabolism and signaling in health and chronic kidney disease. Kidney Int 2016; 90: 1184–1190 [DOI] [PubMed] [Google Scholar]
- 14. Ureña P, Kong XF, Abou-Samra AB. et al. Parathyroid hormone (PTH)/PTH-related peptide receptor messenger ribonucleic acids are widely distributed in rat tissues. Endocrinology 1993; 133: 617–623 [DOI] [PubMed] [Google Scholar]
- 15. Usdin TB, Bonner TI, Harta G. et al. Distribution of parathyroid hormone-2 receptor messenger ribonucleic acid in rat. Endocrinology 1996; 137: 4285–4297 [DOI] [PubMed] [Google Scholar]
- 16. Bergwitz C, Klein P, Kohno H. et al. Identification, functional characterization, and developmental expression of two nonallelic parathyroid hormone (PTH)/PTH-related peptide receptor isoforms in Xenopus laevis (Daudin). Endocrinology 1998; 139: 723–732 [DOI] [PubMed] [Google Scholar]
- 17. Ureña P, Iida-Klein A, Kong XF. et al. Regulation of parathyroid hormone (PTH)/PTH-related peptide receptor messenger ribonucleic acid by glucocorticoids and PTH in ROS 17/2.8 and OK cells. Endocrinology 1994; 134: 451–456 [DOI] [PubMed] [Google Scholar]
- 18. Massy Z, Drueke T.. Adynamic bone disease is a predominant bone pattern in early stages of chronic kidney disease. J Nephrol 2017; 30: 629–634 [DOI] [PubMed] [Google Scholar]
- 19. Vervloet MG, Massy ZA, Brandenburg VM. et al. Bone: a new endocrine organ at the heart of chronic kidney disease and mineral disorders. Lancet Diabetes Endocrinol 2014; 2: 427–436 [DOI] [PubMed] [Google Scholar]
- 20. Olauson H, Lindberg K, Amin R. et al. Parathyroid-specific deletion of Klotho unravels a novel calcineurin-dependent FGF23 signaling pathway that regulates PTH secretion. PLoS Genet 2013; 9: e1003975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ott SM. Bone cells, sclerostin, and FGF23: what's bred in the bone will come out in the flesh. Kidney Int 2015; 87: 499–501 [DOI] [PubMed] [Google Scholar]
- 22. Komaba H, Goto S, Fujii H. et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int 2010; 77: 232–238 [DOI] [PubMed] [Google Scholar]
- 23. Avci HX, Lebrun C, Wehrle R. et al. Thyroid hormone triggers the developmental loss of axonal regenerative capacity via thyroid hormone receptor alpha1 and kruppel-like factor 9 in Purkinje cells. Proc Natl Acad Sci USA 2012; 109: 14206–14211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chiu SL, Chen CM, Cline HT.. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron 2008; 58: 708–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de la Monte SM, Tong M.. Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem Pharmacol 2014; 88: 548–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dou JT, Chen M, Dufour F. et al. Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn Mem 2005; 12: 646–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feng L, Li AP, Wang MP. et al. Plasticity at axon initial segment of hippocampal CA3 neurons in rat after status epilepticus induced by lithium-pilocarpine. Acta Neurochir (Wien) 2013; 155: 2373–2380; discussion 2380 [DOI] [PubMed] [Google Scholar]
- 28. Juntti SA, Tollkuhn J, Wu MV. et al. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron 2010; 66: 260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liston C, Cichon JM, Jeanneteau F. et al. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci 2013; 16: 698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oury F, Khrimian L, Denny CA. et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell 2013; 155: 228–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tyzio R, Cossart R, Khalilov I. et al. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science 2006; 314: 1788–1792 [DOI] [PubMed] [Google Scholar]
