Abstract
Background
A sub-analysis of a Phase III study was conducted to identify factors that might predict increased ferritin levels during long-term sucroferric oxyhydroxide (SO) treatment in hemodialysis patients.
Methods
The open-label, multicenter, Phase III study assessed the efficacy and safety of SO 750–3000 mg/day for 52 weeks in Japanese patients with chronic renal failure and hyperphosphatemia. A total of 125 of 161 patients from the Phase III trial, and who had data for ferritin levels after 28 weeks of SO treatment, were evaluated.
Results
Baseline ferritin was the strongest contributor (P < 0.0001) to ferritin increases during SO treatment. By Week 28, there were significant differences (P < 0.05/3) in ferritin increases between patients with higher [quartile 4 (Q4)] versus lower (Q1, Q2 and Q3) baseline ferritin. An erythropoiesis-stimulating agent dosage reduction was observed in patients with the lowest baseline ferritin level (Q1), and only slight reductions were noted in the other patient subsets. SO dosages administered to patients in baseline ferritin quartiles Q2, Q3 and Q4 were comparable throughout the study with slight fluctuations. SO dosages in Q1 were considerably lower than those in the other quartiles.
Conclusions
In summary, of the baseline variables found to predict increased ferritin, and changes in iron-related parameters, during SO treatment in Japanese chronic kidney disease patients undergoing hemodialysis, baseline ferritin was the most relevant variable.
Keywords: dialysis, end-stage kidney disease, ferritin, phosphate binder, sucroferric oxyhydroxide
INTRODUCTION
Sucroferric oxyhydroxide (SO) is an iron-based phosphate binder with high phosphate-binding capacity in vitro [1] and low iron release [2]; thus, it is a minimal source of iron in addition to normal iron absorption in humans. Based on data from several clinical trials evaluating the safety, efficacy and tolerability of SO [1, 3–5], SO is currently approved in 37 countries for the treatment of hyperphosphatemia in patients with chronic kidney disease (CKD) undergoing dialysis. Various studies have reported increases over baseline ferritin levels during SO treatment. In a multicenter, open-label, randomized, parallel-group, active-controlled, Phase III study comparing SO and sevelamer carbonate [4], ferritin levels and transferrin saturation (TSAT) increased with both treatments; however, these increases were greater in patients receiving SO. A post hoc analysis of this 24-week trial [4] and its 28-week extension [5] concluded that long-term changes in iron parameters were likely due to minimal iron release from SO [6]. In a long-term, Phase III study of SO in Japanese dialysis patients, a dose-dependent ferritin increase was also observed [7]. However, iron-based phosphate binders, such as ferric citrate, also increase ferritin and TSAT. Previous studies have reported that ferric citrate increased iron parameters and reduced intravenous iron and erythropoiesis-stimulating agent (ESA) use while maintaining hemoglobin levels [8–10].
In a long-term, Japanese, Phase III study, ferritin levels increased in line with SO dosage; however, the ferritin level remained stable after 24 weeks of SO administration at ∼200 ng/mL, even when the maximum SO dosage was 2250–3000 mg/day [7]. Therefore, factors other than dosage may also contribute to ferritin dynamics after SO administration. If such patient background factors can be identified, along with the corresponding time course, then prediction of changes in iron parameters during SO administration will be possible. We considered it useful to determine if any dose-dependent increase in serum ferritin levels occurs because of SO treatment, whether this relates to any patient characteristics and, if so, what these relationships imply. Thus, we conducted a sub-analysis of a Phase III study to identify factors that might predict increased ferritin levels during long-term SO treatment.
MATERIALS AND METHODS
The design of the main Phase III, open-label, long-term, multicenter study, on which the present sub-analysis is based, has been detailed elsewhere [7]. Briefly, the efficacy and safety of SO administered three times daily (at dosages of 750–3000 mg/day) were evaluated for 52 weeks. The study protocol was approved by the ethical review boards of all 15 participating centers, and all patients provided written informed consent to participate. The study was conducted in accordance with the latest version of the Declaration of Helsinki.
