Abstract
Background
Inherited nephrogenic diabetes insipidus (NDI) is a rare disorder characterized by impaired urinary concentrating ability. Little clinical data on long-term outcome exists.
Method
This was a single-centre retrospective medical record review of patients with a diagnosis of NDI followed between 1985 and 2017. We collected available data on growth, weight, school performance, complications and comorbidities.
Results
We identified 36 patients with available data and a clinical diagnosis of NDI, which was genetically confirmed in 33 of them. Patients presented at a median age of 0.6 years and median length of follow-up was 9.5 years. Chief symptoms at presentation were faltering growth, vomiting/feeding concerns, polyuria/polydipsia, febrile illness and hypernatraemic dehydration. Median weight standard deviation scores (SDS) improved from −2.1 at presentation to 0.2 at last follow-up. In contrast, height SDS remained essentially unchanged at −1.1 at presentation and −0.9 at last follow-up. Most patients were treated with prostaglandin synthesis inhibitors and thiazides, yet weaned off during school age without an obvious change in urine output. Median estimated glomerular filtration rate at last follow-up was 81 mL/min/1.73 m2. Urological complications were noted in 15 patients, constipation in 11 and learning difficulties in 5. Median age at resolution of nocturnal enuresis was 11 years. Estimated median daily fluid intake at median age of 13 years was 3800 mL/m2.
Conclusion
The overall prognosis in inherited NDI is favourable with regular treatment. As expected, most complications were related to polyuria. There is an apparent loss of efficacy of medications during school age. Our data inform the prognosis and management of patients with NDI.
Keywords: AVPR2, AQP2, congenital nephrogenic diabetes insipidus, hypernatraemia, polyuria
INTRODUCTION
Inherited nephrogenic diabetes insipidus (NDI) is a rare disorder characterized by an insensitivity of the kidneys to arginine-vasopressin (AVP) and a consequent inability to concentrate the urine [1]. There are primary and secondary forms of inherited NDI [2]. In primary inherited NDI, we distinguish between an X-linked form, due to mutations in the gene encoding the vasopressin type 2 receptor, namely arginine vasopressin receptor-2 (AVPR2) and an autosomal form, due to mutations in the gene encoding the water channel aquaporin2 (AQP2). In addition to NDI, patients with the X-linked form also have an impaired haemodynamic and coagulation response to AVPR2 stimulation [3]. Both forms are typically inherited in a recessive fashion, although rare dominant cases have been described [4]. Approximately 90% of cases are due to mutations in AVPR2 and to date more than 250 mutations have been described. As with most rare diseases, little long-term clinical data exists to inform management and prognosis. Previous reports have highlighted the potential complications of flow uropathy, behavioural abnormalities, such as attention deficit disorders as well as severe developmental delay, sometimes associated with intracranial calcifications [5–7]. Some of these, such as the developmental delay and the intracranial calcification, are considered preventable by appropriate treatment. Here we review the clinical course of patients in the tubulopathy clinic at Great Ormond Street Hospital (GOSH) for Children NHS Foundation Trust.
MATERIALS AND METHODS
We performed a retrospective review of the medical records of patients with a clinical diagnosis of primary congenital NDI who were followed in the specialized clinic for tubular disorders at GOSH between 1985 and 2017. We ascertained available molecular, as well as clinical data, including symptoms and age at presentation and last follow-up, family history, medical treatments, growth indicators, complications, comorbidities and biochemical parameters (plasma sodium, creatinine and osmolality, as well as urine osmolality) at the time of presentation and at last follow-up. Patients without genetic confirmation of the diagnosis were excluded from the main analysis to maintain a clearly defined cohort. Standard deviation scores (SDS) for anthropometric measures were calculated using growth charts based on National Health and Nutrition Examination Survey (NHANES), center for disease control (CDC)/National Center for Health Statistics. Estimated glomerular filtration rate (eGFR) was calculated with the Schwartz formula modified for GOSH, using a k-value of 33 [8]. Statistical calculations were performed using Microsoft Excel 2011 and GraphPad Prism software.
RESULTS
Patients
We identified 41 patients with a clinical diagnosis of NDI. Five patients were excluded, due to untraceable records. The remaining 36 patients all had genetic testing performed and the diagnosis was confirmed in 33, of which 29 were male. Median follow-up was 9.5 years (range 0.8–16.8) and median age at last follow-up was 11.9 years (range 0.8–17).
Details of selected genetic and clinical data are presented in Table 1.
Table 1.
