Abstract
Background
Acute kidney injury (AKI) is associated with decreased survival, future risk of chronic kidney disease and longer hospital stays. Electronic alerts (e-alerts) for AKI have been introduced in the UK in order to facilitate earlier detection and improve management. The aim of this study was to establish if e-alerts in primary care were acted on by examining timing of repeat creatinine testing.
Methods
The National Health Service England Acute Kidney Injury electronic alert algorithm was introduced in April 2015 across both primary and secondary care in NHS Tayside accompanied by a programme of education. Data from a 12-month period (2012) predating introduction of the e-alerts were compared with a 12-month period following implementation of e-alerts for AKI. Biochemistry testing following the AKI episode, timing of repeat tests and numbers of patients hospitalized within 7 days of episode were compared between the two time periods.
Results
During the 12 months after e-alert introduction, 9781 AKI e-alerts were generated. Of these, 1460 (14.9%) alerts were generated in primary care. Median duration to repeat blood testing for these primary care alerts was 5 days for AKI Stage 1 [interquartile range (IQR) 2–10], 2 days for Stage 2 (IQR 1–5) and 1 day (IQR 0–2) for Stage 3. During 2012 (prior to e-alert implementation) 8812 AKI episodes were identified. Of these, 2650 tests (30.1%) were requested by primary care staff. Median duration to repeat creatinine testing was longer: 55 days (IQR 20–142) for Stage 1, 38 days (IQR 15–128) for Stage 2 was and 53 days (IQR 20–137) for Stage 3. More patients had biochemistry tests repeated within 7 days of AKI onset, pre-alert implementation; 252 (9.5%) versus 857 (58.7%) (P < 0.001). Rates of hospitalization within 7 days of AKI increased from 342 (12.9%) pre-implementation to 372 (25.5%) post-implementation (P < 0.001).
Conclusions
Within primary care, e-alert implementation was associated with higher rates of creatinine monitoring, but also higher rates of hospitalization.
Keywords: acute kidney injury, electronic alert, primary care
INTRODUCTION
Acute kidney injury (AKI) is common, affecting 15% of hospitalized patients, with a significant proportion originating in the community [1, 2]. It is associated with increased length of hospital stays, higher healthcare resource utilization and mortality [3, 4]. Identification is a key first step in implementing strategies to prevent more severe AKI. Identifying those patients at increased risk of AKI whilst they are in the community may facilitate earlier detection and hence timely implementation of prevention and mitigation measures such as medication review, thus potentially avoiding both worsening of AKI and hospital admission.
Electronic alerts (e-alerts) for AKI are increasingly being implemented in hospitals to facilitate earlier identification. Implementation of e-alerts for AKI in secondary care is mandatory in England and Wales, with extension to primary care planned. There have been a number of studies evaluating the impact of e-alerts for AKI in hospitalized patients, with conflicting findings. A recent meta-analysis showed that e-alerts did not improve survival or reduce utilization of renal replacement therapy [5]. However, the context in which alerts were implemented and the processes of care were variable. In contrast, there is very little work examining the impact of introducing AKI e-alerts to primary care health systems.
E-alerts for AKI were implemented in both primary and secondary care in the National Health Service (NHS) Tayside region of Scotland in April 2015, accompanied by a programme of education to raise awareness. The aim of our study was to measure the impact of e-alerts in primary care by comparing whether repeat biochemistry sampling was performed and the time frame over which it was performed in patients with AKI pre- and post-alert implementation. Rates of hospitalization were also examined.
MATERIALS AND METHODS
Study population
The study population comprised two cohorts: all adults over 18 years of age resident in the NHS Tayside region of Scotland with AKI generated in primary care between 1 January 2012 and the 31 December 2012 and between 30 April 2015 and 1 May 2016. The year 2012 was selected arbitrarily to allow comparison prior to e-alert implementation. Patients with AKI detected from a biochemistry sample requested from hospital locations, out-patient clinics and community hospitals were excluded, limiting the cohort to AKI detected in samples requested by primary care.
