Abstract
Background
The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend targets based on multiples of the upper limit of normal (ULN) of parathyroid hormone (PTH) concentration. However, the ULN has not always been correctly established by manufacturers. While it is known that the ULN is supposed to be higher in African Americans than in Caucasians, it is largely unknown in Africans.
Methods
We established the ULN of PTH concentration in a population of 240 healthy Ivorians using second- and third-generation PTH assays before and after supplementation with 100 000 IU of cholecalferol. We measured the levels of PTH, bone alkaline phosphatase, 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in 100 haemodialysed Ivorian patients.
Results
The prevalence of vitamin D deficiency in Ivory Coast is low. The ULN obtained using the third-generation PTH assay was similar to that obtained in Caucasians but was higher when PTH was measured using the second-generation PTH assay. According to the KDIGO guidelines, ∼20% of the haemodialysed patients were below twice the ULN and 30% were above nine times the ULN. Approximately 25% of the patients were even >12 times the ULN. We observed a discrepancy in the results between the two PTH assays (14%) that was relatively more important than what we observed from previous studies in Caucasians using the same strategy.
Conclusions
We found a low prevalence of vitamin D deficiency in a tropical country like Ivory Coast. We also established the PTH reference range, which could prove useful for the follow-up of haemodialysed patients, particularly for the large number of patients suffering from secondary hyperparathyroidism who are at high risk of adverse bone events.
Keywords: bone alkaline phosphatase, haemodialysis, parathyroid hormone, reference range, vitamin D
INTRODUCTION
Parathyroid hormone (PTH) measurement is routinely performed in patients suffering from chronic kidney disease (CKD) for the diagnosis and management of CKD mineral and bone disorder. In dialysis patients, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend maintaining serum PTH levels within two to nine times the upper limit of normal (ULN) [1]. The definition of the ULN for PTH concentration is therefore of utmost importance for the care of these patients, and this raises a question about the inclusion/exclusion criteria that should be applied when recruiting a reference population to establish normal PTH values [2].
The exclusion criteria should take into consideration any situation potentially inducing an increase or a decrease in PTH concentration. This includes a low serum 25-hydroxyvitamin D [25(OH)D] concentration, which is very common in the general population [3, 4] and is thus likely to be prevalent in an apparently healthy group recruited to establish normal PTH values. Excluding these subjects has been strongly recommended in the two most recent guidelines on the diagnosis and management of asymptomatic primary hyperparathyroidism published in 2009 [5] and 2014 [6]. However, excluding vitamin D–deficient subjects from the reference group requires measuring the 25(OH)D concentration beforehand in all subjects, a practice that complicates the establishment of PTH reference values and is not considered in most studies that provided serum PTH reference values for different immunoassays [7–11]. We recently demonstrated the importance of such exclusion when determining the ULN of PTH concentration [12]. Moreover, the inclusion criteria should take into account the renal function when establishing PTH reference values. It is known that the PTH concentration may increase in some patients when the estimated glomerular filtration rate decreases [1].
Another issue concerning PTH reference values is whether the reference population should be stratified according to various factors such as age, sex, menopausal status, body mass index (BMI) and race. This latter parameter is of importance since it has been shown that serum PTH levels are higher in African Americans (AAs) than in their Caucasian counterparts [13–16]. However, it is not clear whether this is due to the high prevalence of vitamin D deficiency in AAs [17], since 25(OH)D has not always been measured in these studies, or if it is linked to recognition of PTH fragments by the second-generation (‘intact’) PTH assays [16]. Unfortunately, very little information is available on the PTH reference range in subjects of African descent, and even less in black individuals living in Africa. In this study we evaluated the PTH reference range for a second- and a third-generation PTH assay in a population of healthy individuals living in Abidjan, Ivory Coast before and after supplementation with a single dose of 100 000 IU cholecalciferol. Since very limited data are available in haemodialysis (HD) patients from this area of the world, we also measured the levels of PTH, bone alkaline phosphatase (bAP) and 1,25-dihydroxy vitamin D [1,25(OH)2D] in a population of 100 patients undergoing HD and classified them according to the KDIGO guidelines.