Clinical and molecular details of the 33 patients with identified causative mutations
| Genetic details |
Clinical details |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nucleotide | Protein | Sex | At presentation |
At last follow-up |
Meds | Remarks (complications/ comorbidities) | |||||||||
| Age (years) | Plasma osmolality (mOsm/kg) | eGFR (mL/min/ 1.73 m2) | Uosm after DDAVP (mOsm/kg) | Weight SDS | Height SDS | Age (years) | Weight SDS | Height SDS | eGFR (mL/min/ 1.73 m2) | ||||||
| Patients with mutations in AVPR2 | |||||||||||||||
| 1 | c.851G>A | p.(Trp284*) | M | 0.25 | 304 | 43 | 65 | −2.62 | −1.25 | 11.9 | −0.02 | −1.68 | 90 | Indomethacin, thiazide | Feeding difficulty, esophagitis |
| 2.1 | c.999dup | p.(Ser334Leufs*23) | M | 0.016 | 300 | 44 | 73 | −0.44 | 0.51 | 15.3 | −1.03 | −1.16 | 76 | Indomethacin, thiazide | Transient hydronephrosis, large bladder, PVR, constipation |
| 2.2 | c.999dup | p.(Ser334Leufs*23) | M | 0.021 | 310 | 34 | 53 | 0.84 | 0.89 | 16.7 | −0.34 | −0.53 | 75 | Indomethacin, thiazide | Hydronephrosis, constipation |
| 2.3 | c.999dup | p.(Ser334Leufs*23) | M | 0.08 | 310 | 45 | 50 | 0.73 | −1.06 | 17 | 0.08 | −1.13 | 77 | Indomethacin, thiazide | ADHD, impaired concentration, mild hydronephrosis |
| 2.4 | c.999dup | p.(Ser334Leufs*23) | M | 0.16 | 307 | 38 | 89 | −0.87 | −0.54 | 17 | −0.01 | −0.86 | 65 | Indomethacin, thiazide | Mild hydronephrosis, migraine |
| 3 | c.871C>T | p.(Glu291*) | M | 0.83 | 304 | 54 | 111 | 0.91 | −0.76 | 16.3 | 0.29 | −0.89 | 79 | Indomethacin, thiazide | |
| 4 | c.27_54del | p.(Val10Cysfs*18) | M | 0.3 | 301 | 43 | 129 | −3.01 | −0.86 | 14.1 | 1.49 | 0.92 | 76 | Indomethacin, thiazide | Transient hydronephrosis, growth hormone deficiency, impaired concentration |
| 5 | c.299del | p.(Lys100Argfs*16) | M | 0.58 | 325 | 44 | 40 | 2.61 | −2.02 | 11.8 | 0.74 | −0.7 | 72 | Indomethacin, thiazide | Constipation |
| 6 | c.332T>C | p.(Leu211Pro) | M | 0.66 | 354 | 34 | 197 | −4.08 | −3.19 | 14.2 | 1.11 | −2.4 | 106 | Indomethacin, thiazide | Constipation |
| 7.1 | c.262G>A | p.(Val88Met) | M | 2 | 294 | 83 | 570 | −2.8 | −2.87 | 1 | −0.51 | −0.37 | 81 | Indomethacin, thiazide | Hydronephrosis, large bladder, PVR, constipation |
| 7.2 | c.262G>A | p.(Val88Met) | M | 0.04 | 292 | 26 | 63 | −1.88 | −1.59 | 10 | −0.27 | 0.01 | 92 | Indomethacin, thiazide | ADHD, impaired concentration/school performance |
| 8 | c.316C>T | p.(Arg106Cys) | M | 2.9 | 294 | 140 | 173 | −1.78 | −1.56 | 17 | 0.31 | 0.97 | 85 | Indomethacin, thiazide | |
| 9 | c.809_810del | p.(Val270Glyfs*86) | M | 0.5 | 319 | 69 | 173 | −3.38 | −2.25 | 10 | 0.19 | 0.66 | 95 | Indomethacin, thiazide | Mild hydronephrosis, constipation |
| 10.1 | c.316C>T | p.(Arg106Cys) | M | 7 | 298 | 63 | 50 | 0.9 | 0.45 | 15 | 0.37 | 0.27 | 70 | Indomethacin, thiazide | Hydronephrosis, large bladder, PVR, PUV with left VUR |
| 10.2 | c.316C>T | p.(Arg106Cys) | M | 9 | N/A | 89 | 220 | 1.39 | 0.62 | 14 | 0.82 | −0.78 | 80 | Indomethacin, thiazides | Hydronephrosis, constipation |
| 11.1 | c. (-1069_1007) del ins168 | p.? | M | 0.15 | 320 | 54 | 114 | −0.96 | −1.17 | 9 | 1.57 | −0.38 | 66 | Indomethacin, thiazide | |
