Abstract
Background
Patients with frequently relapsing (FR), steroid-dependent (SD) and steroid-resistant (SR) nephrotic syndrome are a therapeutic challenge with limited treatment options. Here, we retrospectively analyze the efficacy and safety of rituximab-induced continuous B-cell depletion in these populations.
Methods
Patients were included if they were at least 18 years of age and had FR, SD or SR minimal change disease (MCD) or primary focal segmental glomerulosclerosis (FSGS) and were treated with a strategy of continuous B-cell depletion. Partial remission (PR) was defined as a urinary protein:creatinine ratio (UPCR) of ≤3.5 g/g and a 50% reduction in the UPCR from baseline. Complete remission (CR) was defined as a UPCR ≤0.3 g/g.
Results
We identified 20 patients with MCD (n = 13) or FSGS (n = 7) who fulfilled the inclusion criteria. All patients had either SD (n = 12), SR (n = 7) or FR (n = 1) disease. Patients received a median of nine rituximab doses [interquartile range (IQR) 7.5, 11] and were treated for a median time of 28 months (IQR 23, 41). Prednisone was weaned from a median of 60 mg daily (IQR 40, 60) at rituximab initiation to 4.5 mg daily (IQR 0, 5.5) by 12 months. All patients achieved PR. CR occurred in 11 of 13 patients with FR or SD disease, but only 1 of 7 patients with SR disease (logrank P = 0.01). Four relapses occurred, all in patients with SR disease. Three serious infections occurred over 70.3 patient-years.
Conclusion
Continuous B-cell depletion is a therapeutic option in the management of complicated nephrotic syndrome. Additional studies are needed to clarify the utility of this strategy.
Keywords: focal segmental glomerulosclerosis, minimal change disease, rituximab
INTRODUCTION
Minimal change disease (MCD) and primary focal segmental glomerulosclerosis (FSGS) are podocytopathies that cause the nephrotic syndrome. Initial treatment for both disease processes is typically glucocorticoid monotherapy [1]. In a significant number of patients, treatment with glucocorticoids is complicated by recurrent relapses when therapy is discontinued [frequently relapsing (FR) disease], relapse during steroid tapering [steroid-dependent (SD) disease] and complete lack of response [steroid-resistant (SR) disease] [2, 3]. The inability to maintain remission predisposes patients to the risks of nephrotic syndrome and ultimately end-stage renal disease [4].
Most patients with SD and SR disease are treated with calcineurin inhibitors with or without low-dose prednisone [1, 5–7]. Remissions with calcineurin inhibitors are often partial rather than complete, and relapse is common upon discontinuation of therapy [3, 5]. The high relapse rate necessitates prolonged and often indefinite treatment. Some patients are able to successfully maintain remission on low-doses of a calcineurin inhibitor without significant treatment-related complications. However, in other patients prolonged therapy is associated with hypertension, diabetes mellitus, cosmetic effects, and irreversible renal fibrosis and chronic kidney disease [8, 9].
Rituximab is an anti-CD 20 monoclonal antibody that depletes B-cell lymphocytes and also possesses podocyte-specific effects [10]. Prior retrospective series in adults and randomized controlled trials in the pediatric population have demonstrated the efficacy of rituximab in patients with FR or SD disease, although relapses are not uncommon with B-cell reconstitution [11–17]. Patients with SR disease appear to have a less favorable response [18]. However, in most reports rituximab therapy was not continued if an initial response was not appreciated. Here, we present our experience of patients with FR, SD and SR nephrotic syndrome treated with prolonged continuous B-cell depletion.
MATERIALS AND METHODS
Study population
We performed a single-center retrospective analysis of adult patients with FR, SD or SR nephrotic syndrome who initiated treatment with rituximab-induced continuous B-cell depletion between September 2008 and June 2016 at the Massachusetts General Hospital Vasculitis and Glomerulonephritis Center. All patients had adult-onset disease and had a median age of 54 years at the time of rituximab initiation. Importantly, all patients with complicated nephrotic syndrome during this period were treated with a strategy of continuous B-cell depletion, minimizing concerns of a selection bias. Patients were included if they had biopsy proven MCD or primary FSGS and a urinary protein:creatinine ratio (UPCR) of >3.5 g/g at initiation of treatment. The study was approved by the Partners HealthCare Human Research Committee and performed in accordance with the Declaration of Helsinki.
Clinicians experienced in the diagnosis and management of glomerular disease differentiated primary from secondary FSGS based on the integration of clinical and pathologic features. Specifically, only patients with a history of nephrotic syndrome [UPCR >3.5 g/g, serum albumin (Alb) <3.5 mg/dL and edema] and widespread or diffuse effacement of the visceral epithelial cell foot processes on electron microscopy were considered to have primary FSGS (Supplementary data, Table S1) [19]. In addition, patients were excluded if they had an identifiable secondary cause of FSGS (e.g. unilateral renal agenesis). FR disease was defined as three or more relapses in the past year. SD disease was defined as relapse during steroid taper or within 2 weeks of steroid discontinuation. SR disease was defined as failure of prednisone at 1 mg/kg for ≥12 weeks to induce a remission or failure of glucocorticoids and a calcineurin inhibitor if glucocorticoids were administered at 1 mg/kg for <12 weeks due to glucocorticoid-related side effects (e.g. uncontrolled diabetes mellitus).