MATERIALS AND METHODS
Healthy individuals
A total of 203 healthy blood donors (including 122 women) from Abidjan, Ivory Coast (5°18′ N, 4°00′ W, 10 m above sea level and with 2100 h of yearly sunshine) were recruited and gave their informed consent. None of these subjects had been previously supplemented with cholecalciferol. The participants underwent a medical examination and blood sampling. Healthy status was confirmed by interview and clinical evaluation to exclude hypertension and diabetes and all subjects had normal blood results [normal haemoglobin and platelet and leucocyte counts; negative serology for human immunodeficiency virus (HIV) and hepatitis B and C; absence of significant proteinuria]. After blood sampling, the subjects took a single dose of 100 000 IU cholecalciferol (D-Cure, SMB Laboratories, Brussels, Belgium) and returned 1 week later for a second blood sampling.
Haemodialysed patients
A total of 100 HD patients (including 50 women) from the Centre Public d’Hémodialyse in Abidjan agreed to participate in the study and gave their informed consent. These patients underwent HD sessions of 4 h duration, twice a week. For the study they underwent a single blood sampling before the HD session.
Methods
Samples were centrifuged within 1.5 h of collection and aliquoted into separate vials that were kept at –80°C. After completion of the study, samples were shipped on dry ice to the Centre Hospitalier Universitaire (CHU) de Liège by a specialized transporter and measured within a month. Third-generation assays for PTH (whole PTH) and 25(OH)D were conducted using the Fujirebio Lumipulse G 1200 analyser (Fujirebio, Tokyo, Japan). Performance of the 25(OH)D assay has been recently described [18]. The third-generation PTH assay does not cross-react with N-truncated PTH fragments that accumulate in CKD patients [19], since the antibody used only recognizes the first four amino acids of the peptide. The coefficient of variation of the method is <7% and the expected range, established from 133 healthy Caucasians presenting with 25(OH)D concentrations >30 ng/mL, is 5.5–31.9 pg/mL in serum. Second-generation (‘intact’) PTH assays were also performed using Roche Elecsys (Mannheim, Germany) before cholecalciferol supplementation in healthy individuals and in HD patients. The expected range for this assay is 15–65 pg/mL and was established in a population of 72 healthy blood donors from Boston in 1987 [9]. bAP (Ostase) levels were measured in HD patients using the iSYS automated platform (IDS, Boldon, UK). We also measured 1,25(OH)2D levels in HD patients using the DiaSorin Liaison instrument (Saluggia, Italy). Calcium and phosphorus concentrations were determined locally using a Roche Cobas autoanalyser (Mannheim, Germany).
Statistics
Medcalc (Mariakerke, Belgium) was used to perform the Mann–Whitney and Wilcoxon tests. The reference interval was calculated using the non-parametric percentile method according to the Clinical and Laboratory Standards Institute (CLSI) C28-A3 guideline.
Ethics
The study was approved by the Comité National d’Ethique et de la Recherche (CNER) of the Ministère de la Santé et de l’Hygiène Publique of the Republic of Ivory Coast under the reference 072/MSHP/CNER-kp.
RESULTS
The mean age of healthy participants was 37.0 ± 10.3 years (no difference between men and women). The median BMI was 24.0 kg/m2 [95% confidence interval (CI) 23.4–24.9], with women presenting with significantly higher BMIs [24.2 kg/m2 (95% CI 23.5–25.9); P < 0.05] than men [23.5 kg/m2 (95% CI 22.9–24.6)]. Before cholecalciferol administration, the median third-generation PTH assay result was 15.0 pg/mL (95% CI 13.8–16.0) and the median second-generation PTH assay result was 33.3 pg/mL (95% CI 31.0–35.4). The reference range obtained with the Fujirebio Lumipulse third-generation PTH assay was from 6.1 (90% CI 5.4–8.0) to 35.8 (90% CI 31.4–40.9) pg/mL. The reference range using the Roche second-generation PTH assay was from 15.9 (90% CI 14.3–19.1) to 71.7 (90% CI 64.4–84.2) pg/mL. The median 25(OH)D level was 25.9 ng/mL (95% CI 24.9–27.0). Twenty subjects (9.0%) presented with 25(OH)D levels <20 ng/mL and 68 subjects (30.6%) naturally had 25(OH)D levels >30 ng/mL (Figure 1). There was no significant difference (P = 0.09) between median PTH levels observed in individuals presenting with 25(OH)D levels between 20 and 30 ng/mL and those with 25(OH)D levels >30 ng/mL.
FIGURE 1.