- 32. Villeda SA, Luo J, Mosher KI. et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011; 477: 90–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Villeda SA, Plambeck KE, Middeldorp J. et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med 2014; 20: 659–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aslan D, Andersen MD, Gede LB. et al. Mechanisms for the bone anabolic effect of parathyroid hormone treatment in humans. Scand J Clin Lab Invest 2012; 72: 14–22 [DOI] [PubMed] [Google Scholar]
- 35. Karsenty G, Kronenberg HM, Settembre C.. Genetic control of bone formation. Annu Rev Cell Dev Biol 2009; 25: 629–648 [DOI] [PubMed] [Google Scholar]
- 36. Dotzenrath CME, Kaetsch AK, Pfingsten H. et al. Neuropsychiatric and cognitive changes after surgery for primary hyperparathyroidism. World J Surg 2006; 30: 680–685 [DOI] [PubMed] [Google Scholar]
- 37. Numann PJ, Torppa AJ, Blumetti AE.. Neuropsychologic deficits associated with primary hyperparathyroidism. Surgery 1984; 96: 1119–1123 [PubMed] [Google Scholar]
- 38. Walker MD, McMahon DJ, Inabnet WB. et al. Neuropsychological features in primary hyperparathyroidism: a prospective study. J Clin Endocrinol Metab 2009; 94: 1951–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chiang CY, Andrewes DG, Anderson D. et al. A controlled, prospective study of neuropsychological outcomes post parathyroidectomy in primary hyperparathyroid patients. Clin Endocrinol (Oxf) 2005; 62: 99–104 [DOI] [PubMed] [Google Scholar]
- 40. Chou F-F, Chen J-B, Hsieh K-C. et al. Cognitive changes after parathyroidectomy in patients with secondary hyperparathyroidism. Surgery 2008; 143: 526–532 [DOI] [PubMed] [Google Scholar]
- 41. Gilli P, De Bastiani P.. Cognitive function and regular dialysis treatment. Clin Nephrol 1983; 19: 188–192 [PubMed] [Google Scholar]
- 42. Schram MT, Trompet S, Kamper AM. et al. Serum calcium and cognitive function in old age. J Am Geriatr Soc 2007; 55: 1786–1792 [DOI] [PubMed] [Google Scholar]
- 43. Bühler G, Balabanova S, Milowski S. et al. Detection of immunoreactive parathyroid hormone-related protein in human cerebrospinal fluid. Exp Clin Endocrinol Diabetes 1997; 105: 336–340 [DOI] [PubMed] [Google Scholar]
- 44. Joborn C, Hetta J, Niklasson F. et al. Cerebrospinal fluid calcium, parathyroid hormone, and monoamine and purine metabolites and the blood-brain barrier function in primary hyperparathyroidism. Psychoneuroendocrinology 1991; 16: 311–322 [DOI] [PubMed] [Google Scholar]
- 45. Lourida I, Thompson-Coon J, Dickens CM. et al. Parathyroid hormone, cognitive function and dementia: a systematic review. PLoS One 2015; 10: e0127574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Usdin TB, Gruber C, Bonner TI.. Identification and functional expression of a receptor selectively recognizing parathyroid hormone, the PTH2 receptor. J Biol Chem 1995; 270: 15455–15458 [DOI] [PubMed] [Google Scholar]
- 47. Weaver DR, Deeds JD, Lee K. et al. Localization of parathyroid hormone-related peptide (PTHrP) and PTH/PTHrP receptor mRNAs in rat brain. Brain Res Mol Brain Res 1995; 28: 296–310 [DOI] [PubMed] [Google Scholar]
- 48. Weir EC, Brines ML, Ikeda K. et al. Parathyroid hormone-related peptide gene is expressed in the mammalian central nervous system. Proc Natl Acad Sci USA 1990; 87: 108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lepage R, Roy L, Brossard JH. et al. A non-(1-84) circulating parathyroid hormone (PTH) fragment interferes significantly with intact PTH commercial assay measurements in uremic samples. Clin Chem 1998; 44: 805–809 [PubMed] [Google Scholar]
