Abstract
Background
Sub-Saharan Africans exhibit a higher frequency of chronic kidney disease (CKD) than other populations. In this study, we sought to determine the frequency of apolipoprotein L1 (APOL1) genotypes in hypertension-attributed CKD in Kinshasa, Democratic Republic of the Congo.
Methods
We performed a case–control study identifying 162 subjects: 79 with hypertension-attributed CKD and 83 controls living in Kinshasa who were genotyped for APOL1 risk variants between July 2013 and November 2016. We selected control subjects from the general population and matched them with the cases according to age. Logistic regression analysis was used to examine the relationship between APOL1 high-risk genotypes and CKD.
Results
The frequencies of the APOL1 G1 and G2 alleles were 19.1 and 7.1%, respectively. The number of individuals with the G1 and G2 risk alleles was significantly higher in the CKD group (12.7%) than in the control group (2.4%), particularly in individuals with end-stage kidney disease (14.3%). Subjects carrying two risk alleles was strongly and independently associated with hypertension-attributed nephropathy, with an adjusted odds ratio of 7.7 (95% confidence interval 1.5–39.7; P = 0.014). The high-risk APOL1 genotypes were G1/G1 and G1/G2, whereas G2/G2 was not found in the study population.
Conclusions
The results of this study demonstrate the association of high-risk APOL1 genotypes with kidney disease in Kinshasa. The absence of G2/G2 may be consistent with powerful selective sweeps induced by Trypanosoma brucei gambiense infection. In contrast, the presence of APOL1 G2/G2 among individuals of African ancestry in the USA may indicate relaxation of natural selection in a trypanosome-free environment.
Keywords: APOL1 risk variants, CKD, hypertension, Kinshasa, Trypanosoma brucei gambiense
INTRODUCTION
The identification of risk variants in the gene encoding apolipoprotein L1 (APOL1) in 2010 provided a population genetics explanation for the higher rate of kidney disease observed in Americans of African ancestry [1, 2]. Two allelic variants (designated G1 and G2) in the last exon of the gene were shown to be highly associated with end-stage kidney disease (ESKD) risk, with a high odds ratio (OR). The APOL1 G1 variants rs73885319 and rs60910145 result in Ser342Gly and Ile384Met amino acid substitutions, respectively. G2 (rs71785313) is a lower frequency mutation consisting of a 6 bp in-frame deletion that removes amino acids Asn388–Tyr389 [1, 2]. These variants predispose African-Americans to several common kidney diseases, including clinically diagnosed hypertension-attributed kidney disease [1, 2], biopsy-proven focal segmental glomerulosclerosis (FSGS) [3] and human immunodeficiency virus-associated nephropathy (HIVAN) [3]. The OR associated with carrying two APOL1 risk loci is ∼7–10 for hypertension-attributed ESKD, whereas that for biopsy-proven FSGS is ∼10–17 and that for HIVAN, the kidney disease most strongly associated with APOL1, is ∼29–89 [1–4].
APOL1 causes lysis of Trypanosoma brucei, conferring humans with protection against illness from this species of the parasite, which does infect and cause illness in many other species [5]. On the other hand, both T.b. rhodesiense and T.b. gambiense cause human African trypanosomiasis in East and West Africa, respectively [6]. In vitro studies performed using serum from patients with APOL1 G2 (and, to a lesser extent, G1) have shown lysis of the trypanosome T.b. rhodesiense [2], and increased survival of APOL1 G2 transgenic mice infected with this parasite has been reported [7]. Therefore, a heterozygous advantage model was proposed, wherein dominant resistance to T.b. rhodesiense [2] provides a selective advantage, while individuals with two APOL1 alleles are at increased risk for chronic kidney disease (CKD) [1, 2].
Recent studies by Cooper et al. [8] conducted in East Africa (Uganda) and in West Africa (Guinea) have provided important new insights and raised further questions regarding trypanosomal infection and APOL1 variants [9]. These studies indicated that neither the APOL1 G1 nor the G2 allele provides trypanolytic protection against infection by T.b. gambiense, which is the cause of West African sleeping sickness [2, 7]. However, the APOL1 G1 allele is associated with a latent aparasitemic state and a reduced risk for active T.b. gambiense illness. In contrast, the G2 genotype is associated with an increased risk for active, virulent T.b. gambiense infection [2, 7]. As demonstrated in previous in vivo and in vitro studies, G2, but not G1, showed a 5-fold protective association against East African sleeping sickness mediated by T.b. rhodesiense [8, 9]. The protective role of G1 against active T.b. gambiense infection may explain the high frequency of this allele in West Africa. However, G2 is still frequent in some areas of West Africa despite the fact that it increases the risk for symptomatic T.b. gambiense infection. Trypanosoma brucei gambiense infection causes more deaths in the Democratic Republic of the Congo (DRC) than in any other country in Africa [10]. Hence, we reasoned that the examination of the distribution of APOL1 risk alleles in the DRC may provide further clarification.