Treatment regimens
The rituximab dosing protocol was designed to maintain continuous B-cell depletion. Rituximab was initially administered as two 1000 mg IV doses separated by 2–4 weeks. Thereafter, rather than waiting for B-cell reconstitution or clinical relapse, rituximab was administered as one 1000 mg IV dose every 4 months. This dosing interval has been demonstrated to achieve continuous B-cell depletion in >95% of patients [20–22]. B-cell depletion was monitored prior to each dose with flow cytometry by examining the population of CD19+CD20+ lymphocytes. Patients with B-cell reconstitution at a 4-month dosing interval were administered rituximab every 3 months. If rituximab was continued for >2 years, then stretching the dosing interval to every 6 months was attempted. Prednisone and other immunosuppressive agents were initially administered with rituximab in most patients. Tapering of prednisone/immunosuppressive agents and the duration of rituximab therapy were at the discretion of the treating physician.
Outcomes
Partial remission (PR) was defined as a spot UPCR of ≤3.5 g/g and a 50% reduction in the UPCR from baseline confirmed on two consecutive measurements. Complete remission (CR) was defined as a UPCR ≤0.3 g/g and normalization of Alb on two consecutive occasions. Relapse after remission was defined as a UPCR >3.5 g/g on two occasions after remission was attained or on one occasion if there was any escalation in therapy. Serious adverse events (SAEs) were defined as events that were life threatening, led to hospitalization, caused persistent disability or permanent damage, or resulted in death. Serious infections (SIs) were defined as infections that required hospitalization or intravenous antibiotics. SAEs and SIs were determined by review of our electronic medical records and flow sheets maintained as part of patient management.
Statistical analyses
All analyses were carried out using Stata version 14 (College Station, TX, USA). Patient characteristics were stratified by histologic diagnosis and phenotype (i.e. FR, SD or SR). Continuous variables are presented as median [interquartile range (IQR)] and categorical variables are presented as percentages. Differences between continuous variables were compared using the Wilcoxon rank-sum test. Time to remission was examined using the Kaplan–Meier method and differenced between curves were compared with the log-rank test. Poisson regression was used to generate confidence intervals for SAEs and SIs.
RESULTS
Baseline characteristics
Twenty patients with either MCD (n = 13) or FSGS (n = 7) met inclusion criteria (Table 1). The majority of patients had SD (n = 12) or SR (n = 7) disease. All patients were previously treated with prednisone and 14 patients had failed additional immunosuppressive therapies (Table 1). Median (IQR) UPCR at initiation of treatment was 9.1 g/g (5.9, 17.5) and 5.8 g/g (4.1, 11.4) in patients with MCD and FSGS, respectively. The baseline median Alb was 2.6 g/dL (IQR 2.2, 2.9).
Table 1.
Baseline characteristics
| Patient | Age (years) | Sex | Diagnosis | Phenotype | Failed therapies | Proteinuriaa (g/g) | Alb (g/dL) | Cr (mg/dL) |
|---|---|---|---|---|---|---|---|---|
| 1 | 32 | M | MCD | FR | Pred | 12.1 | 2.2 | 1.0 |
| 2 | 46 | M | MCD | SD | Pred, Cyc, Aza | 9.0 | 2.7 | 1.0 |
| 3 | 23 | F | MCD | SD | Pred, FK, MMF | 17.5 | 1.8 | 0.5 |
| 4 | 60 | M | MCD | SD | Pred, CsA, MMF | 3.6 | 2.1 | 1.4 |
| 5 | 61 | M | MCD | SD | Pred | 18.0 | 1.7 | 1.2 |
| 6 | 53 | M | MCD | SD | Pred, Cyc, MMF | 5.9 | NA | 0.9 |
| 7 | 61 | F | MCD | SD | Pred, CsA | 18.5 | 3.1 | 4.4 |
| 8 | 34 | M | MCD | SD | Pred | 9.1 | NA | 0.8 |
| 9 | 40 | M | MCD | SR | Pred | 8.4 | 2.8 | 1.0 |
| 10 | 50 | M | MCD | SR | Pred, CsA, FK | 5.6 | 2.7 | 1.8 |
| 11 | 49 | M | MCD | SR | Pred, CsA | 17.5 | 2.0 | 1.3 |
| 12 | 22 | M | MCD | SR | Pred | 25.6 | 2.3 | 1.0 |
| 13 | 30 | M | MCD | SR | Pred, CsA | 5.0 | 2.6 | 1.1 |
| 14 | 56 | M | FSGS | SD | Pred, CsA, FK, MMF | 4.1 | 3.2 | 1.1 |
| 15 | 67 | M | FSGS | SD | Pred, MMF | 3.9 | NA | 1.3 |
| 16 | 67 | F | FSGS | SD | Pred, CsA | 5.8 | 3.2 | 1.2 |
| 17 | 72 | F | FSGS | SD | Pred | 11.4 | 2.9 | 1.1 |
| 18 | 54 | F | FSGS | SD | Pred, CsA, FK, MMF, Abat | 4.9 | 2.2 | 0.8 |
| 19 | 65 | F | FSGS | SR | Pred, CsA, MMF | 13.8 | 2.3 | 1.6 |
| 20 | 66 | F | FSGS | SR | Pred, Cyc, MMF | 8.0 | 3.1 | 1.3 |
Expressed as UPCR.