- 50. Souberbielle J-C, Boutten A, Carlier M-C. et al. Inter-method variability in PTH measurement: implication for the care of CKD patients. Kidney Int 2006; 70: 345–350 [DOI] [PubMed] [Google Scholar]
- 51. Souberbielle JC, Friedlander G, Cormier C.. Practical considerations in PTH testing. Clin Chim Acta 2006; 366: 81–89 [DOI] [PubMed] [Google Scholar]
- 52. Bilezikian JP, Brandi ML, Eastell R. et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab 2014; 99: 3561–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cavalier E, Delanaye P, Vranken L, et al. Interpretation of serum PTH concentrations with different kits in dialysis patients according to the KDIGO guidelines: importance of the reference (normal) values. Nephrol Dial Transplant 2012; 27: 1950–1956 [DOI] [PubMed] [Google Scholar]
- 54. Tepel M, Armbruster FP, Grön HJ. et al. Nonoxidized, biologically active parathyroid hormone determines mortality in hemodialysis patients. J Clin Endocrinol Metab 2013; 98: 4744–4751 [DOI] [PubMed] [Google Scholar]
- 55. Seiler-Mussler S, Limbach AS, Emrich IE. et al. Association of nonoxidized parathyroid hormone with cardiovascular and kidney disease outcomes in chronic kidney disease. Clin J Am Soc Nephrol 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Danese MD, Kim J, Doan QV. et al. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis 2006; 47: 149–156 [DOI] [PubMed] [Google Scholar]
- 57. Jadoul M, Albert JM, Akiba T. et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2006; 70: 1358–1366 [DOI] [PubMed] [Google Scholar]
- 58. Wakasugi M, Kazama JJ, Taniguchi M. et al. Increased risk of hip fracture among Japanese hemodialysis patients. J Bone Miner Metab 2013; 31: 315–321 [DOI] [PubMed] [Google Scholar]
- 59. Coco M, Rush H.. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis 2000; 36: 1115–1121 [DOI] [PubMed] [Google Scholar]
- 60. Stehman-Breen CO, Sherrard DJ, Alem AM. et al. Risk factors for hip fracture among patients with end-stage renal disease. Kidney Int 2000; 58: 2200–2205 [DOI] [PubMed] [Google Scholar]
- 61. Kazama JJ, Iwasaki Y, Fukagawa M.. Uremic osteoporosis. Kidney Int Suppl (2011) 2013; 3: 446–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kazama JJ, Matsuo K, Iwasaki Y. et al. Chronic kidney disease and bone metabolism. J Bone Miner Metab 2015; 33: 245–252 [DOI] [PubMed] [Google Scholar]
- 63. Maruyama Y, Taniguchi M, Kazama JJ. et al. A higher serum alkaline phosphatase is associated with the incidence of hip fracture and mortality among patients receiving hemodialysis in Japan. Nephrol Dial Transplant 2014; 29: 1532–1538 [DOI] [PubMed] [Google Scholar]
- 64. Floege J, Kim J, Ireland E. et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 2011; 26: 1948–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lau WL, Kalantar-Zadeh K, Kovesdy CP. et al. Alkaline phosphatase: better than PTH as a marker of cardiovascular and bone disease? Hemodial Int 2014; 18: 720–724 [DOI] [PubMed] [Google Scholar]
- 66. Pilz S, Tomaschitz A, Drechsler C. et al. Parathyroid hormone level is associated with mortality and cardiovascular events in patients undergoing coronary angiography. Eur Heart J 2010; 31: 1591–1598 [DOI] [PubMed] [Google Scholar]
- 67. Vaidya A, Brown JM, Williams JS.. The renin-angiotensin-aldosterone system and calcium-regulatory hormones. J Hum Hypertens 2015; 29: 515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McMahon DJ, Carrelli A, Palmeri N. et al. Effect of parathyroidectomy upon left ventricular mass in primary hyperparathyroidism: a meta-analysis. J Clin Endocrinol Metab 2015; 100: 4399–4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Moe SM, Chertow GM, Parfrey PS. et al. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) trial. Circulation 2015; 132: 27–39 [DOI] [PubMed] [Google Scholar]