The surfeit of nondiabetic CKD patients in healthcare and hemodialysis centers in Kinshasa provided a unique opportunity to test whether the prevalence of the APOL1 kidney risk alleles G1 and G2 is affected by the persistent presence of T.b. gambiense [11]. The prevalence of CKD among hypertensive Congolese individuals was previously reported to be 26% [11]. The present study was aimed at assessing the APOL1 risk allele frequency in Kinshasa among individuals with hypertension-attributed CKD and control individuals. The Kinshasa CKD and control populations allowed us to draw conclusions regarding the presence of APOL1 alleles under intense natural selection due to T.b. gambiense trypanosomal disease.
MATERIALS AND METHODS
Design, sampling and study setting
We conducted a case–control study between July 2013 and November 2016 in Kinshasa, a multiethnic megacity and the capital of the DRC (Figure 1 and Table 1). The Kinshasa city-province covers a vast area; over 90% of the city-province’s land mass is rural, with the urban area occupying only a small section but expanding on the western side. Kinshasa represents Africa’s third largest urban area. The majority of the inhabitants of Kinshasa are of Bantu ethnicity. However, it was not possible to identify the precise tribe or ethnicity of all participants. Many people living in the city of Kinshasa, which is currently home to ∼11 million people, speak one of the four native languages associated with the following regions of the country: (i) the Kikongo area in the southwest; (ii) the Lingala area in the northwest; (iii) the Tshiluba area in the center; and (iv) the Swahili area in the east.
FIGURE 1.
Map of the Democratic Republic of the Congo. The frequencies of APOL1 alleles in the study population are: G1, 19.13%; G2, 7.09%. The identified countries exhibited higher APOL1 G1 allele frequencies than this in at least one study.
Table 1.
Descriptive characteristics of participants by CKD status
| Cases, N = 79 | Controls, N = 83 | P-value | |
|---|---|---|---|
| Male gender, n (%) | 52 (72.2) | 39 (47.0) | 0.001 |
| FH-CKD, n (%) | 17 (21) | 8 (8.9) | 0.041 |
| HT, n (%) | 79 (100) | 14 (16.9) | <0.0001 |
| Duration HT, years | 8.7 ± 6.4 | 4.6 ± 5.6 | 0.039 |
| Native language, n (%) | 0.065 | ||
| Kikongo | 38 (56.7) | 55 (67.1) | |
| Tshiluba | 15 (22.4) | 11 (13.4) | |
| Swahili | 10 (14.9) | 5 (6.1) | |
| Lingala | 4 (6) | 11 (13.4) | |
| Age range, years, n (%) | 0.520 | ||
| 18–39 | 11 (13.9) | 7 (8.4) | |
| 40–59 | 46 (58.2) | 53 (63.9) | |
| >60 | 22 (27.8) | 23 (27.7) | |
| Age, years | 51.9 ± 11.8 | 53 ± 10.8 | 0.497 |
| SBP, mmHg | 150.1 ± 21.2 | 132.9 ± 22.6 | <0.0001 |
| DBP, mmHg | 87.0 ± 13.9 | 79.1 ± 13.0 | 0.001 |
| BMI, kg/m2 | 24.7 ± 4.7 | 27.4 ± 6.0 | 0.103 |
| Proteinuria, g/24 h | 370 ± 262 | 0 | <0.0001 |
| S Creatinine, mg/dL | 13.4 ± 8.5 | 0.89 ± 0.19 | <0.0001 |
| CKD status, n (%) | |||
| Stage 1 | 0 | 0 | |
| Stage 2 | 10 (12.7) | 0 | |
| Stage 3 | 6 (7.6) | 0 | |
| Stage 4 | 0 | 0 | |
| ESKD (Stage 5) | 63 (79.7) | 0 | |
| Setting | |||
| General population, n (%) | 5 (6.3) | 79 (96.2) | <0.0001 |
| Hospital, n (%) | 74 (93.6) | 4 (4.8) | <0.0001 |
Four of the controls were from hospital clinics. Values are expressed as numbers and proportions in parentheses or mean ± SD, as appropriate. Percentages are based on the total participant number, except regarding native language or the number responding. For 13 subjects, it was not possible to identify the precise native language (missing data).
HT, hypertension; SBP, systolic blood pressure; DBP, diastolic blood pressure; S creatinine, serum creatinine; FH, family history.