Abat, abatacept; Aza, azathioprine; F, female; FK, tacrolimus; M, male; Pred, prednisone.
Treatments
All patients were treated with rituximab-induced continuous B-cell depletion and a prednisone taper (Table 2). Patients received a median of 9 (IQR 7.5, 11) rituximab doses and were treated for a median time of 28 months (IQR 23, 41). Other medications used initially with rituximab included cyclophosphamide (Cyc) (n = 12), mycophenolate mofetil (MMF) (n = 2), cyclosporine (CsA) (n = 1) and tacrolimus (n = 1). The initial prednisone dose was 60 mg/day (IQR 40, 60). Prednisone was tapered such that the median (IQR) daily prednisone dose was 8.75 mg (5, 13.75) at 4 months, 5.0 mg (1.875, 10.0) at 8 months and 4.5 mg (0, 5.5) at 12 months (Figure 1). By 1 year, all patients with FR and SD disease were on ≤7.5 mg/day, whereas three of seven patients with resistant disease remained on 10 mg/day or more (maximum = 15 mg/day).
Table 2.
Treatment regimens and outcomes
| Patient | Initial Pred (mg/day) | Other meds/duration (months) | RTX doses (n) | Duration of RTX (months) | Outcome | Follow-up (months) | Follow-up after last RTX (months) |
|---|---|---|---|---|---|---|---|
| 1 | 60 | Cyc/2 | 9 | 26 | CR | 46 | 20 |
| 2 | 60 | Aza/21 | 28 | 71 | CR | 71 | 0 |
| 3 | 80 | None | 14 | 54 | CR | 60 | 6 |
| 4 | 60 | CsA/9 | 8 | 32 | CR | 55 | 24 |
| 5 | 60 | Cyc/2 | 8 | 28 | CR | 46 | 19 |
| 6 | 40 | Cyc/2 | 7 | 24 | CR | 39 | 14 |
| 7 | 60 | Cyc/3 | 9 | 34 | CR | 34 | 0 |
| 8 | 60 | None | 8 | 26 | CR | 32 | 7 |
| 9 | 10 | None | 20 | 90 | PR | 90 | 0 |
| 10 | 60 | Cyc/10 | 11 | 36 | PR | 36 | 0 |
| 11 | 80 | Cyc/6 | 9 | 28 | PR | 32 | 5 |
| 12 | 80 | Cyc/2 | 9 | 20 | PR | 22 | 2 |
| 13 | 40 | Cyc/6 | 4 | 11 | CR | 16 | 5 |
| 14 | 20 | FK/44 | 11 | 46 | CR | 83 | 37 |
| 15 | 5 | MMF/22 | 15 | 70 | PR | 70 | 0 |
| 16 | 40 | Cyc/2 | 8 | 25 | CR | 31 | 6 |
| 17 | 60 | Cyc/4 | 5 | 13 | PR | 14 | 1 |
| 18 | 60 | Cyc/3 | 6 | 13 | CR | 13 | 0 |
| 19 | 40 | Cyc/3 | 10 | 29 | PR | 29 | 0 |
| 20 | 15 | MMF/24 | 7 | 21 | PR | 24 | 3 |
Aza, azathioprine; FK, tacrolimus; Meds, medications; Pred, prednisone; RTX, rituximab.
FIGURE 1.
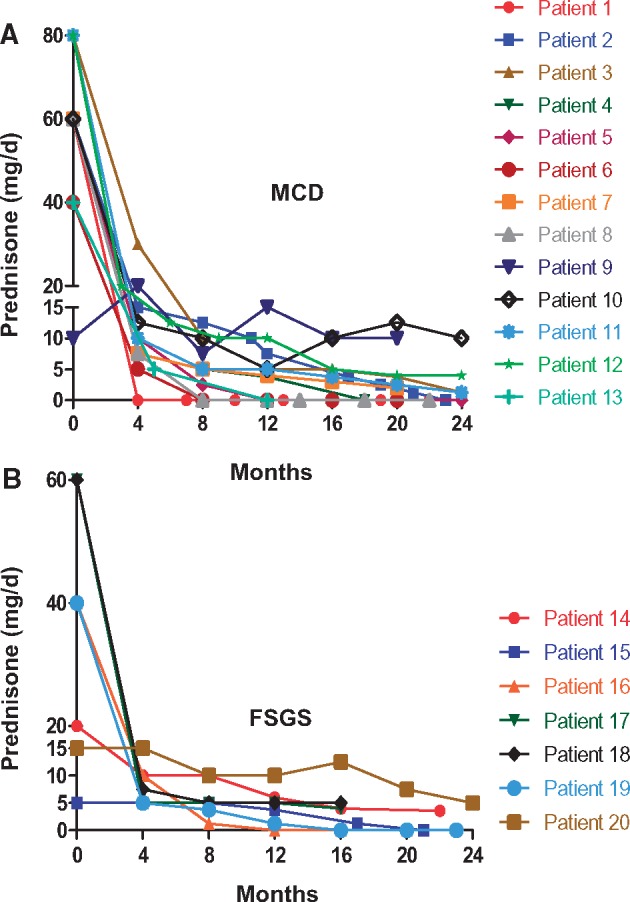
Prednisone dose. Shown is the prednisone dose for individual patients with MCD (A) and FSGS (B).