The inclusion and exclusion criteria have been published elsewhere [7]. Briefly, patients were adult Japanese men and women aged ≥20 years with chronic renal failure and hyperphosphatemia [predialysis serum phosphorus concentration 3.5–10.0 mg/dL (patients receiving phosphate binders) or >6.0 to ≤10.0 mg/dL (patients not treated with phosphate binders)] undergoing hemodialysis three times weekly for ≥12 weeks. Patients with a history of hemochromatosis, or any other iron overload disorder, or patients with a serum ferritin level >800 ng/mL or TSAT >50% before Week −2 were excluded. The use of intravenous iron was permitted if the investigator considered it necessary. The investigator adjusted SO dosage after Week 2, based on the predialysis serum phosphorus concentration on that week’s first dialysis day and in accordance with criteria detailed previously [7].
Patients who received other phosphate binders prior to SO treatment continued their treatment without any change in dose until they switched to SO treatment. SO treatment was started with 750 mg/day, and the dose increased in increments of 750 mg/day to a maximum dose of 3000 mg/day, during which serum phosphorus concentrations were monitored.
The change in ferritin level from baseline to Week 28 was established as the response variable and the correlation analysis between baseline variables [age, sex, bodyweight, dialysis duration, baseline ferritin, TSAT, hemoglobin, mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), serum phosphorus, corrected calcium, intact parathyroid hormone, albumin, normalized protein catabolic rate (nPCR) and baseline ESA dosage] was used as explanatory variables. There were few examples of intravenous iron use, so this parameter was not added to the explanatory factors.
Pearson’s product-moment correlation coefficients were calculated in the correlation analysis of explanatory variables. Associations between investigated factors and changes in ferritin levels were evaluated with single regression analysis, and a P-value was obtained for each investigated factor. Among factors with an absolute value for the correlation coefficient of ≥0.6, either factor with a large P-value was excluded. A multiple regression analysis with stepwise method was performed, with change in ferritin level as the response variable and each factor not excluded as an explanatory variable, to identify significantly associated factors.
Patients were stratified into groups according to quartiles (Q) of baseline level of explanatory variable with the greatest influence. A stratified analysis was conducted of iron-related parameter changes during treatment.
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical tests were performed using two-sided tests at the P < 0.05/3 significance level, with multiple adjustments using the Bonferroni correction.
RESULTS
Of the 181 patients originally screened for the Phase III study, 161 received treatment. In these 161 patients, mean (± SD) age was 58.5 (±11.1) years, mean dialysis duration was 85.3 (±63.2) months and most patients were male (70.6%; n = 113). Only two patients (1.3%) had not received previous treatment with phosphate binders. For the sub-analysis population (n = 125) who had ferritin level data after 28 weeks of SO treatment, mean age was 59.4 (±10.5) years, mean dialysis duration was 84.3 (±64.4) months, mean baseline ferritin level was 70.5 (±77.1) ng/mL and most patients were male (71.2%; n = 89) (Table 1).
Table 1.
Baseline demographic and clinical characteristics for the sub-analysis population (n = 125)a
| Baseline characteristics | Patients |
|---|---|
| Age, years | 59.4 ± 10.5 |
| Sex, male, n (%) | 89 (71.2) |
| Bodyweight, kg | 63.2 ± 12.8 |
| Dialysis duration, months | 84.3 ± 64.4 |
| Baseline ferritin (min, max), ng/mL | 70.5 ± 77.1 (5.0, 455.0) |
| TSAT, % | 23.0 ± 9.2 |
| Hemoglobin, g/dL | 10.5 ± 0.8 |
| MCH, pg | 31.6 ± 1.6 |
| MCV, fL | 96.6 ± 4.3 |
| Serum phosphorus, mg/dL | 5.4 ± 1.0 |
| Corrected calcium, mg/dL | 9.3 ± 0.7 |
| Intact PTH, pg/mL | 194.9 ± 119.4 |
| Albumin, g/dL | 3.9 ± 0.3 |
| nPCR, g/kg/day | 0.9 ± 0.1 |
| ESA dosage,b IU/week (n) | 4150 ± 2641 (115) |
Data are presented as mean ± SD, unless otherwise indicated.