| 11.2 | c.(-1069_1007) del ins168 | p.? | M | 0.5 | 328 | 41 | 179 | −2.66 | −0.33 | 5 | 0.92 | −0.68 | 67 | Indomethacin, amiloride, thiazide | ADHD, impaired concentration |
| 12 | c.del970 | p.(Ile324Serfs*112) | M | 8 | 298 | 69 | 55 | 2.6 | 1.59 | 17 | 0.92 | −1.42 | 56 | Thiazide | Hydronephrosis, single kidney, large bladder + PVR |
| 13 | c.491G>A | p.(W146X) | M | 1.83 | 277 | 103 | 44 | −2.55 | −2.13 | 15 | 0.7 | 0.23 | 86 | Indomethacin, thiazide | Large bladder, PVR, ADHD, dyslexia mild hydronephrosis |
| 14 | c.357G>C | p.(Glu119His) | M | 0.16 | 359 | 45 | 50 | −2.25 | −1 | 4 | −0.98 | −1.54 | 91 | Indomethacin, thiazides | |
| 15 | c.599G>A | p.(Trp200*) | M | 1.66 | 290 | 102 | 147 | −1.98 | −1.02 | 3 | 1.64 | 1.3 | 114 | Indomethacin, thiazide, amiloride | Transient neurological impairment associated with acute hypernatraemia |
| 16 | c.604C>T | p.(Arg202Cys) | M | 0.58 | 287 | 111 | 76 | −2.83 | −0.33 | 2.2 | −1.65 | −3.5 | 90 | Amiloride, thiazide | |
| 17 | c.(?-1) _(*1_?) del | p.? | M | 0.03 | 337 | 49 | 185 | 0.34 | 0.89 | 0.83 | −1.06 | −0.76 | 89 | Celecoxib, thiazide | |
| 18 | c.348C>G | p.(Lys116Asn) | M | 0.91 | 298 | 73 | 95 | −4.47 | −4.02 | 3.6 | −1.89 | −4.15 | 81 | Indomethacin, thiazide, amiloride | IUGR, necrotizing enterocolitis |
| 19 | c.830T>C | p.(Val277Ala) | M | 8 | 338 | 41 | 73 | 2.2 | 1.9 | 16 | 2.3 | 2.1 | 83 | Indomethacin, thiazide | |
| 20 | c.332T>C | p.(Leu111Pro) | M | 0.01 | 293 | 95 | 65 | −2.42 | −1.06 | 6.1 | 1.43 | 0.34 | 66 | Celecoxib, thiazide, amiloride | Rhomboencephalo-synapsis |
| Patients with mutations in AQP2 | |||||||||||||||
| 21 | c.377C>T | p.(Thr126Met) | M | 0.58 | 354 | 62 | 95 | −2.8 | −2.97 | 13 | 0.23 | −0.9 | 60 | Indomethacin, thiazide | Global developmental delay |
| 22 | c.253C>T | p.(Arg85*) | M | 3.6 | 304 | 85 | 177 | 1.49 | −1.86 | 17 | 3.06 | −1.39 | 81 | Ibuprofen, thiazide | Left hydronephrosis with 10% divided function, large bladder, PVR |
| 23.1 | c.337C>T | p.(Arg113Cys) | F | 0.33 | 340 | 45 | 158 | −2.83 | −2.23 | 7.9 | −2.58 | −2.85 | 67 | Indomethacin, thiazide | Constipation |
| 23.2 | c.337C>T | p.(Arg113Cys) | F | 0 | 280 | 52 | 114 | −2.55 | −1.33 | 3 | −2.75 | −1.72 | 97 | Indomethacin, thiazide | Constipation |
| 24 | c.211G>A | p.(Val71Met) | F | 0.25 | 350 | 69 | 158 | −2.1 | −0.59 | 16 | 1.54 | −1.67 | 72 | Indomethacin, thiazide | Hydronephrosis |
| 25 | c.299G>T/ c.763C>T | p.(Gly100Val)/ p.(Gln255*) | M | 6.5 | 284 | 50 | 86 | −4.65 | −2.02 | 10.5 | −1.51 | −3.02 | 84 | Celecoxib | |
| 26 | c.211G>A | p.(Val71Met) | M | 1.75 | 295 | 107 | 100 | −8.25 | −4.94 | 5 | −1.92 | −1.32 | 129 | Indomethacin, thiazide | Mild hydronephrosis, constipation |
Shown are pertinent molecular and clinical details. Mutations in AQP2 are all homozygous, except for Patient 25, who is compound heterozygous.
‘?’ is standard genetic annotation for Unknown.
Uosm, urine osmolality; PUV, posterior urethral valves; VUR, vesico-ureteric reflux; IUGR, intra-uterine growth retardation.
Molecular details
Twenty-six patients had mutations in AVPR2 (all boys) and seven in AQP2 (four boys, three girls) (Table 1). All were inherited in a recessive fashion. Mutations and selected clinical details for some patients have been reported previously [9–12].