Data sources
Data from 2012, prior to the implementation of e-alerts, were linked using the Health Informatics Centre (HIC) at the University of Dundee [6]. HIC enables anonymized health record linkage from the population of Tayside (400 000), Scotland, using a unique identifying Community Health Index (CHI) number. Data were linked between the following data sets: Scottish Morbidity Record of hospital admissions (SMR01), laboratory results and the Scottish Care Initiative-Diabetes Collaboration. Age, sex and heart failure data were obtained from SMR01. Creatinine measurements were obtained from the biochemistry laboratory system and the presence of diabetes from the Scottish Care Initiative-Diabetes Collaboration system.
The NHS Tayside biochemistry laboratory reporting system was used to identify all e-alerts generated between 30 April 2015 and 1 May 2016. Data on age, sex, stage of AKI, diabetes, heart failure and repeat biochemistry testing following the generation of the e-alert, and timing of this, and hospital admission were collated.
Outcomes
AKI was defined using the NHS England algorithm based on the Kidney Disease: Improving Global Outcomes criteria [7, 8]. Baseline was taken as either: (i) the median creatinine level in the period between 8 and 365 days prior to the index creatinine measurement; (ii) the lowest level in the period 0–7 days prior to the index measurement; or (iii) the lowest level between 0 and 2 days prior to the index measurement. If several alerts were generated, the first alert was included. A further alert following recovery to baseline renal function was treated as a new episode. The same definition based on the NHS England algorithm was applied to the 2012 cohort.
E-alerts were implemented in both primary and secondary care in April 2015 in Tayside, with education delivered to both areas. Real-time alerts are generated on the laboratory reporting system. Following an e-alert, clinicians are prompted to intervene and signposted to local AKI guidelines—either hospital or primary care guidelines dependent on where the sample was requested. E-mails are sent to requesting clinicians for AKI Stages 2 and 3.
Statistical methods
Data were analysed in Microsoft Excel and SPSS v24 (IBM, Armonk, New York, USA). Categorical variables are presented as percentages. Groups were compared using Pearson chi-squared tests.
Ethical statement
Anonymized record linkage was conducted according to HIC Standard Operating Procedure (SOP). The Tayside Research Ethics Committee does not require submission of individual studies that follow this SOP. We obtained permission from NHS Tayside’s Caldicott Guardian to examine patients with e-alerts.
RESULTS
Demographics
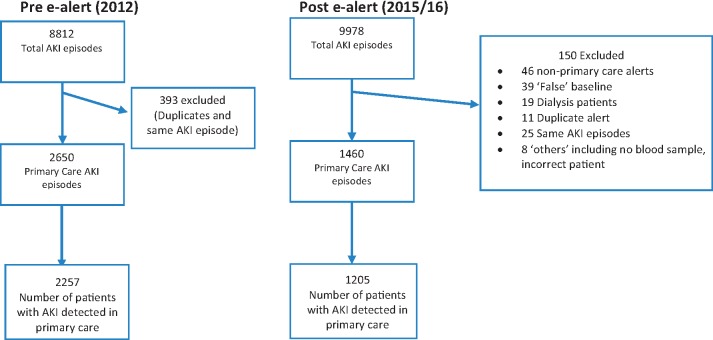
A flowchart outlining formation of study cohorts is shown in Figure 1. Demographics of patients’ pre- and post-alert implementation are shown Table 1.
FIGURE 1.
Flowchart of e-alerts forming study cohorts. Duplicate alert is defined as replicate e-alert with the same patient, date and time. ‘Same AKI episode’ is defined as e-alert for same patient occurring within 7 days of previous alert or improving AKI stages from initial e-alert.
Table 1.