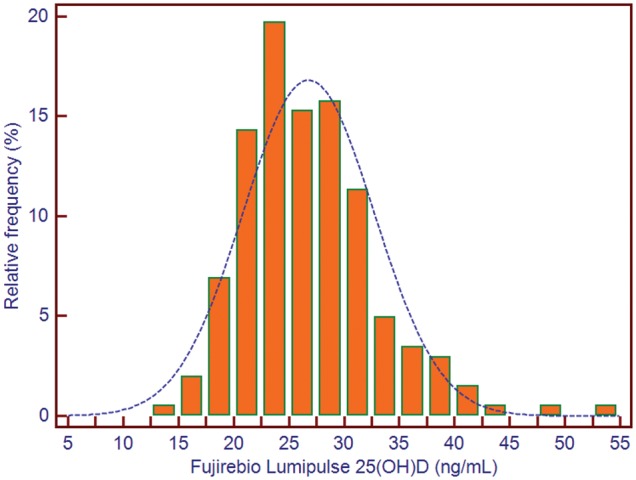
Distribution of 25(OH)D concentrations in 240 healthy individuals from Abidjan, Ivory Coast.
Supplementation with 100 000 IU cholecalciferol significantly increased 25(OH)D levels, with an increased median of 36.1 ng/mL (95% CI 34.8–37.7; P < 0.0001). A single subject still presented with 25(OH)D levels <20 ng/mL and 190 subjects (85.6%) had 25(OH)D levels >30 ng/mL. This increase was significantly inversely proportional to the BMI (r = –0.03; P < 0.0001). However, the increase in 25(OH)D levels had no impact on third-generation PTH assay results, and the median remained at 15.3 pg/mL (95% CI 14.4–16.5) after cholecalciferol supplementation. At baseline there was a significant difference (P = 0.001) between PTH concentrations observed in men [median 13.4 pg/mL (95% CI 12.6–14.8)], compared with women [median 16.0 pg/mL (95% CI 15.1–17.9)]. There was a non-significant trend (P = 0.07), in terms of sex difference, in the 25(OH)D status in women [25.7 ng/mL (95% CI 23.7–27.0)] versus men [26.4 ng/mL (95% CI 25.3–28.2)]. After cholecalciferol supplementation, the PTH concentration according to sex remained significant (P = 0.02) for PTH levels, but also became significant (P = 0.02) for 25(OH)D levels, with median values of 34.8 ng/mL (95% CI 33.7–36.9) in women versus 37.7 ng/mL (95% CI 35.9–39.9) in men. We observed a significant correlation in healthy patients between PTH and 25(OH)D levels, both before (ρ = −0.165; P = 0.0185) and after (ρ = −0.272; P = 0.0001) cholecalciferol supplementation.
The mean age of HD patients was 44.7 ± 12.7 years. Dialysis vintage was 38.5 months (range 1–236) and 61 patients were hypertensive. None of the patients were treated with a phosphate binder other than calcium carbonate. All patients were prescribed native vitamin D supplements, but compliance with both calcium and vitamin D supplementation was poor, since only 42% of the patients acknowledged taking the supplements, due to financial issues. Calcium levels ranged from 1.15 to 3.23 mmol/L, with a median of 2.15 mmol/L (95% CI 2.06–2.19); serum phosphate levels ranged from 0.43 to 4.72 mmol/L, with a median of 1.53 mmol/L (95% CI 1.39–1.75). The median 25(OH)D concentration was 40.2 ng/mL (95% CI 37.3–43.6). Five per cent of the HD patients presented with 25(OH)D levels <20 ng/mL, whereas 80% had 25(OH)D levels >30 ng/mL. The median concentration obtained with the third-generation PTH assay was 165 pg/mL (95% CI 132–234) and with the median second-generation PTH assay it was 403 pg/mL (95% CI 304–514). Taking into consideration the ULN of PTH concentration established in the healthy population, the KDIGO range for the third-generation PTH assay was established at 72–324 pg/mL and for the second-generation PTH assay at 144–648 pg/mL. With the third-generation (second-generation) assay, 22% (19%) of the patients presented with levels <72 (<144) pg/mL, 30% (32%) of which were higher than nine times the ULN. Fourteen per cent of the patients were misclassified, even if the correlation between both methods was very high (ρ = 0.939; P < 0.0001).