Presenting features
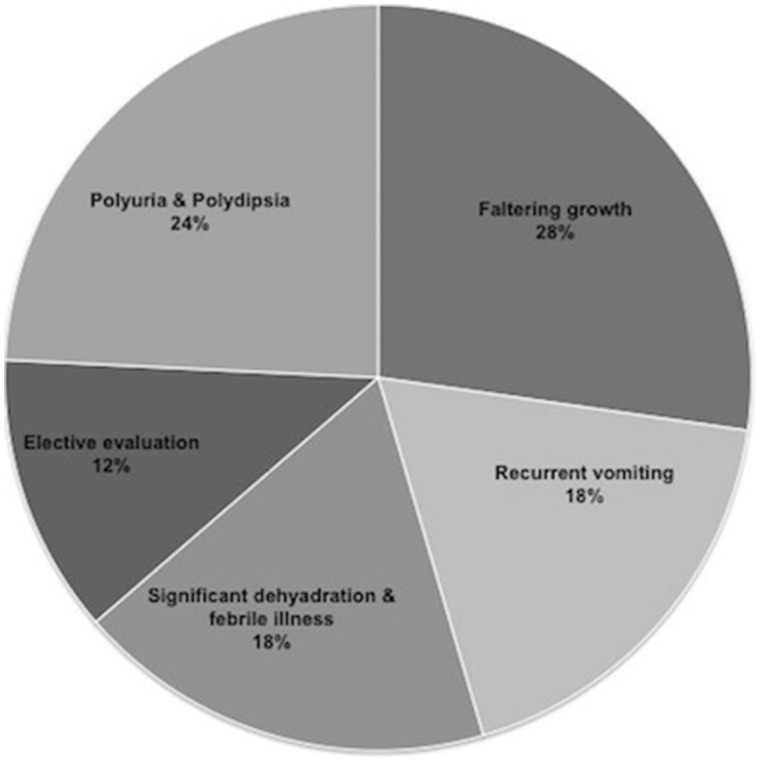
Congenital NDI was diagnosed during the first year of life in 23 (69%) patients. The median age of diagnosis was 0.6 years (range 0.01–9). A family history of NDI was noted in 11 patients. One patient (23.2) had a prenatal diagnosis. Chief complaints at time of admission were faltering growth, vomiting, polyuria/polydipsia and febrile illness with hypernatraemic dehydration (Figure 1). Four (12%) were investigated due to a positive family history.
FIGURE 1.
Symptoms at initial presentation. Shown is the frequency of the chief complaints at presentation.
Ten patients were treated for a different diagnosis before the establishment of NDI, most commonly gastroesophageal reflux disease (n = 5). A pyloric web was diagnosed in one case (26), based on vomiting and hypernatraemia, and surgically corrected. One patient (19) was given an initial diagnosis of central DI at his local hospital based on a partial response to 1-desamino-8-D-arginine vasopressin (DDAVP) (maximal urine osmolality 602 mOsm/kg), but was subsequently found to have a clinical diagnosis of partial NDI, confirmed by genetic testing. Interestingly, a repeat DDAVP test at the age of 10 years showed no response to DDAVP. Two premature neonates (18, 20) were diagnosed in the neonatal intensive care unit based on polyuria. One patient (21) presented with global developmental delay and hypernatraemic dehydration at the age of 0.6 years. Magnetic resonance imaging of the brain had been performed in five patients. One patient (15) showed signs suggestive of myelinolysis after experiencing severe acute hypernatraemia in the context of treatment with 0.9% saline [12].
Median SDS or score and range for height at presentation was −1.06 (−4.94 to 1.9) and for weight: −2.1 (−8.25 to 2.6). Weight for length (for children <3 years), SDS was −1.98 (−7.5 to 2.3).
Feeding
Treatment included input from a renal dietician and an osmotic load <15 mOsm/kg was targeted. Tube feeding was performed in 34% (n = 12) of which 24% (n = 8) were fed via gastrostomy. The indication for tube feeding was persistent growth failure in all (weight <0.4th percentile for age and no evidence of catch-up growth). Initially, a nasogastric tube was placed and, if tube feeding was deemed necessary for several months, a gastrostomy was considered. Tube feeding was discontinued at a median age of 2 years (range 2–4) once growth failure had resolved and oral intake was deemed adequate.
Medications
For drug treatment, all patients bar one (25) received thiazides at diagnosis and 28 (84%) received an additional prostaglandin synthesis inhibitor (PSI), mostly indomethacin (n = 25), or, alternatively, celecoxib (n = 2) or ibuprofen (n = 1). PSI was discontinued in 10 (indomethacin in 9, ibuprofen in 1) because of perceived lack of efficacy at median age 12 years (range 4–16). In addition, it was changed to celecoxib in one patient (15, at age 3 years) due to a gastrointestinal bleed and stopped in another one (7.1) due to concerns over renal function associated with an obstructive uropathy (at age 12 years). Five patients (2.3, 6, 7.2, 20 and 25) switched from indomethacin to ibuprofen due to difficulties with medication availability. One patient (25) developed nausea after commencement of celecoxib and it was changed to ibuprofen. One patient (2.2) developed severe hydronephrosis and a distended bladder with a volume of ∼1500 mL and post-void residual (PVR), which was noted for a first few months after stopping indomethacin. The drug was restarted but without apparent change in urine volume and the hydronephrosis persisted. The median daily dose of indomethacin at the time of discontinuation due to perceived lack of efficacy was available in eight patients and was 1.1 mg/kg (range 0.4–1.7). Those patients continuing indomethacin (n = 7) were prescribed at last follow-up a median daily dose of 1.4 mg/kg.