- 70. Lehner S, Todica A, Vanchev Y. et al. In vivo monitoring of parathyroid hormone treatment after myocardial infarction in mice with [(68)Ga]Annexin A5 and [(18)F]Fluorodeoxyglucose positron emission tomography. Mol Imaging 2014; 13: 7290201400035 [DOI] [PubMed] [Google Scholar]
- 71. Brunner S, Weinberger T, Huber BC. et al. The cardioprotective effects of parathyroid hormone are independent of endogenous granulocyte-colony stimulating factor release. Cardiovasc Res 2012; 93: 330–339 [DOI] [PubMed] [Google Scholar]
- 72.KDIGO. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009;113: S1–S130 [DOI] [PubMed] [Google Scholar]
- 73. Agarwal R, Georgianos PI.. Con: nutritional vitamin D replacement in chronic kidney disease and end-stage renal disease. Nephrol Dial Transplant 2016; 31: 706–713 [DOI] [PubMed] [Google Scholar]
- 74. Goldsmith DJ. Pro: should we correct vitamin D deficiency/insufficiency in chronic kidney disease patients with inactive forms of vitamin D or just treat them with active vitamin D forms? Nephrol Dial Transplant 2016; 31: 698–705 [DOI] [PubMed] [Google Scholar]
- 75. Sprague SM, Crawford PW, Melnick JZ. et al. Use of extended-release calcifediol to treat secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Am J Nephrol 2016; 44: 316–325 [DOI] [PubMed] [Google Scholar]
- 76. Sprague SM, Strugnell SA. . Bishop CW. Extended-release calcifediol (Rayaldee) for secondary hyperparathyroidism. Med Lett Drugs Ther 2017; 59: 36–37 [PubMed] [Google Scholar]
- 77. Block GA, Martin KJ, de Francisco ALM. et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 2004; 350: 1516–1525 [DOI] [PubMed] [Google Scholar]
- 78. Behets GJ, Spasovski G, Sterling LR. et al. Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int 2015; 87: 846–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chertow GM, Parfrey PS.. Cinacalcet for cardiovascular disease in patients undergoing dialysis. N Engl J Med 2013; 368: 1844–1845 [DOI] [PubMed] [Google Scholar]
- 80. Raggi P, Bellasi A, Gamboa C. et al. All-cause mortality in hemodialysis patients with heart valve calcification. Clin J Am Soc Nephrol 2011; 6: 1990–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Investigators TET. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 2012; 357: 2482–2494 [DOI] [PubMed] [Google Scholar]
- 82. Block GA, Bushinsky DA, Cunningham J. et al. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA 2017; 317: 146–155 [DOI] [PubMed] [Google Scholar]
- 83. Block GA, Bushinsky DA, Cheng S. et al. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA 2017; 317: 156–164 [DOI] [PubMed] [Google Scholar]
- 84. Tentori F, Wang M, Bieber BA. et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol 2015; 10: 98–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kim SM, Long J, Montez-Rath ME. et al. Rates and outcomes of parathyroidectomy for secondary hyperparathyroidism in the United States. Clin J Am Soc Nephrol 2016; 11: 1260–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wetmore JB, Liu J, Do TP. et al. Changes in secondary hyperparathyroidism-related biochemical parameters and medication use following parathyroidectomy. Nephrol Dial Transplant 2016; 31: 103–111 [DOI] [PubMed] [Google Scholar]
- 87. Mazzaferro S, Pasquali M, Farcomeni A. et al. Parathyroidectomy as a therapeutic tool for targeting the recommended NKF-K/DOQI ranges for serum calcium, phosphate and parathyroid hormone in dialysis patients. Nephrol Dial Transplant 2008; 23: 2319–2323 [DOI] [PubMed] [Google Scholar]