Mean dosage in administered patients only (×200 equivalent was taken for darbepoetin and epoetin beta pegol). PTH, parathyroid hormone.
Analyses of correlations (r ≥ 0.6) among explanatory variables showed a correlation between MCH and MCV (r = 0.932). Therefore, MCV was excluded from the explanatory variables used in the multiple regression analysis because P-values in the single regression analysis of correlations between baseline explanatory variables and change in ferritin level at Week 28 were 0.888 for MCH and 0.900 for MCV (Table 2). Results from the multiple regression analysis of correlations between baseline explanatory variables and change in ferritin level at Week 28 are shown in Table 3. In the stepwise multiple regression analysis, baseline ferritin, baseline ESA dosage, age and nPCR were identified as factors influencing ferritin levels during treatment. Of these factors, baseline ferritin was the strongest contributing factor (P < 0.0001) to ferritin increase during 28 weeks of SO treatment.
Table 2.
Single regression analysis of correlations between baseline explanatory variables and change in ferritin level at Week 28
| Baseline explanatory variable | Regression coefficient (B) (95% confidence interval) | P-value |
|---|---|---|
| MCH | 0.013 (−0.163, 0.188) | 0.888 |
| MCV | 0.011 (−0.165, 0.187) | 0.900 |
Table 3.
Stepwise multiple regression analysis of correlations between baseline explanatory variables and change in ferritin level at Week 28
| Baseline explanatory variable | Regression coefficient (B) (95% confidence interval) | P-value |
|---|---|---|
| Ferritin | −0.544 (−0.758, −0.331) | <0.0001 |
| ESA dosage | 0.010 (0.004, 0.016) | 0.001 |
| Age | −1.940 (−3.553, −0.327) | 0.019 |
| nPCR | 135.6 (21.96, 249.2) | 0.020 |
| Corrected calcium | 19.51 (−2.483, 41.50) | 0.082 |
| Hemoglobin | 17.68 (−2.275, 37.63) | 0.082 |
| Bodyweight | 1.009 (−0.294, 2.311) | 0.128 |
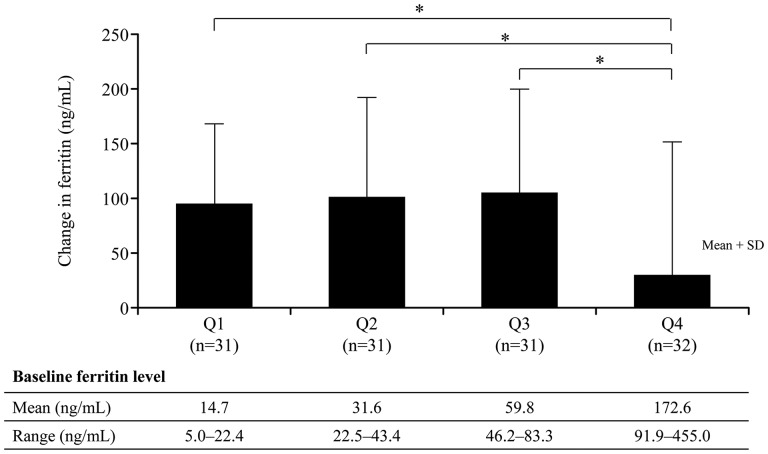
Patients’ baseline characteristics are shown by quartiles (Q) of baseline ferritin level in Table 4. Mean (±SD) baseline ferritin levels were lowest among patients in Q1, Q2 and Q3 (14.7 ± 4.3, 31.6 ± 6.6 and 59.8 ± 9.9 ng/mL, respectively) and highest among patients in Q4 (172.6 ± 90.1 ng/mL). Figure 1 shows changes in ferritin from baseline to Week 28 by quartiles of baseline ferritin. By Week 28, there were significant differences (P < 0.05/3) in ferritin increases between patients with higher (Q4) versus lower (Q1, Q2 and Q3) baseline ferritin.