Remission
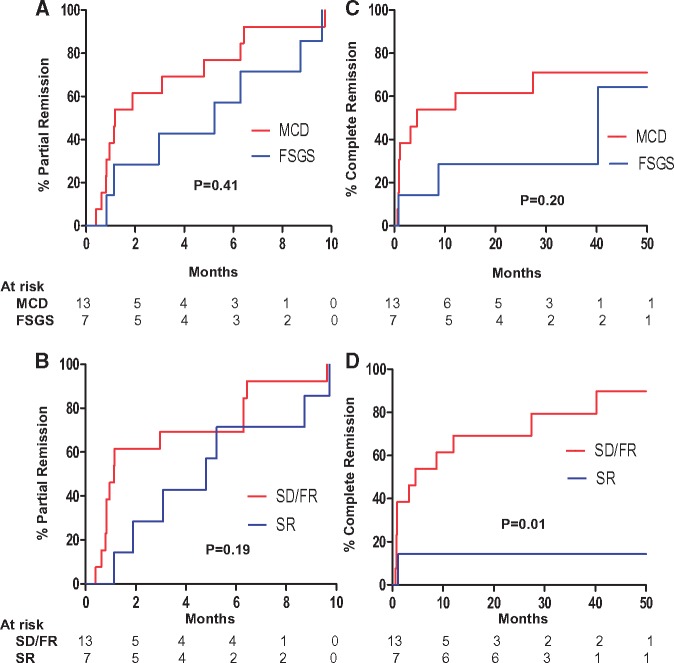
All patients achieved partial remission (PR) at a median time of 2.4 months (IQR 0.9, 6.3) (Figure 2A). Median time to PR was longer in patients with a diagnosis of FSGS [5.2 months (IQR 1.1, 8.7)] compared with MCD [1.2 months (IQR 0.8, 4.8)] (Wilcoxon rank-sum P = 0.20). Regardless of histologic diagnosis, patients with SR disease had a longer median time to PR compared with patients who had SD or FR disease [1.1 months (IQR 0.8, 6.3) versus 4.8 months (IQR 1.9, 8.7); Wilcoxon rank-sum P = 0.10] (Figure 2B).
FIGURE 2.
CR and PR. Shown are Kaplan–Meier curves for PR stratified by histologic diagnosis (A) and phenotype (B), and for CR stratified by histologic diagnosis (C) and phenotype (D). P-values were derived from the log-rank test.
In patients with MCD, 9 of 13 patients achieved CR at a median time of 1.1 months (IQR 0.9, 4.5), whereas 3 of 7 patients with FSGS achieved CR at a median time of 8.7 months (range 0.8–40.2) (Figure 2C). CR occurred in 11 of 13 patients with FR or SD disease, but only 1 of 7 patients with SR disease (Figure 2D; logrank P = 0.01).
Relapse after remission
Four patients (Patients 9, 10, 11 and 20) relapsed at a median time of 5 months (IQR 4, 8) after attaining PR. All relapsing patients had SR disease. The other 16 patients maintained remission without relapse, with a median follow-up time of 35 months (IQR 19, 57) following PR. All relapsing patients subsequently obtained a PR at median time of 2 months (IQR 2, 9) following relapse (Supplementary data, Table S2). No relapsing patients, however, went on to obtain a CR.
Rituximab therapy was discontinued in five patients (Patients 1, 4, 5, 6 and 14) during the study period after a median of 28 months (IQR 26, 32) of rituximab, none of whom had SR disease. B-cell recovery was documented in all patients, except in Patient 1 (not checked) who had 20 months of follow-up after the last rituximab. All of these patients maintained CR at a median follow-up time of 20 months (IQR 19, 24) after the last rituximab dose.
Changes in renal indices over time
Changes in proteinuria, Alb and serum creatinine (Cr) for individual patients during the first 2 years of therapy are shown in Figures 3–5, respectively. Response to treatment at 1 year overall and stratified by histologic diagnosis/disease phenotype is presented in Table 3. Cr did not change significantly during the first year. Proteinuria decreased and Alb increased significantly in all groups. After 1 year of treatment, UPCR had fallen to 0.1 g/g (IQR 0.1, 0.6) in patients with FR and SD disease versus 3.1 g/g (IQR 2.0, 3.9) in patient with SR disease (P = 0.01).
FIGURE 3.