- 88. Hernandes FR, Canziani MEF, Barreto FC. et al. The shift from high to low turnover bone disease after parathyroidectomy is associated with the progression of vascular calcification in hemodialysis patients: A 12-month follow-up study. PLoS One 2017; 12: e0174811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Isaksson E, Ivarsson K, Akaberi S. et al. The effect of parathyroidectomy on risk of hip fracture in secondary hyperparathyroidism. World J Surg 2017; 41: 2304–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ishani A, Liu J, Wetmore JB. et al. Clinical outcomes after parathyroidectomy in a nationwide cohort of patients on hemodialysis. Clin J Am Soc Nephrol 2015; 10: 90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Komaba H, Taniguchi M, Wada A. et al. Parathyroidectomy and survival among Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int 2015; 88: 350–359 [DOI] [PubMed] [Google Scholar]
- 92. Scialla JJ, Wolf M.. When there will never be a randomized controlled trial. Kidney Int 2015; 88: 220–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang L, Xing C, Shen C. et al. Diagnostic accuracy study of intraoperative and perioperative serum intact PTH level for successful parathyroidectomy in 501 secondary hyperparathyroidism patients. Sci Rep 2016; 6: 26841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Konturek A, Barczyński M, Stopa M. et al. Subtotal parathyroidectomy for secondary renal hyperparathyroidism: a 20-year surgical outcome study. Langenbecks Arch Surg 2016; 401: 965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vulpio C, Bossola M, De Gaetano A. et al. Parathyroid gland ultrasound patterns and biochemical findings after one-year cinacalcet treatment for advanced secondary hyperparathyroidism. Ther Apher Dial 2010; 14: 178–185 [DOI] [PubMed] [Google Scholar]
- 96. Hindié E, Urenã P, Jeanguillaume C. et al. Preoperative imaging of parathyroid glands with technetium-99m-labelled sestamibi and iodine-123 subtraction scanning in secondary hyperparathyroidism. Lancet 1999; 353: 2200–2204 [DOI] [PubMed] [Google Scholar]
- 97. Karipineni F, Sahli Z, Somervell H. et al. Are preoperative sestamibi scans useful for identifying ectopic parathyroid glands in patients with expected multigland parathyroid disease? Surgery 2018; 163: 35–41 [DOI] [PubMed] [Google Scholar]
- 98. Hindié E, Ugur O, Fuster D. et al. 2009 EANM parathyroid guidelines. Eur J Nucl Med Mol Imaging 2009; 36: 1201–1216 [DOI] [PubMed] [Google Scholar]
- 99. Krakauer M, Wieslander B, Myschetzky PS. et al. A prospective comparative study of parathyroid dual-phase scintigraphy, dual-isotope subtraction scintigraphy, 4D-CT, and ultrasonography in primary hyperparathyroidism. Clin Nucl Med 2016; 41: 93–100 [DOI] [PubMed] [Google Scholar]
- 100. Fuster D, Ybarra J, Ortin J. et al. Role of pre-operative imaging using 99mTc-MIBI and neck ultrasound in patients with secondary hyperparathyroidism who are candidates for subtotal parathyroidectomy. Eur J Nucl Med Mol Imaging 2006; 33: 467–473 [DOI] [PubMed] [Google Scholar]
- 101. Hindié E, Urena P, Melliere D. et al. Technetium-99m-sestamibi and iodine-123 subtraction scanning in primary and secondary hyperparathyroidism. Adv Nephrol Necker Hosp 1999; 29: 221–240 [PubMed] [Google Scholar]
- 102. Hindié E, Zanotti-Fregonara P, Just P-A. et al. Parathyroid scintigraphy findings in chronic kidney disease patients with recurrent hyperparathyroidism. Eur J Nucl Med Mol Imaging 2010; 37: 623–634 [DOI] [PubMed] [Google Scholar]