Table 4.
Baseline variables stratified by quartiles (Q) of baseline ferritin levela
| Baseline variables | Q1 (n = 31) | Q2 (n = 31) | Q3 (n = 31) | Q4 (n = 32) |
|---|---|---|---|---|
| Ferritin (min, max), ng/mL | 14.7 ± 4.3 (5.0, 22.4) | 31.6 ± 6.6 (22.5, 43.4) | 59.8 ± 9.9 (46.2, 83.3) | 172.6 ± 90.1 (91.9, 455.0) |
| Age, years | 62.1 ± 9.5 | 58.5 ± 9.4 | 60.2 ± 11.1 | 57.0 ± 11.4 |
| Male, n (%) | 24 (77.4) | 21 (67.7) | 23 (74.2) | 21 (65.6) |
| Bodyweight, kg | 64.6 ± 13.2 | 62.7 ± 10.5 | 61.7 ± 11.1 | 63.8 ± 15.9 |
| Dialysis duration, months | 77.5 ± 61.0 | 96.1 ± 70.6 | 88.1 ± 74.7 | 75.6 ± 50.0 |
| TSAT, % | 19.5 ± 6.9 | 22.8 ± 9.4 | 23.7 ± 8.0 | 25.7 ± 11.1 |
| Hemoglobin, g/dL | 10.3 ± 0.9 | 10.8 ± 0.7 | 10.4 ± 0.8 | 10.3 ± 0.8 |
| MCH, pg | 31.0 ± 1.6 | 31.7 ± 1.4 | 31.7 ± 1.7 | 31.8 ± 1.5 |
| MCV, fL | 95.7 ± 4.6 | 96.9 ± 3.8 | 96.6 ± 4.7 | 97.1 ± 4.1 |
| Serum phosphorus, mg/dL | 5.5 ± 0.9 | 5.7 ± 1.1 | 5.3 ± 1.0 | 5.3 ± 1.0 |
| Corrected calcium, mg/dL | 9.2 ± 0.6 | 9.4 ± 0.8 | 9.2 ± 0.7 | 9.2 ± 0.7 |
| Intact PTH, pg/mL | 170.8 ± 91.5 | 176.9 ± 126.8 | 208.0 ± 136.4 | 223.1 ± 116.0 |
| Albumin, g/dL | 3.9 ± 0.3 | 3.9 ± 0.3 | 3.9 ± 0.2 | 4.0 ± 0.3 |
| nPCR, g/kg/day | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.2 |
| ESA dosage,b IU/week (n) | 4147 ± 2299 (29) | 3295 ± 1816 (28) | 4000 ± 2893 (27) | 5056 ± 3131 (31) |
Data are presented as mean ± SD, unless otherwise indicated.
Mean dosage in administered patients only (×200 equivalent was taken for darbepoetin and epoetin beta pegol). PTH, parathyroid hormone.
FIGURE 1.
Changes in ferritin from baseline to Week 28, according to quartiles (Q) of baseline ferritin. *P < 0.05/3; Q4 versus Q1, Q2 and Q3 (two-sample t-test, adjusted by Bonferroni multiple-comparison correction method).