Populations with AKI episodes pre-and post-e-alert introduction
| 2012 | 2015–16 | |
|---|---|---|
| Total number of individuals with AKI | 6604 | 5852 |
| Mean age of individuals with AKI (years) (SD) | 72.0 (16.1) | 71.1 (17.4) |
| Female sex—individuals with AKI (%) | 3328 (50.4) | 3124 (53.4) |
| Total number of AKI episodes | 8812 | 9978 |
| AKI detection sample requested by GP (% of all AKI) | 2650 (30.1) | 1460 (14.8) |
| Number of patients with AKI detected from GP sample | 2257 | 1205 |
| Mean age of individuals with AKI from GP sample (years) (SD) | 73.4 (14.5) | 73.0 (16.9) |
| Female sex—individuals with AKI from GP sample (%) | 1166 (51.7) | 706 (58.6) |
| Diabetes Mellitus (%) | 628 (27.8) | 320 (26.6) |
| Congestive Heart Failure (%) | 372 (16.5) | 201 (16.7) |
| eGFR <60 mL/min/1.73 m2 (%) | 1281 (56.8) | 429 (34.5) |
| GP-detected AKI Stage 1 (percentage of all GP-detected AKI) | 2483 (93.7) | 1167 (79.9) |
| GP-detected AKI Stage 2 (percentage of all GP-detected AKI) | 110 (4.2) | 181 (12.4) |
| GP-detected AKI Stage 3 (percentage of all GP-detected AKI) | 57 (2.1) | 112 (7.7) |
There were 8812 AKI episodes in 2012 over a 12-month period (1 January 2012 to 31 December 2012; pre-alert implementation). Of these, 2650 (30.1%) originated in primary care with 2483 (93.7%) Stage 1 AKI, 110 (4.2%) Stage 2 and 57 (2.1%) Stage 3 AKI.
Following implementation of e-alerts, there were 9978 AKI episodes e-alerts during the 12-month study period (30 April 2015–1 May 2016). A total of 150 alerts were excluded due to false positives, dialysis patients and same AKI episode. As a proportion of the total alerts generated, fewer alerts were generated in primary care: 1460 (14.8%) compared with 2650 (30.1%), P < 0.001. Of these, 1167 (79.9%) were Stage 1 AKI, 181 (12.4%) Stage 2 and 112 (7.7%) Stage 3 AKI.
Actions in response to AKI
Number of patients with repeat creatinine testing within 7 days of an AKI episode and number of patients admitted to hospital within 7 days of an AKI episode before and after implementation of e-alerts are shown in Table 2. Repeat creatinine testing was higher across all stages of AKI following e-alert introduction. In 2012, the rate of repeat testing within 7 days was 9.4, 11.8 and 7.0% for Stages 1, 2 and 3, respectively, compared with 53.4, 79.6 and 80.4% for Stages 1, 2 and 3 following implementation of e-alerts in 2015, P < 0.001. In 2012, the median duration to repeat creatinine testing for AKI Stage 1 was 55 days [interquartile range (IQR) 20–142], for AKI Stage 2 was 38 days (IQR 15–128) and for AKI Stage 3 was 53 days (IQR 20–137).
Table 2.
Actions associated with AKI occurrences, pre- and post-e-alert introduction (number and percentage)
| Number (%) | 2012 | 2015–16 | P |
|---|---|---|---|
| Number with repeat creatinine within 7 days: | |||
| All AKI episodes (%) | 252/2650 (9.5) | 857/1460 (58.7) | <0.001 |
| AKI Stage 1 (%) | 235/2483 (9.4) | 623/1167 (53.4) | <0.001 |
| AKI Stage 2 (%) | 13/110 (11.8) | 144/181 (79.6) | <0.001 |
| AKI Stage 3 (%) | 4/57 (7.0) | 90/112 (80.4) | <0.001 |
| Number admitted to hospital within 7 days: | |||
| All AKI episodes (%) | 342/2650 (12.9) | 372/1460 (25.5) | <0.001 |
| AKI Stage 1 (%) | 270/2483 (10.9) | 218/1167 (18.7) | <0.001 |
| AKI Stage 2 (%) | 43/110 (39.1) | 77/181 (42.5) | 0.56 |
| AKI Stage 3 (%) | 29/57 (50.9) | 77/112 (68.8) | 0.02 |
Median duration to repeat creatinine testing in 2015–16 was 5 days for AKI Stage 1 (IQR 2–10), 2 days for AKI Stage 2 (IQR 1–5) and 1 day (IQR 0–2) for AKI Stage 3.
More patients were admitted to hospital within 7 days of an AKI episode following introduction of e-alerts: 342 (12.9%) versus 372 (25.5%), P < 0.001, compared with 2012 prior to e-alert implementation. Almost double the number of patients with Stage 1 AKI were admitted to hospital within 7 days following e-alert implementation (10.9% versus 18.7%).