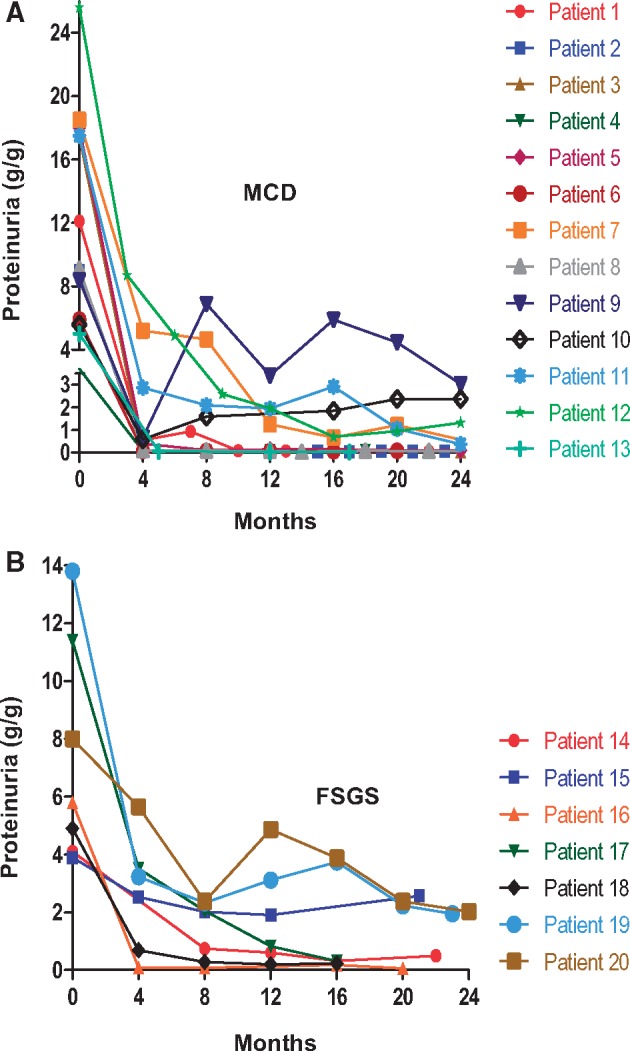
Proteinuria. Shown is the UPCR for individual patients with MCD (A) and FSGS (B).
Table 3.
Treatment outcomes at 1 year
| Overall |
MCD |
FSGS |
SD/FR |
SR |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Initial | 1 year | P | Initial | 1 year | P | Initial | 1 year | P | Initial | 1 year | P | Initial | 1 year | P |
| Proteinuria (g/g) | 8.7 (5.3, 15.7) | 0.4 (0.1, 2.0) | <0.01 | 9.1 (5.9, 17.5) | 0.1 (0.1, 2.0) | <0.01 | 5.8 (4.1, 11.4) | 0.8 (0.2, 3.1) | <0.01 | 9.0 (4.9, 12.1) | 0.1 (0.1, 0.6) | <0.01 | 8.4 (5.6, 17.5) | 3.1 (2.0, 3.9) | <0.01 |
| Albumin (g/dL) | 2.6 (2.2, 2.9) | 4.2 (3.8, 4.4) | <0.01 | 2.5 (2.1, 2.8) | 4.3 (3.6, 4.4) | <0.01 | 3.0 (2.3, 3.2) | 4.0 (3.8, 4.2) | <0.01 | 2.7 (2.1, 3.2) | 4.2 (4.2, 4.4) | <0.01 | 2.6 (2.3, 2.8) | 3.6 (3.2, 4.0) | <0.01 |
| Creatinine (mg/dL) | 1.1 (0.9,1.3) | 1.1 (0.9,1.4) | 0.83 | 1.0 (0.9, 1.3) | 1.1 (1.0,1.3) | 0.72 | 1.2 (1.1,1.3) | 1.0 (0.8, 1.4) | 0.56 | 1.1 (0.8, 1.3) | 1.0 (0.9,1.3) | 0.84 | 1.3 (1.0, 1.6) | 1.3 (1.1, 1.5) | 0.90 |
| Prednisone (mg/day) | 60 (40, 60) | 4.5 (0, 5.5) | <0.01 | 60 (60, 60) | 4 (0, 5) | <0.01 | 40 (15, 60) | 5 (1.25, 6) | <0.01 | 60 (40, 60) | 3.75 (0, 5) | <0.01 | 40 (15, 80) | 5 (1.25, 10) | <0.01 |
Data are presented as median (IQR). P-values obtained using the Wilcoxon rank-sum test.
FIGURE 4.