Similarly, thiazides were discontinued in eight patients at a median age of 14 years (range 5–17). At this age, all eight were receiving bendroflumethiazide with a median daily dose of 0.08 mg/kg (range 0.06–0.11). In those continuing bendroflumethiazide (n = 15), the median prescribed daily dose was 0.1 mg/kg. Urine output was not formally measured before and after stopping medications, but patients reported no apparent change.
Amiloride was given in four patients (Table 1) to maintain normokalaemia. Apart from these drugs, potassium chloride was prescribed in 19% of patients. Other prescribed medications mostly included laxatives, antacids and prokinetics. Five patients were able to stop all medications at median age of 14 years (12.5 ± 3.8).
Biochemistries
Biochemistries at presentation were consistent with the clinical diagnosis of NDI: median (range) for urine osmolality was 86 (46–177) mOsm/kg, for plasma osmolality 303 (277–359) mOsm/kg and for plasma sodium 148 (133–177) mmol/L. Median eGFR at presentation was 54 mL/min/1.73 m2 (range 26–140).
Interestingly, urine osmolality in patient 7.1 increased after DDAVP to 570 mOsm/kg, consistent with a diagnosis of partial NDI [9].
Biochemistries at last follow-up showed persistent NDI with a median (range) urine osmolality of 84 (31–215) mOsm/kg, but now with normal plasma values: median osmolality 292 (281–307) mOsm/kg and sodium 143 (133–149) mmol/L.
Growth
Median SDS or z-score for weight (range) improved from: −2.1 (−8.3 to 2.6) at presentation to 0.2 (−2.8 to 3) at last follow-up (Table 2). In contrast, height SDS (range) remained essentially unchanged: −1.06 (−4.94 to 1.9) at presentation and −0.9 (−4.2 to 1.3) at last follow-up.
Table 2.
Comparison between patient with AVPR2 and AQP2 mutations
| At presentation |
At last follow-up |
No. with urological complication (%) | Age of resolution of nocturnal enuresis | No. with reported school/behavioural complications | |||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Median height SDS (range) | Median weight SDS (range) | Median eGFR (mL/min/ 1.73 m2) (range) | Median height SDS (range) | Median weight SDS (range) | Median eGFR (mL/min/ 1.73 m2) (range) | |||
| AVPR2 | −1.0 (−4 to 1.9) | −1.9 (−4.5 to 2.6) | 51 (26–140) | −0.7 (−4.2 to 1.3) | 0.3 (−1.9 to 1.6) | 80.5 (56–114) | 10 (38.5) | 10 (4–15) | 5 |
| AQP2 | −2.0 (−4.9 to − 0.6) | −2.8 (−8.3 to 1.5) | 61 (45–107) | −1.7 (−3 to −0.9) | −1.5 (−2.8 to 3) | 81 (59.6–129) | 5 (71) | 12.4 (9.4–14) | 0 |
| Combined | −1.1 (−4.9 to 1.9) | −2.1 (−8.3 to 2.6) | 54 (26–140) | −0.9 (−4.2 to 1.3) | 0.2 (−2.8 to 3) | 81 (56–129) | 15 (46) | 11.3 (4–15) | 5 |
Shown are selected data for patients with X-linked versus autosomal NDI. No significant differences were seen.
Urological problems
Urological complications were noted in 46% (n = 15) of patients. Nocturnal enuresis and incomplete voiding were the most common concerns. Large bladder capacity and incomplete bladder emptying was noted in eight patients. During follow-up of mild-to-moderate severity, unilateral or bilateral hydronephosis was noticed in 14 patients and 7 of these had persistence of hydronephrosis and a large bladder capacity, whereas in 7 the dilatation was only mild and transient (Figure 2). Resolution of nocturnal enuresis was seen in 19 patients at a median age of 11.3 (range 5–16) years. Interestingly, one child (7.1) with partial NDI had resolution of nocturnal enuresis with desmopressin therapy [9]. One patient (10.1) with hydronephrosis and multiple urinary tract infection was found to have posterior urethral valves, and had a left nephroureterectomy and Mitrofanoff formation [10]. His brother (10.2) shared the diagnosis of NDI but had no evidence of an obstructive uropathy.