The age and gender distributions of the Kinshasa population are almost identical to those of the DRC as a whole. Patients with hypertension-attributed nephropathy were recruited from four dialysis facilities (Ngaliema Medical Center, Afia Medical Center, Belgian Medical Center of Kinshasa and Kinshasa University Hospital) and the Church Outpatient Medical Clinics. Hypertension-attributed nephropathy was diagnosed in individuals exhibiting known hypertension with persistent proteinuria <1 g/24 h (or dipstick proteinuria 1+ or 2+, if quantitative proteinuria data were not available, or if the individual was being treated with maintenance chronic hemodialysis). Ten cases had an estimated glomerular filtration rate (eGFR) between 60 and 89 mL/min/1.73 m2, six had an eGFR between 30 and 59 mL/min/1.73 m2 and 63 had an eGFR <15 mL/min/1.73 m2 or ESKD. Individuals with diabetes, polycystic kidney disease, active glomerulonephritis (or proteinuria >1 g/24 h), urologic disease, sickle cell anemia and HIV were excluded. In the dialysis facilities of Kinshasa, hypertension-attributed nephropathy accounts for 25% of subjects [12]. The control population sample set was recruited from the general population and from the medical services divisions of the hospitals. The criteria for the controls included the minimum eGFR based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula (>60 mL/min/1.73 m2) and the maximum negative dipstick values for proteinuria and hematuria (quantitative proteinuria <200 mg/24 h, if available). The subjects were enrolled sequentially and were not related to each other.
Sample collection
The obtained clinical data included the date of birth, gender, medications taken, province of origin, native language, history of risk factors related to CKD [first-degree family history (FH) of CKD, hypertension, diabetes, sickle cell anemia, obesity, HIV infection] and data recorded during physical examination [height, weight, body mass index (BMI), blood pressure]. The diagnoses were confirmed based on written patient charts. Blood pressure was measured in the right or left arm at heart level, using the average value from readings obtained in the sitting position with an automated sphygmomanometer (Omron HEM-7114, USA), which were repeated after 5 min. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg and/or concomitant use of antihypertensive medications [13]. The laboratory analyses included urine dipstick testing, quantitative proteinuria, serum creatinine and HIV testing.
The control participants provided a urine sample in which protein was detected using urinary strips for the Combur 10 test (Roche Diagnostics, Rotkreuz, Switzerland). Female subjects were instructed to void a random urine specimen outside of their menstrual period. Quality control was performed regularly to ensure the reliability of the dipstick results. Serum creatinine was measured via the calibrated isotope dilution mass spectrometry traceable enzymatic method at the University of Liege. To estimate the eGFR, we employed the CKD-EPI formula [14] using the African-American ethnic correction presumed to be appropriate at that time [15, 16]. Based on present knowledge that was unavailable at the time of the study, the ethnic correction overestimates measured GFR [16].
CKD was defined as the presence of either persistent (>3 months) proteinuria (≥300 mg/24 h) or an eGFR <60 mL/min/1.73 m2. The clinical stages of CKD were classified according to Kidney Disease: Improving Global Outcomes [17].
DNA extraction
Genomic DNA was obtained from peripheral blood samples using the salting out method at the genetic laboratory of the University of Kinshasa [18]. All DNA samples were then transported to the Rappaport Faculty of Medicine and Research Institute of the Technion, Haifa, Israel, for genotyping.
Genotyping
Genomic DNA was used for the direct sequencing of APOL1 mutations, including G1 (rs73885319 and rs60910145), G2 (rs71785313) and the single nucleotide polymorphism (SNP) rs16996616 [1, 2]. Wild-type or G0 represents a chromosome that does not carry G1 or G2. The DNA analysis was masked for identity regarding the case or control status of each sample. For the SNP rs16996616, in addition to genotyping, the TOPO® TA Cloning® Kit (Invitrogen) and Sanger sequencing were utilized to determine the background in which the mutation arose.
Comparison of APOL1 G2/G2 genotype frequencies in CKD patients in Kinshasa, Nigeria and the USA
Inspection of the data revealed very low rates of APOL1 G2/G2 homozygous genotypes in CKD patients in Kinshasa and Nigeria [19]. These results were compared with the rate of G2/G2 genotypes in the USA [1, 2].
Statistical analyses
All data were analyzed using SPSS for Windows version 18.00, 2009. The results are presented as numbers and percentages or the mean ± SD. The two-sample Student’s t-test and Chi-squared test were used for comparisons of means and proportions where appropriate. To increase statistical power, we combined the ESKD (CKD Stage 5) patients (n = 63) with CKD Stages 2 (n = 10) and 3 (n = 6) patients and compared the entire group with the controls (n = 83). A multivariate logistic regression model was fitted to identify the independent determinants of CKD, including carrying two APOL1 risk alleles (G1/G1 or G1/G2 or G2/G2). The confounding variables that were included in the model were age, gender and FH-CKD. The association of the G2/G2 variant with geographical area among kidney disease patients was tested using Fisher’s exact test of independence. The geographic region was first considered by listing countries, which were then combined into regions (Africa or USA). The results were validated by repeating the test for the control subjects. P ≤ 0.05 were considered statistically significant.
The study protocol was approved by the University of Kinshasa Institutional Review Board (ESP/CE/010/11). Written informed consent was obtained from all participants.