DISCUSSION
Our results demonstrate that the introduction of e-alerts for AKI in primary care was associated with higher rates of early repeat blood sampling. We also found that rates of hospitalization within 7 days were higher following introduction of e-alerts.
The 2009 National Confidential Enquiry into Patient Outcome and Death report highlighted deficiencies in recognizing AKI [9]. E-alerts are considered a feasible way to improve early detection in both hospital and community settings [10, 11], although it has become increasingly evident that in isolation these do not improve patient outcomes [5, 11–14]. However, e-alerts could be a powerful adjunct in improving mortality and reducing progression of AKI when coupled with care bundles and change in clinician behaviour [14, 15]. The implementation of e-alerts in Tayside was supported by a programme of education in both primary and secondary care to increase awareness. There was subsequent signposting to a specific primary care AKI guideline, along with an e-mail. This system did not replace already existing advisory telephone calls from laboratory staff for significant results.
We found that the total number of AKI episodes in primary care was significantly higher in 2012 compared with 2015–16 (30.1% versus 14.8%). This may be due to increased awareness of AKI and use of ‘Medicines Sick Day Rule cards’, which were also rolled out in 2015–16 in Tayside. This may have also led to a lower threshold for hospitalization of patients, patients were being admitted earlier in their illness prior to detection of AKI in the community. A repeat creatinine was measured early (within 7 days) in 58.7% of the post-e-alert cohort compared with 9.5% in the pre-e-alert cohort. This suggests that most primary care physicians are actively responding to the alerts in a timely manner. This is reassuring as a Welsh study has shown that patients with community-acquired AKI who had an early repeat creatinine measurement (<7 days) had better outcomes than those who had creatinine repeated later (>7 days) [16]. The introduction of alerts in association with change in clinician behaviour has recently been shown elsewhere to reduce mortality, length of hospitalization and rates of dialysis [17]. It has also been demonstrated that the early detection of AKI in primary care has the potential to prevent more severe AKI and reduce overall mortality [13]. This therefore highlights the importance of early action in patients with AKI detected in the community, and raises the notion that a clinical review, or referral, together with a repeat measurement of creatinine within 7 days would be a prudent response to AKI e-alerts in primary care.
25.5% of patients with AKI detected in the community were admitted within 7 days, in contrast to 12.9% in the pre-e-alert cohort. This admission rate is in line with a study by Holmes et al., who examined e-alerts generated in primary care in a large Welsh cohort [16]. They found that 22% of patients with AKI from primary care were admitted to hospital within the same period [16]. This is despite a smaller number of AKI being detected in primary care in the post-e-alert cohort (1460 versus 2650) and thus a higher number of AKI cases being detected during, or at the point of, hospital admission. One explanation for this could be greater awareness of the danger of AKI by general practitioners (GPs), leading to a lower threshold for admitting patients before their illness progresses to the point where AKI becomes manifest. An alternative explanation is that our results reflect a trend of hospitalization for less severe disease that is unrelated to AKI. Similar trends have been seen in hospitalization rates for community-acquired pneumonia; hospitalization rates have risen sharply with no change in severity, comorbidity or outcomes [18]. Early hospitalization for a range of illnesses would tend to shift the point of diagnosis for AKI from community to hospital, but would also explain the higher rate of hospitalization following a community diagnosis of AKI. Disentangling the causes for this observation will require further studies.
A limitation of our work was that we arbitrarily selected 2012 as the period prior to alert implementation. The increase in testing may also be related to the differences between these two populations and increased awareness of AKI unrelated to the introduction of e-alerts. Examination of another year would ensure that this year is truly representative of practice prior to e-alert implementation. A further limitation of our study is that we are unable to present mortality data. This may have had an impact on our findings as people may have died prior to repeat testing. Furthermore, data on mortality would help establish whether the two populations are comparable. Further work examining mortality rates and rates of renal progression are required to establish whether e-alerts have had a beneficial effect on mortality. We were also unable to electronically interrogate GP records. Thus, we could not ascertain the clinical actions undertaken by primary care staff. These could have included withholding high-risk medicines, advising on hydration, referring to tertiary renal services or appropriate imaging. It is also important to remember that repeated blood sampling might have been inappropriate for some patients, for example those who were approaching the end of their lives.