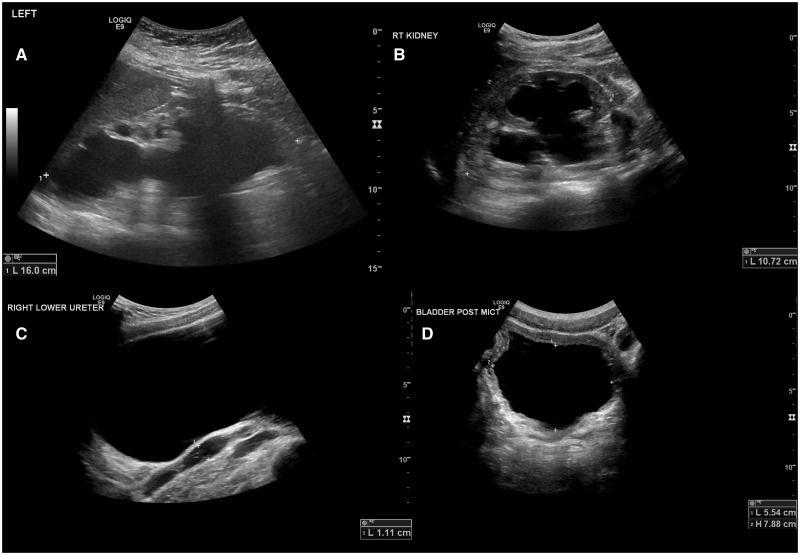
FIGURE 2.
Examples of flow uropathy. Shown are ultrasound images detailing flow uropathy. (A) Marked hydronephrosis of single right kidney with loss of renal cortex in Patient 22 (at age 17 years). (B) Hydronephrosis, (C) Dilated bladder and ureter, (D) PVR in Patient 2.1 (all images at age 15 years). Hydronephrosis in this patient developed after the age of 8 years.
Estimated glomerular filtration rate
Median eGFR at last follow-up was 81 (range 56–129) mL/min/1.73 m2 and was comparable between patients with AVPR2 and AQP2 mutations (Table 2).
Fluid intake at last follow-up, as estimated by patients and/or family was available in 28 patients. Estimated median daily fluid intake at a median age of 13 years was 3800 mL/m2 (range 1600–9200).
Constipation
Constipation remained a concern in our cohort in 11 patients. It was often noted during the initial years with mild-to-moderate severity. Regular intake of laxatives was prescribed in most of these patients.
Intelligence and school performance
One patient (21) had severe global developmental delay already noted at his presentation at 7 months of age with faltering growth and hypernatraemic (170 mmol/L) dehydration. Data on learning or behaviour problems were available in 5 (17.8%) out of 28 school-age children, who had been diagnosed with attention-deficit hyperactivity disorder (ADHD) and/or learning difficulties and/or impaired concentration (Table 1).
No complaints on school performance were noted in the records of the remaining patients.
Genotype–phenotype correlation
We compared clinical parameters of the 26 patients with AVPR2 mutations to the 7 with AQP2 mutation. No significant difference was seen (see Table 2).
Patients without genetic confirmation
In three patients, no mutation in AVPR2 or AQP2 could be identified: two male siblings and one female child presented at a median age of 0.16 (range 0.01–0.16) years. Further genetic analysis showed that both brothers had inherited the same X-chromosome from their mother. The girl, born to consanguineous parents, was homozygous at the AQP2 locus. Thus, it is possible that these three patients have non-coding mutations in these genes, which were not assessed by the testing procedure in the laboratory. All three were unresponsive to intravenous DDAVP at presentation with median maximum osmolality of 97 (range 91–114) mOsm/kg, consistent with NDI. There were no clinical features that would suggest a diagnosis of secondary NDI [2, 13]. Median plasma sodium at presentation was 154 (150–157) mmol/L and osmolality 309 (307–317) mOsm/kg. Of specific interest was a spontaneous improvement of urinary concentrating ability in the two brothers, with a DDAVP test at 11 and 15 years of age showing a maximum urine osmolality of 536 and 752 mOsm/kg, respectively (Table 3).
Table 3.
Spontaneous improvement in urinary concentration in the two brothers without identified mutation
| Patient | Presentation |
Last follow-up |
||||
|---|---|---|---|---|---|---|
| Age (months) | Posm (mOsm/kg) | Max Uosm after DDAVP | Age (years) | Posm (mOsm/kg) | Max Uosm after DDAVP | |
| 28.1 | 1.5 | 317 | 114 | 15.3 | 298 | 536 |
| 28.2 | 0.1 | 309 | 91 | 11.5 | 285 | 752 |
Shown are data on urinary concentration. Patient 28.1 presented at the age of 1 month with recurrent vomiting and hypernatraemia. His brother 28.2 was electively reviewed at 3 days of age due to the family history. Both received a clinical diagnosis of NDI based on the inappropriately low urine osmolality. Both showed a spontaneous improvement in symptoms and urinary concentration over time.
Uosm, urine osmolality; Posm, plasma osmolality.
DISCUSSION
We here report long-term clinical data on a single-centre cohort of patients with congenital NDI. Overall, our data show a favourable outcome: growth is in the normal range for the vast majority of patients, although median height at follow-up was slightly below the average at −0.9 SDS. Kidney function, as assessed by eGFR at last follow-up, was within chronic kidney disease (CKD) Stages 1 or 2 in all except for Patient 12, who was born with a single kidney.