RESULTS
This study consisted of 162 adults: 79 had hypertension-attributed nephropathy and 83 were healthy subjects (Table 1). No significant differences between the cases and controls in terms of age, native language or BMI were identified. In contrast, significant differences in kidney-related parameters were observed. Blood pressure was higher in the cases whose average blood pressure was 150/87 mmHg, compared with 133/79 mmHg in the controls. Furthermore, the proportion of individuals with a FH of CKD was significantly higher in the cases than in the controls (P < 0.05).
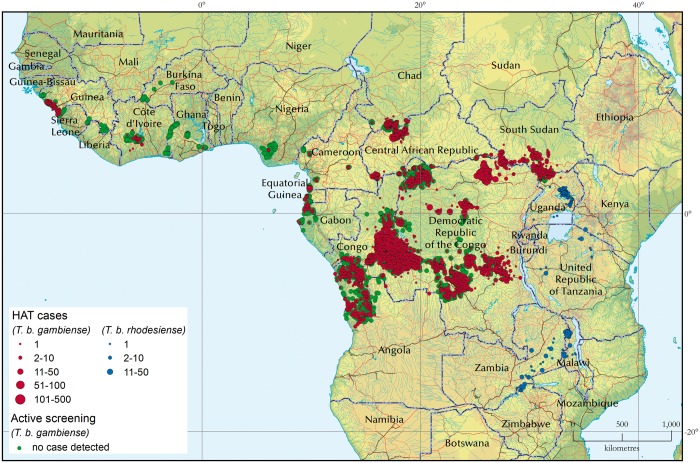
In 7.4% of participants, two APOL1 risk alleles were present (G1/G1, G1/G2 or G2/G2). The overall allele frequencies of G1 and G2 were 19.1 and 7.1%, respectively. Figure 2 shows the geographic regions of the DRC where endemic human African trypanosomiasis is present (Figure 2), providing recent information regarding endemicity for T.b. gambiense infection in the Kinshasa region.
FIGURE 2.
Distribution of human African trypanosomiasis (HAT) from 2010 to 2014. Map obtained with permission from Franco et al. [10]. The overwhelming majority of HAT cases represent T.b. gambiense infections. Red circles (gambiense HAT cases) are plotted and then overlaid with green circles (active screening campaigns in which no HAT cases were detected). As a result, only the green circles located at the fringes of the gambiense HAT distribution are visible. There is a strikingly high occurrence of T.b. gambiense in the DRC, particularly near Kinshasa. This is consistent with our finding of a relative lack of individuals carrying the G2 allele. There was one HAT case in Nigeria.
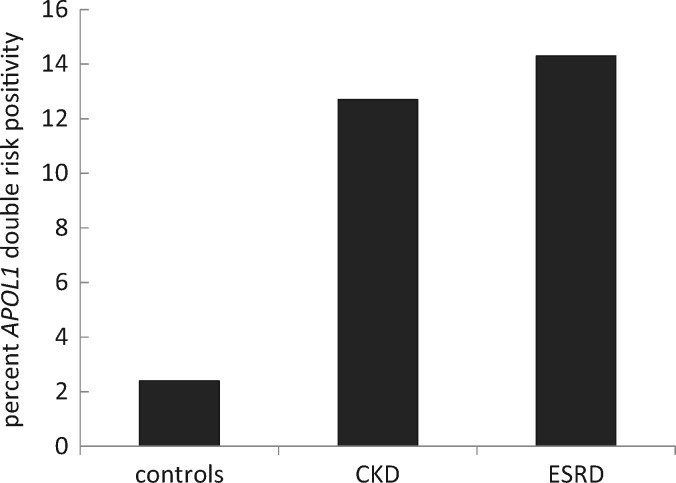
The APOL1 double risk allele frequency was significantly greater in the CKD group than in the control group (12.7 versus 2.4%) and was still higher in the ESKD patients (14.3%) (Figure 3). In light of the previous finding demonstrating that carrying a single APOL1 risk allele is not different than carrying zero alleles and is not associated with increased CKD risk, we decided a priori not to test for this association [1–3].
FIGURE 3.
Frequency of carrying two APOL1 risk alleles among controls (2.4%), CKD individuals (12.7%) and among ESKD individuals (14.3%) from Kinshasa. The occurrence of APOL1 two risk alleles is higher in those with hypertension-attributed CKD compared with controls with no renal disease, and even higher in those with hypertensive ESKD compared with controls (both P < 0.01).
Table 2 lists the incidence of each genotype in cases and controls. Among the cases, 87% of subjects carried one risk allele (G0/G1 or G0/G2) or zero risk allele (G0/G0), versus 97% in the control groups. Two APOL1 risk alleles were detected in 10 of the 79 (13%) cases and 2 of the 83 (2%) controls. The results of logistic regression (Table 3) under a two risk allele inheritance mode showed a high association of CKD with two APOL1 alleles. The unadjusted OR was 5.8 (P = 0.025). Following adjustment for gender and FH of CKD, the adjusted OR was 7.7 (P = 0.014).
Table 2.