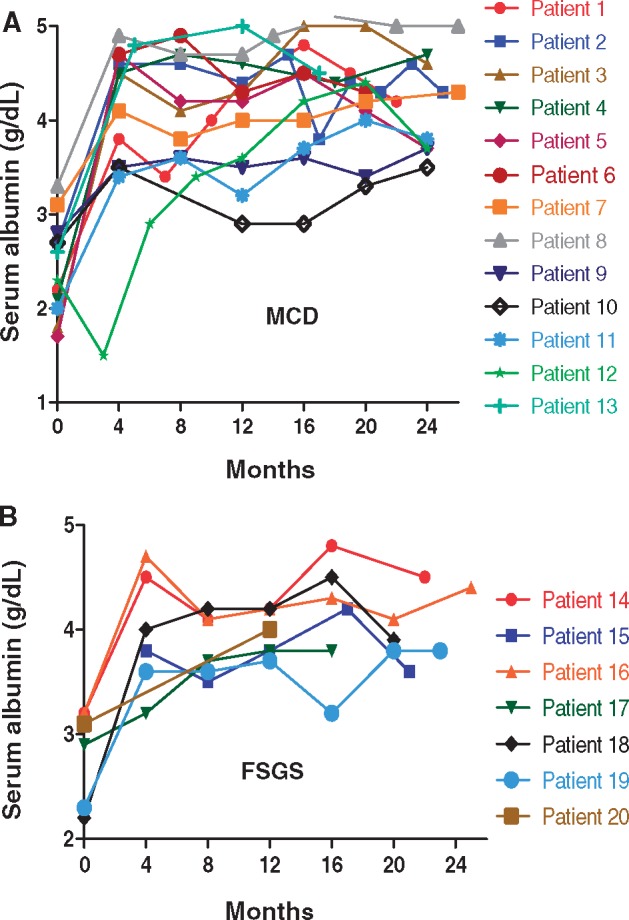
Alb. Shown is the Alb for individual patients with MCD (A) and FSGS (B).
FIGURE 5.
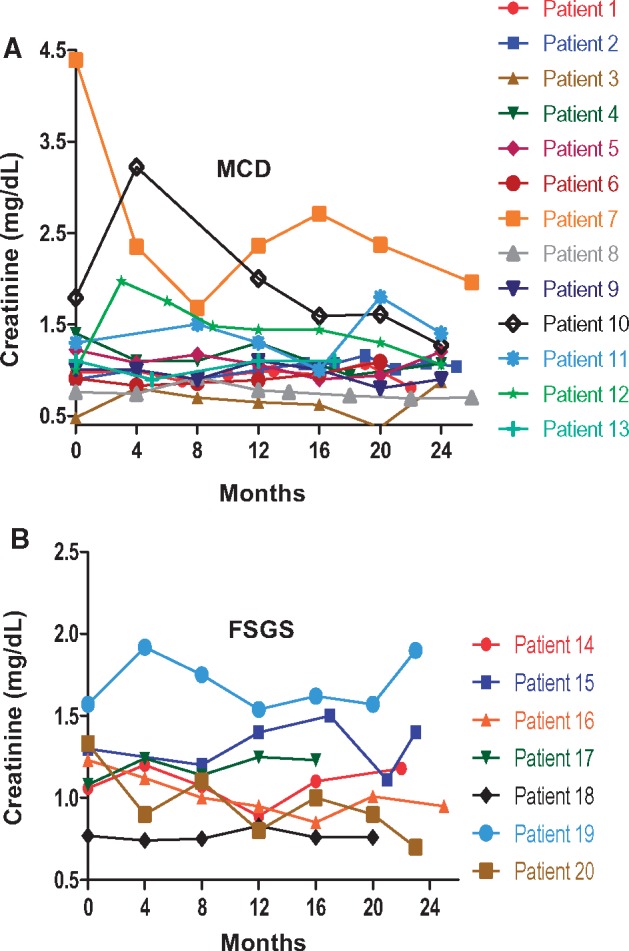
Cr. Shown is the Cr for individual patients with MCD (A) and FSGS (B).
Adverse events
The total exposure time was 70.3 patient-years. SAEs occurred at a rate of 0.10 events per year [95% confidence interval (CI) 0.04, 0.21] and SIs occurred at a rate of 0.04 infections per year (95% CI 0.01, 0.12). SAEs are shown in, Supplementary data, Table S3. Patient 12 developed cellulitis and had blood cultures that were positive for Morganella morganii a few weeks after his first rituximab infusion. Immediately before his first rituximab infusion, he was treated at an outside institution with prednisone 80 mg daily for 4 months without response. Patient 16 developed recurrent Clostridium difficile colitis following a course of amoxicillin for sinusitis after ∼2 years of continuous B-cell depletion. She had multiple recurrences and was ultimately treated successfully with a fecal transplant. One death occurred as a result of a cerebrovascular accident.
DISCUSSION
FR, SD and SR nephrotic syndrome are a therapeutic challenge for the nephrologist. This retrospective series provides evidence that continuous B-cell depletion may be a useful component of treatment in these complicated scenarios. All patients in the study achieved a PR and 12 of 20 patients achieved a CR with weaning of steroids to discontinuation or replacement doses. In agreement with prior reports, patients with FR and SD disease had a better response to rituximab than those with SR disease [12, 18].