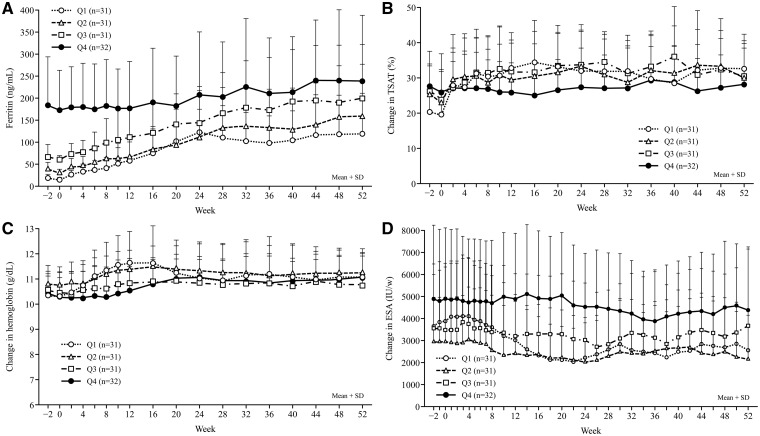
Changes in ferritin from baseline to Week 52, by quartiles of baseline ferritin, are shown in Figure 2A. The ferritin increase was less marked and more gradual in patients with higher (Q4) rather than lower (Q1, Q2 and Q3) baseline ferritin. Despite the sharp increase in ferritin among patients in Q1, Q2 and Q3, ferritin levels for all patients remained stable from Weeks 28 to 52, regardless of baseline ferritin level. In patients with higher baseline ferritin levels (Q4), TSAT remained within a consistent range of 25–30%, whereas in patients with lower baseline ferritin levels (Q1, Q2 and Q3), TSAT increased to ∼30% and then remained constant up to Week 52 (Figure 2B). Overall, no major changes in hemoglobin were noted during the study (Figure 2C). Hemoglobin increased slightly until Week 12 in patients with the lowest baseline ferritin level (Q1). An ESA dosage reduction was observed in patients with the lowest baseline ferritin level (Q1), and only slight reductions were noted in the other patient subsets (Figure 2D).
FIGURE 2.
Changes from baseline to Week 52 in ferritin (A), TSAT (B), hemoglobin (C) and ESA dosage (D), according to quartiles (Q) of baseline ferritin.
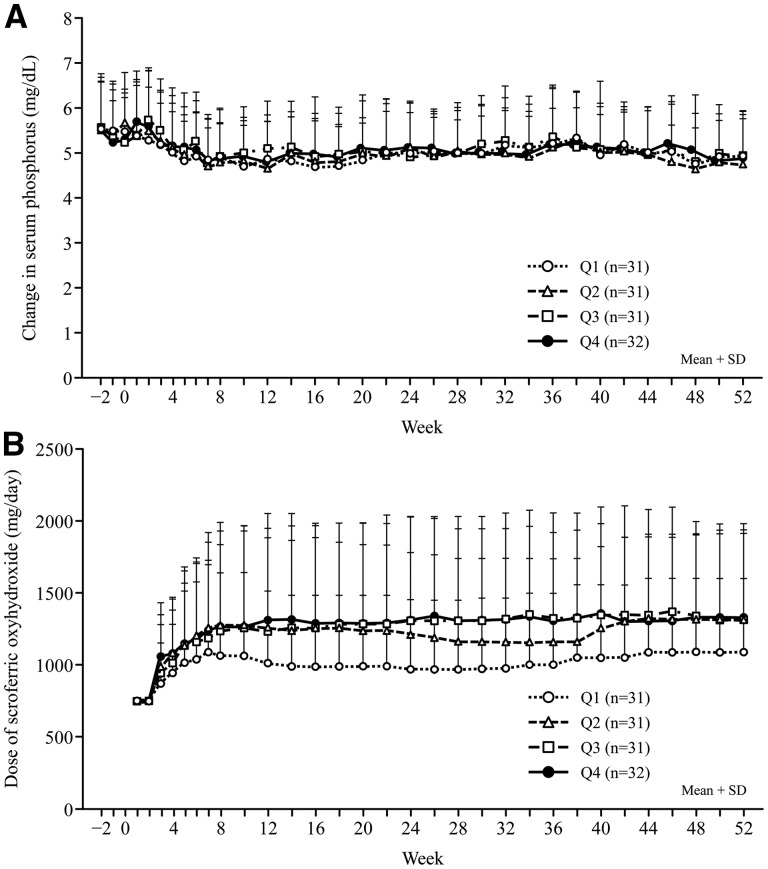
Serum phosphorus improved throughout the study (Figure 3A), regardless of baseline ferritin level (Table 5). SO dosages administered to patients in baseline ferritin quartiles Q2, Q3 and Q4 were comparable throughout the study (Figure 3B), with slight fluctuations, and dosages administered to patients in Q1 were considerably lower than those administered to patients in the other quartiles (Table 5).