The count of each genotype in hypertension-attributed CKD cases and controls
| Cases, N = 79 | Controls, N = 83 | |
|---|---|---|
| Wt/Wt | 45 (0.56) | 44 (0.53) |
| Wt/G1 | 17 (0.21) | 27 (0.32) |
| Wt/G2 | 7 (0.08) | 10 (0.12) |
| G1/G1 | 7 (0.08) | 0 |
| G1/G2 | 3 (0.04) | 1 (0.01) |
| G2/G2 | 0 | 1 (0.01) |
| Zero or one risk alleles | 69 (0.87) | 81 (0.97) |
| Two risk alleles | 10 (0.13) | 2 (0.02) |
Wt or G0 = a chromosome that does not carry G1 or G2; G1 = one chromosome carrying the risk allele G1 (rs73885319 and rs60910145); G2 = one chromosome carrying the risk allele G2 (rs71785313).
Table 3.
Factors associated with hypertension-attributed nephropathy among individuals from Kinshasa
| Determinants | Unadjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value |
|---|---|---|---|---|
| High risk (G1/G1, G1/G2, G2/G2 versus G0/G0) | 5.8 (1.2–27.7) | 0.025 | 7.7 (1.5–39.7) | 0.014 |
| Male gender versus female | 2.9 (1.5–5.6) | 0.001 | 2.7 (1.3–5.7) | 0.006 |
| FH-CKD | 2.7 (1.016–7.3) | 0.045 | 2.5 (0.88–7.1) | 0.08 |
Initially, a separate univariate logistic regression was performed for each independent variable. Three variables were considered: the presence of the high-risk APOL1 genotypes G1/G1, G2/G2 and G1/G2; male gender; and FH-CKD. These variables were statistically significant (P < 0.05), with unadjusted ORs of 5.8, 2.9 and 2.7, respectively. To investigate possible confounding variables and collinearity between independent variables, all significant risk factors from the univariate analysis were subjected to multivariate analysis via stepwise adjustment of all three variables (high-risk alleles, male gender and FH-CKD). The results showed that high-risk APOL1 genotypes were strongly and independently associated with hypertension-attributed nephropathy (adjusted OR). Additionally, male gender and FH-CKD were independently associated with hypertension-attributed nephropathy. CI, confidence interval.
Table 4 lists the genotypes recorded in Kinshasa and compares their frequencies with those in a population from Nigeria [19] and three sample sets from the USA [1, 2]. The APOL1 G2/G2 genotype was notably absent in the sub-Saharan African patients with kidney disease in the populations from Nigeria and Kinshasa, whereas 76 individuals with kidney disease from the USA exhibited this genotype. Statistically significant differences were observed between the proportion of patients with the G2/G2 genotype (0% among sub-Saharan African patients and nearly 5% among USA patients) versus the other disease-causing variants (G1/G1 and G1/G2; P = 0.03) and between G2/G2 and all other APOL1 genotypes (G1/G1, G1/G2, G1/G0, G2/G0 and G0/G0; P = 0.005). In contrast, the sub-Saharan African control subjects without kidney disease exhibited a proportion of the APOL1 G2/G2 genotype similar to that in African-Americans without kidney disease.
Table 4.
Comparison of APOL1 genotype counts between Africa and the USA
| G1/G1 | G1/G0 | G2/G2 | G2/G0 | G1/G2 | G0/G0 | Total | |
|---|---|---|---|---|---|---|---|
| Hypertension-attributed CKD and ESKD | |||||||
| Present report | 7 | 17 | 0 | 7 | 3 | 45 | 79 |
| Ulasi et al. [19] | 14 | 9 | 0 | 3 | 15 | 3 | 44 |
| Tzur et al. [2] | 63 | 82 | 18 | 50 | 59 | 85 | 357 |
| Genovese et al.-a [1] | 60 | 27 | 14 | 9 | 53 | 29 | 192 |
| Genovese et al.-b [1] | 219 | 173 | 44 | 127 | 203 | 239 | 1002 |
| NS | NS | P = 0.005 | NS | NS | NS | ||
| Controls | |||||||
| Present report | 0 | 27 | 1 | 10 | 1 | 44 | 83 |
| Ulasi et al. [19] | 5 | 12 | 1 | 14 | 4 | 7 | 43 |
| Tzur et al. [2] | 7 | 73 | 6 | 75 | 15 | 337 | 513 |
| Genovese et al.-a [1] | 9 | 41 | 5 | 36 | 8 | 77 | 176 |
| Genovese et al.-b [1] | 41 | 250 | 18 | 155 | 50 | 409 | 923 |
| NS | NS | NS | NS | NS | NS | ||
The Kinshasa CKD patients with two APOL1 risk alleles include individuals with the G1/G1 or G1/G2 genotype but not the G2/G2 genotype. This absence of G2/G2 genotypes was observed in the previously investigated Nigerian CKD study cases as well. In contrast, multiple studies among African-Americans have reported G2/G2 genotype frequencies of ∼5%. (i) Data from this present study. (ii) Data from Ulasi et al. [19]. (iii) Data from Tsur et al. [2]. (iv) Data from Genovese et al.-a [1]—dealing with focal segmental glomerulosclerosis in the USA. (v) Data from Genovese et al.-b [1]—dealing with hypertension-attributed nephropathy in the USA.