The mechanism of rituximab in treating MCD and FSGS remains unclear. The leading hypothesis for the development of podocyte dysfunction in MCD and FSGS has been production of a circulating permeability factor by dysregulated T lymphocytes [23, 24]. It is possible that rituximab disrupts important B-cell–T-cell interactions that ultimately lead to production of a permeability factor in some patients. In addition, rituximab may have important podocyte specific effects akin to those observed with CsA [25]. Fornoni et al. demonstrated that rituximab can bind to sphingomyelin-phosphodiesterase-acid-like-3b on podocytes and preserve the structural integrity of podocytes incubated with the serum from patients who developed recurrent FSGS after transplant [10]. The differential effect of rituximab observed among patients with SD and SR disease suggests the possibility of distinct pathogenic mechanisms in these subsets.
Consistent with other reports, patients with FR and SD disease had an excellent response to rituximab therapy, with 11 of 13 patients achieving a sustained CR with weaning of steroids and discontinuation of other immunosuppressants. Within this steroid-responsive subgroup, rituximab appeared more efficacious in patients with a diagnosis of MCD (sustained CR in eight of eight patients) than those with FSGS (sustained CR in three of five patients). The small numbers, however, preclude firm conclusions. Moreover, it is possible that a subset of patients diagnosed with MCD has FSGS and were given a diagnosis of MCD due to sampling error.
In contrast to prior studies, patients with SR disease, most of whom failed a calcineurin inhibitor, achieved a PR with rituximab [18]. There are two likely explanations for this discrepancy. First, patients in our series were also initially treated with alternative immunosuppressants, most commonly Cyc. It is possible that a synergy between rituximab and the other immunosuppressants leads to the improved outcomes. Second, patients in our series were treated with prolonged B-cell depletion via repeated rituximab doses independent of a favorable initial response. Indeed, within the entire cohort, 6 of 20 patients achieved a PR after 6 months of treatment, and 2 patients achieved a PR after 9 months. While only one patient with resistant disease achieved a CR, it has been clearly demonstrated that even transient PRs are associated with improved outcomes [4].
Among patients with FR and SD disease, no relapses occurred over a median of ∼3 years following remission despite withdrawal of prednisone or weaning to replacement doses (≤5 mg per day). In a prior report of patients with SD MCD, 6 of 17 patients relapsed at a median time of approximately 1 year [11]. In all cases of relapse, B-cell reconstitution had occurred. In studies with a longer duration of follow-up, the relapse rate is considerably higher. In a report of 51 patients with FR and SD nephrotic syndrome treated with a single course of rituximab, 94% of patients subsequently sustained a relapse [17]. The low rate of relapse we observed can likely be attributed to our strategy of prolonged B-cell depletion. Further investigation is needed to define the optimal rituximab dosing regimen that balances relapse rate, side effects and cost.
In our series, none of the five patients in whom rituximab was discontinued developed a relapse after a median treatment-free follow-up time of 20 months. It may be that after a prolonged period of B-cell depletion, relapses occur less frequently even when therapy is withdrawn and B-cell reconstitution occurs. Larger studies are needed to test this hypothesis. Among patients with SR disease, rituximab was not discontinued given the concern for disease relapse in a patient subset where achieving remission is often difficult.
Prolonged B-cell depletion was well tolerated in most patients, with only three SIs over 70 patient-years. This favorable side-effect profile is consistent with larger reports of patients with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis treated with prolonged B-cell depletion as maintenance of remission therapy [20, 21]. The low rate of infections and total SAEs appears to be in part due to efficacy of rituximab on disease remission, allowing weaning of steroids. The median prednisone dose at 1 year was <5 mg/day. In addition, the low relapse rate obviates the need for repeated courses of high-dose glucocorticoids and/or prolonged treatment with calcineurin inhibitors, which can predispose to diabetes, osteoporosis, dyslipidemia, neurotoxicity and nephrotoxicity [26].
Our study has several important limitations. The study is retrospective, single-center and contains a relatively small number of patients. Moreover, there was no control group to provide a comparison. However, the near uniform response in a group of patients who had failed multiple prior therapies and had few treatment options corroborates our findings.
Treatment options for patients with complicated MCD and FSGS remain limited. In this retrospective analysis, we demonstrate that continuous B-cell depletion is an effective long-term management strategy for patients with SD disease. In addition, our findings suggest this strategy may be a useful component of therapy for SR disease, particularly when used initially with other immunosuppressants. Additional studies are needed to identify patient subgroups most likely to benefit from this strategy and to define the optimal duration of treatment.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the manuscript as detailed: F.B.C., J.R., K.L. and J.L.N. contributed to conception or design, or analysis and interpretation of data, or both; drafting the article or revising it; and final approval of the version to be published. F.B.C. and J.L.N. provided intellectual content of critical importance to the work described.
CONFLICT OF INTEREST STATEMENT
J.L.N. is currently participating in the Genentech-sponsored Rituximab in ANCA-Associated Vasculitis Registry (RAVER) Study. F.B.C. was previously supported via a fellowship grant from Genentech (G-17505). The results presented in this paper have not been published previously in whole or part, except in abstract form.