Previous reports have highlighted the complications of NDI with regards to intellectual function [6, 14, 15]. Unfortunately, data on school performance were not systematically captured in the medical records and thus may be underestimated in our study, but intellectual and/or behavioural problems were noted in five patients (see Table 1), mostly in the form of a diagnosis of ADHD and/or impaired concentration and learning difficulties. This is considerably less than in a previous report where patients were systematically assessed and almost half had a diagnosis of ADHD and 70% a low score on short-term memory [5]. It has been debated whether ADHD is an intrinsic aspect of the disorder, as there are data suggesting at least temporary expression of AVPR2 in the brain, as well as an effect of AVP on learning and memory [16, 17]. Moreover, AVPR2 knock-out mice show altered expression of genes in the hypothalamus [18]. Alternatively, ADHD and problems with concentration or memory may be secondary to the constant craving for water and the frequent need to void. In this context, it is interesting that we saw this complication only in patients with AVPR2. Yet, this difference in the proportion of patients with noted learning or behavioural problems was not statistically significant between AVPR2 and AQP2 (P = 0.2).
Early reports on patients with NDI suggested severe intellectual impairment as an almost invariant feature of the disease, but subsequent observations indicate that this complication can be prevented in most by adequate treatment [5, 6, 15]. The latter is consistent with our observations here, as we saw severe global developmental delay in only one patient (21). Interestingly, while presenting with hypernatraemic dehydration, his features were not substantially different from other patients in this cohort. It is thus unclear whether for some reason this patient is more susceptible to brain damage, or whether his developmental delay is unrelated to the NDI. Brain imaging to assess for intracerebral calcifications had not been performed.
Overall our data are consistent with the notion that severe intellectual impairment can be prevented with adequate treatment.
Yet, despite the overall favourable prognosis, patients clearly have complications from the polyuria. Almost half of all patients had radiological evidence of a flow uropathy. While this was only transient in some it was severe in others and associated with loss of renal cortex in one (22) (Figure 2). Similarly, nocturnal enuresis was a common problem and resolution was much delayed at a median age of over 11 years. Typical advice and treatment given to patients with nocturnal enuresis, such as decreased fluid intake before bedtime or DDAVP tablets, obviously does not work in patients with NDI.
There were no episodes of hypernatraemic dehydration after diagnosis and start of treatment apparent from the biochemistry results available at GOSH. Yet, since GOSH is a pure tertiary paediatric center without provision of primary or emergency care, acute illnesses would have presented to the respective local hospital and thus would not be systematically captured or reflected in laboratory results obtained at GOSH.
Of interest is the apparent change in response to medical treatment. Thiazide and PSI have been shown to effectively reduce urine output in NDI during the first few years of life [19, 20]. Indeed, one patient (2.1) developed asymptomatic hyponatraemia (130 mmol/L) after commencement of indomethacin and the drug was transiently withheld. Yet, in our experience, many patients stop medications during school age without noticing an appreciable difference in urine output. While Patient 2.2 developed severe hydronephrosis first noted 4 months after stopping indomethacin, this did not improve after restarting the medication. Moreover, his last previous ultrasound, which showed no dilatation, had been 5 years earlier and thus it is likely that the hydronephrosis had developed even before the medication was stopped and was related to voluntary urinary retention, reflected in his large bladder volume. Medications were typically discontinued during teenage years, an age in which adherence to medications is a common problem [21]. Indeed, one patient (2.3) openly admitted that he had stopped taking the medications and in others, either the patient or a parent had reported intermittent missed doses. Thus, whether the apparent lack of efficacy reflects a true change in the response to medications or mainly non-adherence cannot be discerned from our data.
The initial management of Patient 15 with normal saline for his dehydration and subsequent severe hypernatraemia and encephalopathy illustrates the difficulties patients with this rare disease encounter [12]. Most general physicians and even paediatricians will have never have encountered a patient with NDI before and thus follow guidelines for patients in general. Provision of a letter or leaflet that the patient or family can present at an emergency visit, that details the diagnosis and explains emergency management that is critical to prevent such complications.
In conclusion, we show a generally favourable long-term outcome and an apparent loss of efficacy of medical treatment during school age.
FUNDING
Support to DB and RK was provided by Kids Kidney Research, Kidney Research UK, St Peter's Trust for Kidney, Bladder and Prostate Research, The David and Elaine Potter Foundation and the European Union, FP7 (grant agreement 2012-305608 “European Consortium for High-Throughput Research in Rare Kidney Diseases (EURenOmics”). WH, WvH, RK and DB are supported by the Biomedical Research Centre at Great Ordmond Street Hospital/UCL Institute of Child Health.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest and that this work has not been published previously in whole or part.