In the genotyping of APOL1 G1 and G2, we detected a higher allele frequency (20%) of the rs16996616 SNP (Asp337Asn) in ESKD cases and controls in the Kinshasa cohort than the frequency reported for other regions of Africa (8%) [20, 21]. This SNP was not found to be associated with hypertension-attributed nephropathy in Kinshasa or in other studies [20, 21]. In light of the finding that individuals heterozygous for G1 were also heterozygous for Asp337Asn, we sought to determine the haplotype background in which this SNP arose. We performed TA cloning using genomic DNA from informative samples, sequenced the clones and found that this SNP occurred on the background of G0.
DISCUSSION
This study identified a significantly elevated OR for hypertension-attributed CKD among carriers of two APOL1 risk alleles in Kinshasa. We found that the major APOL1 genotypes were G1/G1 and G1/G2, whereas G2/G2 genotype was not present in this study cohort, which may be explained by the prevalence of T.b. gambiense in the DRC [10].
The current study revealed a high frequency of APOL1 double risk allele genotypes in the CKD sample set, which could partly explain the heavy burden of CKD and hypertension in the general population of Kinshasa [11]. The results of the present study do not allow us to distinguish between hypertension as a risk or causative factor of CKD and hypertension as an early manifestation or complication of CKD [22]. However, the association of APOL1 indicates that genetic risk may be proximate to both CKD and hypertension in many Congolese individuals [22].
As in previous studies, most individuals in the CKD sample set did not harbor two high-risk APOL1 variants. Thus, despite the consistent association of two high-risk APOL1 variants with late-stage CKD, other genetic or environmental factors may also be important.
The APOL1 G1 allele frequency found in Kinshasa (19%) is lower than the highest G1 values observed in samples from other continental African cohorts, including cohorts from Ghana (43%) [7] and Nigeria’s Igbo (49%) [7] and Yoruba (38%) tribes [2]. Comparisons between these studies are challenging because they involve small numbers of participants, and their selection criteria and study designs differ substantially. Nonetheless, the presence of both the APOL1 G1 and G2 alleles in Kinshasa is not surprising, since the linguistic core of the Bantu language family (the language of the majority of Kinshasa residents) is a branch of the Niger–Congo language family and emanates from the adjoining regions of Cameroon and Nigeria [23, 24].
The APOL1 G1 risk allele was found to be predominant in this study. Although only a small cohort was examined in this study, the identified individuals with two APOL1 risk alleles all showed G1/G1 or G1/G2 genotypes, whereas no G2/G2 genotypes were observed among the Kinshasa CKD patients. An absence of G2/G2 genotypes was also evident in previously examined Nigerian CKD cases [19]. In contrast, the APOL1 G2/G2 genotype frequency is ∼5% in African-Americans with late-stage CKD or ESKD according to multiple case–control series (Table 4). This discrepancy may be understood by considering the selection pressure imposed by T.b. gambiense infection, as elucidated in recent studies by Cooper et al. [8]. In those studies, T.b. gambiense-infected individuals who carried one copy of the G1 APOL1 variant were found to be 3-fold more likely to be latent asymptomatic carriers [8]. In contrast, the G2 variant was associated with a 3-fold increase in susceptibility to West African trypanosomiasis mediated by the T.b. gambiense species. Moreover, after excluding compound heterozygous (G1/G2) individuals from the analysis, the OR for acute T.b. gambiense infection was increased further (OR = 5.87) [8]. This may explain the absence of G2/G2 in Congolese CKD cases who may have succumbed to virulent T.b. gambiense infection early in life and are therefore absent from the CKD case list. Such selection against individuals with the G2/G2 genotype early in life would not apply in East Africa, where the G2 allele confers protection against T.b. rhodesiense. The moderation of T.b. gambiense disease severity in individuals with the G1 allele could confer survival and reproductive advantages not observed in individuals possessing the G0 or G2 allele, who typically progress to more severe trypanosomal disease (G2 > G0 > G1). It remains puzzling why the G2 risk alleles are present in high frequency in West Africa in light of the increased risk for acute virulent T.b. gambiense infection, suggesting the possibility of protection by G2 against another, as yet unspecified pathogen [25, 26].
In the USA, where trypanosome infections do not occur, the genotypes of CKD patients showed a higher frequency of APOL1 G2/G2, which is present in up to 5% of CKD cases. This situation may indicate relaxation of a natural selective pressure imposed by T.b. gambiense in sub-Saharan Africa. The reason that there was one individual with the APOL1 G2/G2 genotype in the Kinshasa cohort controls is not clear and may be explained by differences in tribal or language matching, or an influx from neighboring populations. These possibilities and other explanations require continued investigation.