AKI e-alerts can help highlight high-risk patients in the community, aiding early detection of AKI. We have demonstrated that their implementation in primary care may have influenced practice with higher levels of timely, repeat testing. There were, however, higher rates of hospitalization. Further work focused on examining the effects of e-alerts on mortality, primary care prescribing and how they alter primary care clinician behaviour is necessary to establish their full impact.
Establishing the resultant burden on biochemistry laboratory and hospital services also deserves further investigation.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Bedford M, Stevens PE, Wheeler TW. et al. What is the real impact of acute kidney injury? BMC Nephrol 2014; 15: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liano F, Pascual J.. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 1996; 50: 811–818 [DOI] [PubMed] [Google Scholar]
- 3. Lafrance JP, Miller DR.. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol 2010; 21: 345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Selby NM, Crowley L, Fluck RJ. et al. Use of electronic results reporting to diagnose and monitor AKI in hospitalized patients. Clin J Am Soc Nephrol 2012; 7: 533–540 [DOI] [PubMed] [Google Scholar]
- 5. Lachance P, Villeneuve PM, Rewa OG. et al. Association between e-alert implementation for detection of acute kidney injury and outcomes: a systematic review. Nephrol Dial Transplant 2017; 32: 265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.University of Dundee. Health Informatics Centre. http://medicine.dundee.ac.uk/health-informatics-centre (November 2012, date last accessed) [Google Scholar]
- 7. England N. Patient Safety Alert on Standardising the Early Identification of Acute Kidney Injury 2014 https://www.england.nhs.uk/2014/06/psa-aki/ (4 October 2014, date last accessed)
- 8.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2012; 2: 1–138 [Google Scholar]
- 9.NCEPOD National Confidential Enquiry into Patient Outcome and Death. Acute kidney injury: adding insult to injury. 2009. https://www.ncepod.org.uk/2009aki.html (7 March 2018, date last accessed)
- 10. Alavijeh OS, Bansal J, Hadfield K. et al. Implementation of an automated primary care acute kidney injury warning system: a quantitative and qualitative review of 2 years of experience. Nephron 2017; 135: 189–195 [DOI] [PubMed] [Google Scholar]
- 11. Wallace K, Mallard AS, Stratton JD. et al. Use of an electronic alert to identify patients with acute kidney injury. Clin Med 2014; 14: 22–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilson FP, Shashaty M, Testani J. et al. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet 2015; 385: 1966–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Porter CJ, Juurlink I, Bisset LH. et al. A real-time electronic alert to improve detection of acute kidney injury in a large teaching hospital. Nephrol Dial Transplant 2014; 29: 1888–1893 [DOI] [PubMed] [Google Scholar]
- 14. Colpaert K, Hoste EA, Steurbaut K. et al. Impact of real-time electronic alerting of acute kidney injury on therapeutic intervention and progression of RIFLE class. Crit Care Med 2012; 40: 1164–1170 [DOI] [PubMed] [Google Scholar]
- 15. McCoy AB, Waitman LR, Gadd CS. et al. A computerized provider order entry intervention for medication safety during acute kidney injury: a quality improvement report. Am J Kidney Dis 2010; 56: 832–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holmes J, Allen N, Roberts G. et al. Acute kidney injury electronic alerts in primary care—findings from a large population cohort. QJM 2017;110: 741–746 [DOI] [PubMed] [Google Scholar]
- 17. Al-Jaghbeer M, Dealmeida D, Bilderback A. et al. Clinical decision support for in-hospital AKI. J Am Soc Nephrol 2018; 29: 654–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Millett ER, De Stavola BL, Quint JK. et al. Risk factors for hospital admission in the 28 days following a community-acquired pneumonia diagnosis in older adults, and their contribution to increasing hospitalisation rates over time: a cohort study. BMJ Open 2015; 5: e008737. [DOI] [PMC free article] [PubMed] [Google Scholar]