REFERENCES
- 1. Bockenhauer D, Bichet DG.. Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus. Nat Rev Nephrol 2015; 11: 576–588 [DOI] [PubMed] [Google Scholar]
- 2. Bockenhauer D, Bichet DG.. Inherited secondary nephrogenic diabetes insipidus: concentrating on humans. Am J Physiol Renal Physiol 2013; 304: F1037–F1042 [DOI] [PubMed] [Google Scholar]
- 3. Bichet DG, Razi M, Lonergan M. et al. Hemodynamic and coagulation responses to 1-desamino[8-D-arginine] vasopressin in patients with congenital nephrogenic diabetes insipidus. N Engl J Med 1988; 318: 881–887 [DOI] [PubMed] [Google Scholar]
- 4. Bichet DG, Bockenhauer D.. Genetic forms of nephrogenic diabetes insipidus (NDI): vasopressin receptor defect (X-linked) and aquaporin defect (autosomal recessive and dominant). Best Pract Res Clin Endocrinol Metab 2016; 30: 263–276 [DOI] [PubMed] [Google Scholar]
- 5. Hoekstra JA, van Lieburg AF, Monnens LA. et al. Cognitive and psychosocial functioning of patients with congenital nephrogenic diabetes insipidus. Am J Med Genet 1996; 61: 81–88 [DOI] [PubMed] [Google Scholar]
- 6. van Lieburg AF, Knoers NV, Monnens LA.. Clinical presentation and follow-up of 30 patients with congenital nephrogenic diabetes insipidus. J Am Soc Nephrol 1999; 10: 1958–1964 [DOI] [PubMed] [Google Scholar]
- 7. Schofer O, Beetz R, Kruse K. et al. Nephrogenic diabetes insipidus and intracerebral calcification. Arch Dis Child 1990; 65: 885–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonzalez Celedon C, Bitsori M, Tullus K.. Progression of chronic renal failure in children with dysplastic kidneys. Pediatr Nephrol 2007; 22: 1014–1020 [DOI] [PubMed] [Google Scholar]
- 9. Bockenhauer D, Carpentier E, Rochdi D, et al. Vasopressin type 2 receptor V88M mutation: molecular basis of partial and complete nephrogenic diabetes insipidus. Nephron Physiol 2010; 114: p1–p10 [DOI] [PubMed] [Google Scholar]
- 10. Jaureguiberry G, Van't Hoff W, Mushtaq I. et al. A patient with polyuria and hydronephrosis: question. Pediatr Nephrol 2011; 26: 1977–1978, 1979–1980 [DOI] [PubMed] [Google Scholar]
- 11. Bichet DG, El Tarazi A, Matar J. et al. Aquaporin-2: new mutations responsible for autosomal-recessive nephrogenic diabetes insipidus—update and epidemiology. Clin Kidney J 2012; 5: 195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bockenhauer D, Bichet DG.. Nephrogenic diabetes insipidus. Curr Opin Pediatr 2017; 29: 199–205 [DOI] [PubMed] [Google Scholar]
- 13. Bockenhauer D, van't Hoff W, Dattani M. et al. Secondary nephrogenic diabetes insipidus as a complication of inherited renal diseases. Nephron Physiol 2010; 116: p23–p29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hillman DA, Neyzi O, Porter P. et al. Renal (vasopressin-resistant) diabetes insipidus; definition of the effects of a homeostatic limitation in capacity to conserve water on the physical, intellectual and emotional development of a child. Pediatrics 1958; 21: 430–435 [PubMed] [Google Scholar]
- 15. Forssman H. Is hereditary diabetes insipidus of nephrogenic type associated with mental deficiency? Acta Psychiatr Neurol Scand 1955; 30: 577–587 [DOI] [PubMed] [Google Scholar]
- 16. de Wied D, Diamant M, Fodor M.. Central nervous system effects of the neurohypophyseal hormones and related peptides. Front Neuroendocrinol 1993; 14: 251–302 [DOI] [PubMed] [Google Scholar]
- 17. Beckwith BE, Petros TV, Couk DI. et al. The effects of vasopressin on memory in healthy young adult volunteers. Theoretical and methodological issues. Ann N Y Acad Sci 1990; 579: 215–226 [DOI] [PubMed] [Google Scholar]
- 18. Schliebe N, Strotmann R, Busse K. et al. V2 vasopressin receptor deficiency causes changes in expression and function of renal and hypothalamic components involved in electrolyte and water homeostasis. Am J Physiol Renal Physiol 2008; 295: F1177–F1190 [DOI] [PubMed] [Google Scholar]
- 19. Monnens L, Jonkman A, Thomas C.. Response to indomethacin and hydrochlorothiazide in nephrogenic diabetes insipidus. Clin Sci (Lond) 1984; 66: 709–715 [DOI] [PubMed] [Google Scholar]
- 20. Pattaragarn A, Alon US.. Treatment of congenital nephrogenic diabetes insipidus by hydrochlorothiazide and cyclooxygenase-2 inhibitor. Pediatr Nephrol 2003; 18: 1073–1076 [DOI] [PubMed] [Google Scholar]
- 21. Beier UH, Green C, Meyers KE.. Caring for adolescent renal patients. Kidney Int 2010; 77: 285–291 [DOI] [PubMed] [Google Scholar]