The mean age of our study subjects with ESKD was 51.9 ± 11.8 years. Hence, these patients were young at the initiation of dialysis, which is consistent with the findings of Tzur et al. [27] and Kanji et al. [28] from the USA showing that African-Americans with nondiabetic ESKD carrying two APOL1 risk alleles initiated dialysis at a mean age that was 9 years younger than that of patients without APOL1 risk alleles [27, 28]. A younger age at the initiation of dialysis is also a prominent characteristic of APOL1 nephropathy with biopsy-proven FSGS [3]. These findings are consistent with the results from Kinshasa showing a higher prevalence of CKD at <70 years of age than in the USA CKD population [11]. The finding of a 12.7% rate of carrying two APOL1 risk alleles in the CKD population of Kinshasa may provide a partial explanation for the younger age of the Congolese individuals with CKD.
The SNP rs16996616 (Asp337Asn) was found to be present during the direct sequencing of APOL1 risk alleles and was found to occur at a high frequency in the Kinshasa cohort in both cases and controls. Through TA cloning and Sanger sequencing, we found that this variant arose on the ancestral G0 haplotype background, which was confirmed by the presence of one individual heterozygous for both the rs16996616 SNP and G1 in the Exome Aggregation Consortium Browser database [29]. In the present study, we identified a 20% frequency of this SNP in the Kinshasa population (cases and controls), compared with the 8% reported in other regions of continental Africa. The SNP showed similar frequencies in cases and controls and was not associated with CKD. Previous studies have indicated that this SNP is not trypanolytic [20]. It is also possible that this variant is independently protective against other pathogens or confers other selective advantages, leading to its higher frequency in the DRC [24]. Further studies are needed to explore the biological significance of this variant.
Multiple limitations exist in the current study, including its case–control study design, the relatively small sample size examined, the consideration of only patients with hypertension-attributed nephropathy, the absence of kidney histopathology and the lack of clinical epidemiological data for correlation with the APOL1 allele frequency. Thus, these findings require confirmation in larger African samples from multiple ethnicities and locations.
CONCLUSIONS
Despite the limitations discussed above, this study demonstrates that G1 APOL1 risk variants are frequent and are associated with hypertension-attributed nephropathy in Kinshasa. These results may explain the especially heavy burden of CKD in this population. The fact that the APOL1 G2/G2 genotype was absent in CKD individuals in areas endemic for T.b. gambiense infection in Kinshasa suggests the possibility that APOL1 G2/G2 susceptibility to severe T.b. gambiense infection might interfere with reproductive fitness and reduce the frequencies of these variants in sub-Saharan Africa. This situation contrasts with that in the USA, where the APOL1 G2/G2 genotype frequency is ∼5% and the trypanosomal pathogen is absent. These differences further highlight the complementary importance of genetic epidemiological studies of health and disease genotypes associated with African ancestry in continental Africa.
ACKNOWLEDGEMENTS
We thank Cathy Songo Mbodo, Prospère Lukusa Tshilobo and Pépé Ekulu Mfutu, the staff of the genetic laboratories of the University of Kinshasa, and Rita Fuhrer-Mor from the Genomics Center Biomedical Core Facility of the Rappaport Faculty of Medicine, Technion Institute of Technology, Haifa, Israel.
FUNDING
This study was partially supported by the Froedtert Hospital Foundation to E.P.C., the Laboratory of University of Liege, Liege, Belgium to E.C. the Rappaport Faculty of Medicine and Research Institute, Israel Science Foundation Grant 890015 to K.S., the Ernest and Bonnie Beutler Foundation and the Kaylie Kidney Health Research Fund at Rambam Medical Center, Israel. The funders had no role in the study design, data collection and analysis or the preparation of the manuscript.