bAP concentrations ranged from 9.1 to 806 µg/L (median 48.4 µg/L) and 1,25(OH)2D vitamin D concentrations ranged from 3.6 to 71.1 pg/mL (median 13.1). According to Salam et al. [42], bAP cut-off values <21 and >31 µg/L (measured on the same instrument) presented the best area under the curve (AUC) for discriminating between low and high bone turnover, respectively. Twenty-three per cent of the HD patients had bAP concentrations <21 µg/L, which is quite similar to the proportion of patients with less than twice the ULN of PTH assays; however, the proportion of patients identified as having high bone turnover was 62%, which is much higher than the proportion of patients with PTH concentrations greater than nine times the ULN. There was no significant correlation between either of the PTH assays and 25(OH)D concentrations in HD patients, but we found a highly significant (P < 0.0001) correlation (ρ = 0.766) between bAP levels and both PTH assays and between 25(OH)D and 1,25(OH)2D levels (ρ = 0.522).
DISCUSSION
In this study we evaluated the 25(OH)D status of healthy blood donors from Abidjan, Ivory Coast, a city located just above the equator, and established the reference range for third- (Fujirebio Lumipulse) and second-generation (Roche Elecsys) PTH assays. We also determined the concentrations of 25(OH)D, PTH, bAP and 1,25(OH)2D vitamin D in a population of HD patients from the same city. In this region of the world, no seasonal variation of 25(OH)D levels can be expected [20] and vitamin D intake in food is unlikely to contribute to 25(OH)D concentrations [21]. Data on the prevalence of vitamin D deficiency or sufficiency in tropical Africa are scarce and results have generally been obtained in small subsets of individuals, mainly children [20]. In this study we found that 9% of healthy subjects presented vitamin D deficiency [defined as 25(OH)D levels <20 ng/mL]. This is much lower than results obtained in a recent study conducted in Abidjan that found the prevalence of vitamin D deficiency in a control population of 110 HIV-negative subjects was 33% [22]. One of the reasons for this discrepancy is the assay used for 25(OH)D measurements. Indeed, some assays, particularly the one used in that study (VIDAS, bioMérieux), are known to underrecover 25(OH)D in African subjects [23], whereas the Fujirebio Lumipulse assay that we used in our study is much less affected [18]. It is not entirely clear why African subjects behave differently in terms of their 25(OH)D profile, but this could be partly due to differences in the polymorphism of vitamin D–binding protein [24]. The Vitamin D Standardization Program has definitely improved the standardization of assays for 25(OH)D measurement (either immunoassays or liquid chromatography–tandem mass spectrometry (LCMS/MS)) and is a benchmark for the evaluation of 25(OH)D assay performance and standardization [25–27]—and this is a very important step. Nevertheless, we have shown that in African subjects, this standardization is lost and spurious results can present to clinicians or hamper epidemiological studies.
Determination of the PTH reference range is of paramount importance in the diagnostic and management of diseases related to phosphocalcic metabolism. We previously showed that exclusion of subjects presenting with PTH levels <20 ng/mL resulted in a lower PTH reference range [28]—and it is now good practice to apply this exclusion criterion [6]. While there are virtually no reports on PTH reference ranges observed in healthy Africans, data obtained in AAs showed that this group presents lower 25(OH)D levels and higher PTH levels than Caucasians. Of note, AA women have a lower bone turnover and a higher bone density than their Caucasian counterparts [29]. Interestingly, the reference range for the Fujirebio third-generation PTH assay in this population of healthy, vitamin D–sufficient African subjects from Ivory Coast (6.1–35.8 pg/mL) is very similar to that obtained in a population of healthy, vitamin D–replete French and Belgian individuals (6.4–41.8 pg/mL) [30]. On the other hand, the ULN obtained with the second-generation PTH assay from Roche in healthy African subjects (71.7 pg/mL) was much higher than what we previously reported in French and Belgian subjects (∼50 pg/mL) from different studies [2, 28]. This discrepancy may reasonably be attributable to interfering PTH fragments that are present in Africans. This finding warrants wider investigation using third-generation PTH assays and also highlights the lack of specificity of second-generation PTH assays, as well as the lack of PTH standardization. This also shows the necessity for each country and laboratory to define their specific reference ranges.
A single dose of 100 000 IU cholecalciferol did not impact on the reference range for the third-generation PTH assay, showing that 25(OH)D concentrations naturally achieved by the participants were adequate for their phosphocalcic status.