FIGURE 3.
Changes from baseline to Week 52 in serum phosphorus concentration (A), and SO dosage (B).
Table 5.
Changesain serum phosphorus and SO dosage from baseline to Week 52, according to quartiles (Q) of baseline ferritinb
| Parameter | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|
| Serum phosphorus,b mg/dL | ||||
| Weeks −2 to 0 | 5.51 ± 0.94 | 5.55 ± 0.94 | 5.42 ± 0.93 | 5.36 ± 0.84 |
| Weeks 1–8 | 5.05 ± 0.83 | 5.09 ± 0.84 | 5.24 ± 0.72 | 5.20 ± 0.80 |
| Weeks 10–28 | 4.87 ± 0.70 | 4.92 ± 0.57 | 5.01 ± 0.67 | 5.02 ± 0.68 |
| Weeks 30–52 | 5.09 ± 0.68 | 4.97 ± 0.67 | 5.16 ± 0.78 | 5.08 ± 0.78 |
| Sucroferric oxyhydroxide dosage,b mg/day | ||||
| Weeks −2 to 0 | – | – | – | – |
| Weeks 1–8 | 940.5 ± 335.0 | 1058.5 ± 331.2 | 1022.2 ± 341.8 | 1057.6 ± 378.2 |
| Weeks 10–28 | 994.4 ± 475.9 | 1231.5 ± 577.0 | 1277.4 ± 694.6 | 1300.8 ± 677.2 |
| Weeks 30–52 | 1045.5 ± 475.6 | 1241.9 ± 548.4 | 1332.7 ± 706.3 | 1343.8 ± 598.4 |
Data shown are mean ± SD.
Quartiles of baseline serum ferritin level (Q1–Q4).
DISCUSSION
This was a sub-analysis of a Phase III, open-label, long-term, multicenter study, in Japanese hemodialysis patients with hyperphosphatemia, designed to identify baseline factors that might influence iron kinetics in patients treated with SO for 52 weeks. In this post hoc analysis, baseline ferritin, baseline ESA dosage, age and nPCR were identified as baseline variables that might influence increases in iron-related parameters, specifically ferritin, during SO treatment in patients with CKD undergoing hemodialysis. Notably, baseline ferritin was the most relevant variable regarding a subsequent influence on ferritin levels during SO treatment. Other significant contributing factors to ferritin increases during SO treatment were baseline ESA dosage, age and nPCR. Treatment compliance was high in this study with most patients (93.8%) taking ≥90% of doses [7]. Only 10 patients had a treatment compliance rate of <90%.
For patients in Q4 with a baseline ferritin level (91.9–455.0 ng/mL), ferritin increased less markedly and more gradually than in patients with lower baseline ferritin (Q1, Q2 or Q3). In Q4, the ferritin increase was probably suppressed by mechanisms inhibiting iron absorption to avoid iron overload [11]. Furthermore, healthy individuals with high ferritin levels absorb iron poorly, and in a study evaluating iron absorption in eight healthy adults, eight nondialysis-dependent CKD patients and eight hemodialysis patients who received 21 days of oral treatment with 59Fe-labeled SO powder, the median (range) blood uptake of labeled iron was 0.43% (0.16–1.25%), 0.06% (0.008–0.44%) and 0.02% (0–0.04%), respectively [1]. Researchers investigating the association between oral iron supplementation and ferritin levels in dialysis patients reported accentuated iron absorption in patients with low ferritin, reduced iron absorption in patients with high ferritin and increased ferritin when iron deficiency was treated with oral iron supplementation [12].