AUTHORS’ CONTRIBUTIONS
E.K.S., N.M.P., E.P.C., K.S., R.S. and W.G.W. conceived and designed the study. E.K.S., P.N.M., J.B.B., J.R.R.M., V.M.M., J.L.L., R.S. and W.G.W. performed the study. E.K.S., K.S., A.R.-B., R.S. and W.G.W. analyzed the data. P.N.M., N.M.N., R.S., E.C. and E.K.-D. contributed reagents, materials and analysis tools. E.K.S., E.P.C., R.S., E.K.-D., A.R.-B., G.B., M.L., K.S. and W.G.W. wrote the paper. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Genovese G, Friedman DJ, Ross MD. et al. Association of trypanolytic APOL1 variants with kidney disease in African Americans. Science 2010; 329: 841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tzur S, Rosset S, Shemer R. et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 2010; 128: 345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kopp JB, Nelson GW, Sampath K. et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 2011; 22: 2129–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kasembeli AN, Duarte R, Ramsay M. et al. APOL1 risk variants are strongly associated with HIV-associated nephropathy in Black South Africans. J Am Soc Nephrol 2015; 26: 2882–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vanhamme L, Paturiaux-Hanocq F, Poelvoorde P. et al. Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature 2003; 422: 83–87 [DOI] [PubMed] [Google Scholar]
- 6. Osafo C, Raji YR, Olanrewaju T. et al. Genomic approaches to the burden of kidney disease in Sub-Saharan Africa: the Human Heredity and Health in Africa (H3Africa) Kidney Disease Research Network. Kidney Int 2016; 90: 2–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thomson R, Genovese G, Canon C. et al. Evolution of the primate trypanolytic factor APOL1. Proc Natl Acad Sci USA 2014; 111: E2130–E2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooper A, Ilboudo H, Alibu VP. et al. APOL1 renal risk variants have contrasting resistance and susceptibility associations with African trypanosomiasis. Elife 2017; 6; e25461: 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaboré JW, Ilboudo H, Noyes H. et al. Candidate gene polymorphisms study between human African trypanosomiasis clinical phenotypes in Guinea. PLoS Negl Trop Dis 2017; 11: e0005833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Franco JR, Cecchi G, Priotto G. et al. Monitoring the elimination of human African trypanosomiasis: Update to 2014. PLoS Negl Trop Dis 2017; 11: e0005585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sumaili EK, Cohen EP, Zinga CV. et al. High prevalence of undiagnosed chronic kidney disease among at-risk population in Kinshasa, the Democratic Republic of Congo. BMC Nephrol 2009; 10: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nlandu Y, Lepira F, Makulo JR. et al. Reverse epidemiology of elevated blood pressure among chronic hemodialysis black patients with stroke: a historical cohort study. BMC Nephrol 2017; 18: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chobanian AV, Bakris GL, Black HR. et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42: 1206–1252 [DOI] [PubMed] [Google Scholar]
- 14. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Omuse G, Maina D, Mwangi J. et al. Comparison of equations for estimating glomerular filtration rate in screening for chronic kidney disease in asymptomatic black Africans: a cross sectional study. BMC Nephrol 2017; 18: 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bukabau JB, Sumaili EK, Cavalier E. et al. Performance of glomerular filtration rate estimation equations in Congolese healthy adults: The inopportunity of the ethnic correction. PLoS One 2018; 13: e0193384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levey AS, Atkins R, Coresh J. et al. Chronic kidney disease as a global public health problem: approaches and initiatives–a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 2007; 72: 247–259 [DOI] [PubMed] [Google Scholar]
- 18. Miller SA, Dykes DD, Polesky HF.. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16: 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ulasi II, Tzur S, Wasser WG. et al. High population frequencies of APOL1 risk variants are associated with increased prevalence of non-diabetic chronic kidney disease in the Igbo people from south-eastern Nigeria. Nephron Clin Pract 2013; 123: 123–128 [DOI] [PubMed] [Google Scholar]
- 20. Limou S, Nelson GW, Lecordier L. et al. Sequencing rare and common APOL1 coding variants to determine kidney disease risk. Kidney Int 2015; 88: 754–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hawkins GA, Friedman DJ, Lu L. et al. Re-sequencing of the APOL1-APOL4 and MYH9 gene regions in African Americans does not identify additional risks for CKD progression. Am J Nephrol 2015; 42: 99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skorecki KL, Wasser WG.. Hypertension-misattributed kidney disease in African Americans. Kidney Int 2013; 83: 6–9 [DOI] [PubMed] [Google Scholar]
- 23. Alves I, Coelho M, Gignoux C. et al. Genetic homogeneity across Bantu-speaking groups from Mozambique and Angola challenges early split scenarios between East and West Bantu populations. Hum Biol 2011; 83: 13–38 [DOI] [PubMed] [Google Scholar]
- 24. Nielsen R, Akey JM, Jakobsson M. et al. Tracing the peopling of the world through genomics. Nature 2017; 541: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Samanovic M, Molina-Portela MP, Chessler AD. et al. Trypanosome lytic factor, an antimicrobial high-density lipoprotein, ameliorates Leishmania infection. PLoS Pathog 2009; 5: e1000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor HE, Khatua AK, Popik W.. The innate immune factor apolipoprotein L1 restricts HIV-1 infection. J Virol 2014; 88: 592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tzur S, Rosset S, Skorecki K. et al. APOL1 allelic variants are associated with lower age of dialysis initiation and thereby increased dialysis vintage in African and Hispanic Americans with non-diabetic end-stage kidney disease. Nephrol Dial Transplant 2012; 27: 1498–1505 [DOI] [PubMed] [Google Scholar]
- 28. Kanji Z, Powe CE, Wenger JB. et al. Genetic variation in APOL1 associates with younger age at hemodialysis initiation. J Am Soc Nephrol 2011; 22: 2091–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karczewski KJ, Weisburd B, Thomas B. et al. The ExAC browser: displaying reference data information from over 60 000 exomes. Nucleic Acids Res 2017; 45: D840–D845 [DOI] [PMC free article] [PubMed] [Google Scholar]