Data on the prevalence of vitamin D deficiency in HD patients from tropical Africa are also lacking [31]. In this study we showed that the prevalence of vitamin D deficiency was very low in this patient group (5%). In contrast, Seck et al. [31] found that 32.6% of HD patients in Dakar, Senegal (14°41′37″ N) presented 25(OH)D levels <15 ng/mL and that 60.8% had 25(OH)D levels ranging between 15 and 30 ng/mL. These Senegalese patients were older (50.3 ± 12.7 years) and there was no information on the method used to determine 25(OH)D levels. Different cultural habits could also explain the differences in the results observed between the two countries. Our patients are generally prescribed calcium and vitamin D supplements; however, since these supplements and the 25(OH)D test are not covered by health insurance, <50% of patients comply with taking the supplements and none have ever been tested for their 25(OH)D level. Of interest, we did not find any difference in 25(OH)D concentrations between those who complied with taking the supplements and those who did not.
According to the KDIGO guidelines, ∼20% of the HD patients were below twice the ULN of PTH concentrations and 30% were greater than nine times the ULN. Approximately 25% of the patients were even >12 times the ULN. We observed a discrepancy in the results obtained from the two PTH assays (14%) that was relatively more important than what we observed from previous studies in Caucasians using the same strategy [2, 30]. Unfortunately, interpretation of such results is difficult in the absence of bone biopsy and in the particular context of this population. Indeed, it is known that racial differences exist between Caucasians and AAs in terms of PTH concentrations, with AAs presenting with higher levels of PTH that are not certainly linked with secondary hyperparathyroidism (SHP) [32–37]. Also, as mentioned above, compliance with supplementation is poor because of the lack of health insurance. Nevertheless, similar levels of SHP have been found in other African countries like Senegal [31, 38], although this is not consistent with some data coming from Nigeria and the definition of SHP is not clear [39]. In HD AA patients, SHP is much more prevalent than low-turnover bone diseases [40], but of course, making comparisons between those population groups might not be accurate or appropriate.
Finally, in this study we report for the first time the bAP and 1,25(OH)2D vitamin concentrations in African HD patients. As expected, we observed a strong correlation between PTH and bAP levels and between 25(OH)D and 1,25(OH)2D vitamin levels. bAP is recommended by the KDIGO as a bone biomarker to be used in the clinic to diagnose and guide the management of renal osteodystrophy [41], and it has been shown to be a good discriminator of low bone turnover [42]. The proportion of patients suffering from high bone turnover according to recently published bAP cut-off values is much higher than if evaluated by traditional KDIGO cut-off values based on nine times the ULN of PTH concentrations.
Our study has limitations, including the lack of bone biopsy and the paucity of clinical data. However, this is the reality of these developing countries. On the other hand, the picture we present here has been obtained with robust analytical and pre-analytical methods and can be useful for the future management of these patients.
CONCLUSION
We showed that vitamin D deficiency is very limited in a tropical country like Ivory Coast, and we established a PTH reference range, which can be useful for the follow-up of HD patients, particularly for the large number of patients suffering from SHPT who are at high risk of adverse bone events.
ACKNOWLEDGEMENTS
Fujirebio kindly provided the reagents for the third-generation PTH assay and 25(OH)D measurements.
CONFLICT OF INTEREST STATEMENT
Etienne Cavalier is consultant for DiaSorin, IDS and Fujirebio. Pierre Delanaye is consultant for IDS.