Supplementary Material
REFERENCES
- 1.Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl 2012; 2: 139–274 [Google Scholar]
- 2. Kyrieleis HA, Lowik MM, Pronk I. et al. Long-term outcome of biopsy-proven, frequently relapsing minimal-change nephrotic syndrome in children. Clin J Am Soc Nephrol 2009; 4: 1593–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Korbet SM. Treatment of primary FSGS in adults. J Am Soc Nephrol 2012; 23: 1769–1776 [DOI] [PubMed] [Google Scholar]
- 4. Troyanov S, Wall CA, Miller JA. et al. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol 2005; 16: 1061–1068 [DOI] [PubMed] [Google Scholar]
- 5. Cattran DC, Appel GB, Hebert LA. et al. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney Int 1999; 56: 2220–2226 [DOI] [PubMed] [Google Scholar]
- 6. Meyrier A, Condamin MC, Broneer D.. Treatment of adult idiopathic nephrotic syndrome with cyclosporin A: minimal-change disease and focal-segmental glomerulosclerosis. Collaborative Group of the French Society of Nephrology. Clin Nephrol 1991; 35 (Suppl 1): S37–S42 [PubMed] [Google Scholar]
- 7. Ponticelli C, Rizzoni G, Edefonti A. et al. A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int 1993; 43: 1377–1384 [DOI] [PubMed] [Google Scholar]
- 8. Meyrier A, Noël L-H, Auriche P. et al. Long-term renal tolerance of cyclosporin A treatment in adult idiopathic nephrotic syndrome. Collaborative Group of the Societe de Nephrologie. Kidney Int 1994; 45: 1446–1456 [DOI] [PubMed] [Google Scholar]
- 9. El-Husseini A, El-Basuony F, Mahmoud I. et al. Long-term effects of cyclosporine in children with idiopathic nephrotic syndrome: a single-centre experience. Nephrol Dial Transplant 2005; 20: 2433–2438 [DOI] [PubMed] [Google Scholar]
- 10. Fornoni A, Sageshima J, Wei C. et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med 2011; 3: 85ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Munyentwali H, Bouachi K, Audard V. et al. Rituximab is an efficient and safe treatment in adults with steroid-dependent minimal change disease. Kidney Int 2013; 83: 511–516 [DOI] [PubMed] [Google Scholar]
- 12. Ochi A, Takei T, Nakayama K. et al. Rituximab treatment for adult patients with focal segmental glomerulosclerosis. Intern Med 2012; 51: 759–762 [DOI] [PubMed] [Google Scholar]
- 13. Colucci M, Carsetti R, Cascioli S. et al. B cell reconstitution after rituximab treatment in idiopathic nephrotic syndrome. J Am Soc Nephrol 2016; 27: 1811–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miyabe Y, Takei T, Iwabuchi Y. et al. Amelioration of the adverse effects of prednisolone by rituximab treatment in adults with steroid-dependent minimal-change nephrotic syndrome. Clin Exp Nephrol 2016; 20: 103–110 [DOI] [PubMed] [Google Scholar]
- 15. Marasa M, Cravedi P, Ruggiero B. et al. Refractory focal segmental glomerulosclerosis in the adult: complete and sustained remissions of two episodes of nephrotic syndrome after a single dose of rituximab. BMJ Case Rep 2014; 2014: doi: 10.1136/bcr-2014-205507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ravani P, Rossi R, Bonanni A. et al. Rituximab in children with steroid-dependent nephrotic syndrome: a multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol 2015; 26: 2259–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamei K, Ishikura K, Sako M. et al. Long-term outcome of childhood-onset complicated nephrotic syndrome after a multicenter, double-blind, randomized, placebo-controlled trial of rituximab. Pediatr Nephrol 2017; 32: 2071–2078 [DOI] [PubMed] [Google Scholar]
- 18. Fernandez-Fresnedo G, Segarra A, Gonzalez E. et al. Rituximab treatment of adult patients with steroid-resistant focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2009; 4: 1317–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sethi S, Zand L, Nasr SH. et al. Focal and segmental glomerulosclerosis: clinical and kidney biopsy correlations. Clin Kidney J 2014; 7: 531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pendergraft WF, Cortazar FB, Wenger J. et al. Long-term maintenance therapy using rituximab-induced continuous B-cell depletion in patients with ANCA vasculitis. Clin J Am Soc Nephrol 2014; 9: 736–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cortazar FB, Pendergraft WF, Wenger J. et al. Effect of continuous B cell depletion with rituximab on pathogenic autoantibodies and total IgG levels in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol 2017; 69: 1045–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cortazar FB, Muhsin SA, Pendergraft WF 3rd. et al. Combination therapy with rituximab and cyclophosphamide for remission induction in ANCA vasculitis. Kidney Int Rep 2017; 3: 394–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koyama A, Fujisaki M, Kobayashi M. et al. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int 1991; 40: 453–460 [DOI] [PubMed] [Google Scholar]
- 24. Sellier-Leclerc A-L, Duval A, Riveron S. et al. A humanized mouse model of idiopathic nephrotic syndrome suggests a pathogenic role for immature cells. J Am Soc Nephrol 2007; 18: 2732–2739 [DOI] [PubMed] [Google Scholar]
- 25. Faul C, Donnelly M, Merscher-Gomez S. et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 2008; 14: 931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med 2004; 351: 2715–2729 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.