Hepcidin is a peptide hormone that suppresses iron absorption from the digestive tract, and regulates iron reutilization [13]. Hepcidin has its synthesis promoted via interleukin-6 in the liver and is cleared via the kidneys. CKD patients have elevated blood hepcidin levels, as renal hepcidin clearance is reduced, and hepcidin production is induced by inflammatory stimuli [14]. In iron deficiency, liver hepcidin expression is reduced, and iron absorption from the gastrointestinal tract is increased. Conversely, significant correlations have been found between absolute hepcidin and ferritin values and between final post-baseline increases in both parameters [15]. Although hepcidin levels were not measured in the current study, it is inferred that hepcidin increased due to the increased ferritin after SO treatment, and that iron absorption from the gastrointestinal tract was reduced. This may be the mechanism involved in the control of iron kinetics during SO treatment in patients with high ferritin levels.
Patients with the lowest baseline ferritin levels (Q1; ≤22.4 ng/mL) showed a slight increase in hemoglobin, particularly during the first 12 weeks of treatment, and a decrease in ESA dosage, throughout the study. It is possible that these patients were receiving higher ESA dosages that maintained normal hemoglobin levels in the presence of iron deficiency. However, a clinically relevant increase in hemoglobin levels for hemodialysis patients who tend to present iron deficiency would be a potential benefit of long-term SO treatment. Moreover, in the earlier post hoc analysis [6], patients who did not receive intravenous iron had reduced ferritin and increased TSAT and hemoglobin, suggesting that SO itself may have a role in inducing hematopoiesis, and the greatest changes in iron parameters with SO occurred during the first 6 months of treatment, whereas changes over the last 6 months were less pronounced. Additionally, patients with high baseline serum ferritin levels had the least pronounced changes in iron parameters [6]. Our study findings are consistent with the earlier post hoc analysis [6], despite the differences in study design.
In our sub-analysis, changes in serum phosphorus and SO dosages were tabulated by quartiles of baseline ferritin. Serum phosphorus concentrations were similar, and adequately managed, for patients in all quartiles of baseline ferritin. Conversely, SO dosages were comparable in Q2, Q3 and Q4, but lower in Q1 although serum phosphorus levels could be properly controlled in any quartile patient. Within each quartile, the SO dosage did not increase in line with increasing ferritin during the study. Furthermore, post-treatment increases in ferritin revealed that ferritin changes were not dependent on SO dosage alone. Other baseline factors (baseline ESA dosage, age and nPCR) were significantly correlated with ferritin increases during SO treatment. Correlation analysis of patient background factors (using the stepwise method) showed that pretreatment ferritin was the most strongly associated factor influencing increased ferritin at Week 28. Based on our findings, it seems relevant to evaluate baseline ferritin levels in patients for whom SO treatment for hyperphosphatemia is planned; such evaluation may be useful for predicting changes in ferritin and other iron-related parameters during treatment.
Our post hoc analysis has some limitations. There were no details of dosage and frequency of intravenous iron administration, but there are a few cases where intravenous iron has been administered in a long-term, Phase III study [7]. So, it was also considered that there was no influence of intravenous iron in this post hoc analysis. There were also no specifications on the use of intravenous iron and ESA. Furthermore, hepcidin was not measured, so detailed analysis of the mechanism of ferritin change was not possible. Given the post hoc design, our findings must be interpreted with caution. Notably, however, our results agree with sub-analyses conducted in the earlier post hoc analysis [6]. Moreover, we examined a limited number of baseline patient demographics and it is possible that some of these unmeasured factors may be confounders.