REFERENCES
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009; (113) (Suppl 13): S1–130 [DOI] [PubMed]
- 2. Cavalier E, Delanaye P, Vranken L. et al. Interpretation of serum PTH concentrations with different kits in dialysis patients according to the KDIGO guidelines: importance of the reference (normal) values. Nephrol Dial Transplant 2011; 27: 1950–1956 [DOI] [PubMed] [Google Scholar]
- 3. Holick MF, Chen TC.. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008; 87: 1080S–1086S [DOI] [PubMed] [Google Scholar]
- 4. Touvier M, Deschasaux M, Montourcy M. et al. Interpretation of plasma PTH concentrations according to 25OHD status, gender, age, weight status, and calcium intake: importance of the reference values. J Clin Endocrinol Metabol 2014; 99: 1196–1203 [DOI] [PubMed] [Google Scholar]
- 5. Khan AA, Bilezikian JP, Potts JT.. The diagnosis and management of asymptomatic primary hyperparathyroidism revisited. J Clin Endocrinol Metabol 2009; 94: 333–334 [DOI] [PubMed] [Google Scholar]
- 6. Bilezikian JP, Brandi ML, Eastell R. et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metabol 2014; 99: 3561–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blind E, Schmidt-Gayk H, Scharla S. et al. Two-site assay of intact parathyroid hormone in the investigation of primary hyperparathyroidism and other disorders of calcium metabolism compared with a midregion assay. J Clin Endocrinol Metabol 1988; 67: 353–360 [DOI] [PubMed] [Google Scholar]
- 8. Endres DB, Villanueva R, Sharp CF. et al. Immunochemiluminometric and immunoradiometric determinations of intact and total immunoreactive parathyrin: performance in the differential diagnosis of hypercalcemia and hypoparathyroidism. Clin Chem 1991; 37: 162–168 [PubMed] [Google Scholar]
- 9. Nussbaum SR, Zahradnik RJ, Lavigne JR. et al. Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem 1987; 33: 1364–1367 [PubMed] [Google Scholar]
- 10. Ratcliffe WA, Heath DA, Ryan M. et al. Performance and diagnostic application of a two site immunoradiometric assay for parathyrin in serum. Clin Chem 1989; 35: 1957–1961 [PubMed] [Google Scholar]
- 11. Gao P, Scheibel S, D'Amour P. et al. Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1-84: implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res 2001; 16: 605–614 [DOI] [PubMed] [Google Scholar]
- 12. Souberbielle J-C, Brazier F, Piketty M-L. et al. How the reference values for serum parathyroid hormone concentration are (or should be) established? J Endocrinal Invest 2016; 40: 241–256 [DOI] [PubMed] [Google Scholar]
- 13. Sawaya BP, Butros R, Naqvi S. et al. Differences in bone turnover and intact PTH levels between African American and Caucasian patients with end-stage renal disease. Kidney Int 2003; 64: 737–742 [DOI] [PubMed] [Google Scholar]
- 14. Bell NH, Greene A, Epstein S. et al. Evidence for alteration of the vitamin-D-endocrine system in Blacks. J Clin Invest 1985; 76: 470–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moore C, Yee J, Malluche H. et al. Relationship between bone histology and markers of bone and mineral metabolism in African-American hemodialysis patients. Clin J Am Soc Nephrol 2009; 4: 1484–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fehmi H, Osman Y, Bhat S. et al. Absence of adynamic bone disease in African-Americans with CKD stage 5 after 3 years of vitamin D therapy guided by iPTH and the PTH-(1-84)/N-terminally truncated PTH fragments ratio. Clin Nephrol 2009; 71: 267–275 [DOI] [PubMed] [Google Scholar]
- 17. Looker AC, Johnson CL, Lacher DA. et al. Vitamin D status: United States, 2001–2006. NCHS Data Brief 2011; 127: 1–8 [PubMed] [Google Scholar]
- 18. Cavalier E, Lukas P, Bekaert A-C.. Analytical and clinical evaluation of the new Fujirebio Lumipulse® G non-competitive assay for 25(OH)-vitamin D and three immunoassays for 25(OH)D in healthy subjects, osteoporotic patients, third trimester pregnant women, healthy African subjects, hemodia. Clin Chem Lab Med 2015; 1–9 [DOI] [PubMed] [Google Scholar]
- 19. D’Amour P, Brossard J-H, Rousseau L. et al. Structure of non-(1-84) PTH fragments secreted by parathyroid glands in primary and secondary hyperparathyroidism. Kidney Int 2005; 68: 998–1007 [DOI] [PubMed] [Google Scholar]
- 20. Prentice A, Schoenmakers I, Jones KS.. Vitamin D deficiency and its health consequences in Africa. Clin Rev Bone Mineral Metabol 2009; 7: 94–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MacKeown JM, Cleaton-Jones PE, Edwards AW. et al. Energy, macro- and micronutrient intake of 5-year-old urban black South African children in 1984 and 1995. Paediatr Perinatal Epidemiol 1998; 12: 297–312 [DOI] [PubMed] [Google Scholar]