- 103. Hindie E, Zanotti-Fregonara P, Tabarin A. et al. The role of radionuclide imaging in the surgical management of primary hyperparathyroidism. J Nucl Med 2015; 56: 737–744 [DOI] [PubMed] [Google Scholar]
- 104. Michaud L, Balogova S, Burgess A. et al. A pilot comparison of 18F-fluorocholine PET/CT, ultrasonography and 123I/99mTc-sestaMIBI dual-phase dual-isotope scintigraphy in the preoperative localization of hyperfunctioning parathyroid glands in primary or secondary hyperparathyroidism: influence of thyroid anomalies. Medicine (Baltimore) 2015; 94: e1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Evenepoel P. Recovery versus persistence of disordered mineral metabolism in kidney transplant recipients. Semin Nephrol 2013; 33: 191–203 [DOI] [PubMed] [Google Scholar]
- 106. Evenepoel P, Bammens B, Claes K. et al. Measuring total blood calcium displays a low sensitivity for the diagnosis of hypercalcemia in incident renal transplant recipients. Clin J Am Soc Nephrol 2010; 5: 2085–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Evenepoel P, Naesens M, Claes K. et al. Tertiary ‘hyperphosphatoninism’ accentuates hypophosphatemia and suppresses calcitriol levels in renal transplant recipients. Am J Transplant 2007; 7: 1193–1200 [DOI] [PubMed] [Google Scholar]
- 108. van Londen M, Aarts BM, Deetman PE. et al. Post-transplant hypophosphatemia and the risk of death-censored graft failure and mortality after kidney transplantation. Clin J Am Soc Nephrol 2017; 12: 1301–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Iyer SP, Nikkel LE, Nishiyama KK. et al. Kidney transplantation with early corticosteroid withdrawal: paradoxical effects at the central and peripheral skeleton. J Am Soc Nephrol 2014; 25: 1331–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Giannini S, Sella S, Silva Netto F. et al. Persistent secondary hyperparathyroidism and vertebral fractures in kidney transplantation: role of calcium-sensing receptor polymorphisms and vitamin D deficiency. J Bone Miner Res 2010; 25: 841–848 [DOI] [PubMed] [Google Scholar]
- 111. Perrin P, Caillard S, Javier RM. et al. Persistent hyperparathyroidism is a major risk factor for fractures in the five years after kidney transplantation. Am J Transplant 2013; 13: 2653–2663 [DOI] [PubMed] [Google Scholar]
- 112. Evenepoel P, Lerut E, Naesens M. et al. Localization, etiology and impact of calcium phosphate deposits in renal allografts. Am J Transplant 2009; 9: 2470–2478 [DOI] [PubMed] [Google Scholar]
- 113. Cruzado JM, Moreno P, Torregrosa JV. et al. A randomized study comparing parathyroidectomy with cinacalcet for treating hypercalcemia in kidney allograft recipients with hyperparathyroidism. J Am Soc Nephrol 2016; 27: 2487–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Trillini M, Cortinovis M, Ruggenenti P. et al. Paricalcitol for secondary hyperparathyroidism in renal transplantation. J Am Soc Nephrol 2015; 26: 1205–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Evenepoel P, Claes K, Kuypers D. et al. Natural history of parathyroid function and calcium metabolism after kidney transplantation: a single-centre study. Nephrol Dial Transplant 2004; 19: 1281–1287 [DOI] [PubMed] [Google Scholar]
- 116. Evenepoel P, Cavalier E, D’Haese PC.. Biomarkers predicting bone turnover in the setting of CKD. Curr Osteoporos Rep 2017; 15: 178–186 [DOI] [PubMed] [Google Scholar]
- 117. Evenepoel P, Behets GJ, Viaene L. et al. Bone histomorphometry in de novo renal transplant recipients indicates a further decline in bone resorption 1 year posttransplantation. Kidney Int 2017; 91: 469–476 [DOI] [PubMed] [Google Scholar]