In summary, of the baseline variables found to predict increased ferritin and changes in iron-related parameters during SO treatment in Japanese CKD patients undergoing hemodialysis, baseline ferritin was the most relevant. Other relevant variables were baseline ESA dosage, age and nPCR. In patients with high baseline ferritin, the ferritin increase during SO treatment was minor and gradual, whereas in patients with low baseline ferritin, slight increases in hemoglobin, and associated ESA dosage reductions, were observed.
ACKNOWLEDGEMENTS
The authors would like to thank Keyra Martinez Dunn, MD, and David Murdoch of Edanz Medical Writing for providing medical writing services, which were funded by Kissei Pharmaceutical Co., Ltd, Japan. Kissei Pharmaceutical Co., Ltd, was also involved in additional sub-analyses and investigations of the study results.
FUNDING
Funding support for this study was provided by Kissei Pharmaceutical Co., Ltd.
CONFLICT OF INTEREST STATEMENT
F.K. was medical advisor for this clinical study, and he has received consulting fee from Kissei Pharmaceutical Co., Ltd.
REFERENCES
- 1. Geisser P, Philipp E.. PA21: a novel phosphate binder for the treatment of hyperphosphatemia in chronic kidney disease. Clin Nephrol 2010; 74: 4–11 [DOI] [PubMed] [Google Scholar]
- 2. Wilhelm M, Gaillard S, Rakov V. et al. The iron-based phosphate binder PA21 has potent phosphate binding capacity and minimal iron release across a physiological pH range in vitro. Clin Nephrol 2014; 81: 251–258 [DOI] [PubMed] [Google Scholar]
- 3. Wuthrich RP, Chonchol M, Covic A. et al. Randomized clinical trial of the iron-based phosphate binder PA21 in hemodialysis patients. Clin J Am Soc Nephrol 2013; 8: 280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Floege J, Covic AC, Ketteler M. et al. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int 2014; 86: 638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Floege J, Covic AC, Ketteler M. et al. Long-term effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant 2015; 30: 1037–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Covic AC, Floege J, Ketteler M. et al. Iron-related parameters in dialysis patients treated with sucroferric oxyhydroxide. Nephrol Dial Transplant 2017; 32: 1363–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koiwa F, Yokoyama K, Fukagawa M. et al. Long-term assessment of the safety and efficacy of PA21 (sucroferric oxyhydroxide) in Japanese hemodialysis patients with hyperphosphatemia: an open-label, multicenter, phase III study. J Ren Nutr 2017; 27: 346–354 [DOI] [PubMed] [Google Scholar]
- 8. Umanath K, Jalal DI, Greco BA. et al. Ferric citrate reduces intravenous iron and erythropoiesis-stimulating agent use in ESRD. J Am Soc Nephrol 2015; 26: 2578–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lewis JB, Sika M, Koury MJ. et al. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol 2015; 26: 493–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yokoyama K, Fukagawa M, Akiba T. et al. Ferritin elevation and improved responsiveness to erythropoiesis-stimulating agents in patients on ferric citrate hydrate. Kidney Int Rep 2017; 2: 359–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wheby MS, Jones LG, Crosby WH.. Studies on iron absorption. intestinal regulatory mechanisms. Clin Invest 1964; 43: 1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eschbach JW, Cook JD, Scribner BH. et al. Iron balance in hemodialysis patients. Ann Intern Med 1977; 87: 710–713 [DOI] [PubMed] [Google Scholar]
- 13. Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003; 102: 783–788 [DOI] [PubMed] [Google Scholar]
- 14. Babitt JL, Lin HY.. Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. Am J Kidney Dis 2010; 55: 726–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaillard CA, Bock AH, Carrera F. et al. Hepcidin response to iron therapy in patients with non-dialysis dependent CKD: An analysis of the FIND-CKD trial. PLoS One 2016; 11: e0157063. [DOI] [PMC free article] [PubMed] [Google Scholar]