- 22. Boyvin L, Aké J, Séri K. et al. 25 (OH) Vitamin D level and calcium/phosphorus metabolism disorders in patients living with HIV in Abidjan. Int J Biochem Res Rev 2017; 17: 1–7 [Google Scholar]
- 23. Moreau E, Bächer S, Mery S.. Performance characteristics of the VIDAS® 25-OH Vitamin D Total assay – comparison with four immunoassays and two liquid chromatography-tandem mass spectrometry methods in a multicentric study. Clin Chem Lab Med 2016; 54: 45–53 [DOI] [PubMed] [Google Scholar]
- 24. Kamboh MI, Ferrell RE.. Ethnic variation in vitamin D-binding protein (GC): a review of isoelectric focusing studies in human populations. Hum Genet 1986; 72: 281–293 [DOI] [PubMed] [Google Scholar]
- 25. Sempos CT, Betz JM, Camara JE. et al. General steps to standardize the laboratory measurement of serum total 25-hydroxyvitamin D. J AOAC Int 2017; 100: 1230–1233 [DOI] [PubMed] [Google Scholar]
- 26. Wise SA, Phinney KW, Tai SS-C. et al. Baseline assessment of 25-hydroxyvitamin D assay performance: a vitamin D standardization program (VDSP) interlaboratory comparison study. J AOAC Int 2017; 100: 1244–1252 [DOI] [PubMed] [Google Scholar]
- 27. Phinney KW, Sempos CT, Tai SS-C.. Baseline assessment of 25-hydroxyvitamin D reference material and proficiency testing/external quality assurance material commutability: a vitamin D standardization program study. J AOAC Int 2017; 100: 1–6 [DOI] [PubMed] [Google Scholar]
- 28. Souberbielle JC, Cormier C, Kindermans C. et al. Vitamin D status and redefining serum parathyroid hormone reference range in the elderly. J Clin Endocrinol Metab 2001; 86: 3086–3090 [DOI] [PubMed] [Google Scholar]
- 29. Aloia JF, Vaswani A, Yeh JK. et al. Risk for osteoporosis in black women. Calcif Tissue Int 1996; 59: 415–423 [DOI] [PubMed] [Google Scholar]
- 30. Cavalier E, Salsé M, Dupuy A-M. et al. Establishment of reference values in a healthy population and interpretation of serum PTH concentrations in hemodialyzed patients according to the KDIGO Guidelines using the Lumipulse® G whole PTH (3rd generation) assay. Clin Biochem 2018; 54: 119–122 [DOI] [PubMed] [Google Scholar]
- 31. Seck SM, Dahaba M, Ka EF. et al. Mineral and bone disease in Black African hemodialysis patients: a report from Senegal. Nephrourol Mon 2012; 4: 613–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang D, Niwa M, Koong AC. Targeting the IRE1α-XBP1 branch of the unfolded protein response in human diseases. Semin Cancer Biol 2015; 33: 48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jovanovich A, Chonchol M, Cheung AK. et al. Racial differences in markers of mineral metabolism in advanced chronic kidney disease. Clin J Am Soc Nephrol 2012; 7: 640–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalantar-Zadeh K, Miller JE, Kovesdy CP. et al. Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J Bone Miner Res 2010; 25: 2448–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gupta A, Kallenbach LR, Zasuwa G. et al. Race is a major determinant of secondary hyperparathyroidism in uremic patients. J Am Soc Nephrol 2000; 11: 330–334 [DOI] [PubMed] [Google Scholar]
- 36. Omije D, Norris K, Wang J. et al. Race is a major determinant of secondary hyperparathyroidism in uremic patients: comparative study of Blacks and Hispanics. Clin Nephrol 2008; 70: 312–318 [DOI] [PubMed] [Google Scholar]
- 37. Gupta A. Renal bone disease in black dialysis patients: are algorithms developed for white dialysis patients valid? Nephrol Dial Transplant 2001; 16: 1518–1519 [DOI] [PubMed] [Google Scholar]
- 38. Seck S, Cisse M, Ka E. et al. Epidemiology of vitamin D deficiency in West African hemodialysis patients: a pilot study from Senegal. Indian J Nephrol 2014; 24: 127–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sanusi AA, Arogundade FA, Oladigbo M. et al. Prevalence and pattern of renal bone disease in end stage renal disease patients in Ile-Ife, Nigeria. West African J Med 2010; 29: 75–80 [DOI] [PubMed] [Google Scholar]
- 40. Malluche HH, Mawad HW, Monier-Faugere MC.. Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res 2011; 26: 1368–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.KDIGO Working Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 42. Salam S, Gallagher O, Gossiel F. et al. Diagnostic accuracy of biomarkers and imaging for bone turnover in renal osteodystrophy. J Am Soc Nephrol 2018; 29: 1557–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]


