Abstract
Establishment and analysis of mean platelet volume (MPV) may be helpful in the discrimination between underproduction or over-destruction of platelets as the causes of thrombocytopenia. The primary objective is to find the cut-off point of MPV for distinguishing causes of thrombocytopenia. The secondary objective is to validate the cut-off value of the MPV by using bone marrow examination. Thrombocytopenic patients were enrolled in a training set and a receiving operating characteristics (ROC) curve was plotted to obtain the cut-off value of MPV. A validation set of patients was recruited to validate the cut-off value. The training set included 240 patients. Half with with underproductive (n=120) and half with over-destructive thrombocytopenia (n=120). The best cut-off value of MPV was 8.8 fL. The validation set included 119 patients in total, again in 2 groups, those with underproductive (n=84) and those with overdestructive thrombocytopenia (n=35). The sensitivity, specificity, PPV and NPV when MPV ≥8.8 fL indicating over-destructive thrombocytopenia were 77%, 89%, 89% and 77%, respectively. MPV is useful for differentiating the cause of thrombocytopenia. The value of MPV ≥8.8 fL has acceptable sensitivity and specificity for diagnosis of over-destructive thrombocytopenia.
Key words: Thrombocytopenia, mean platelet volume, underproductive thrombocytopenia, over-destructive thrombocytopenia
Introduction
Platelets are the major blood component involved in primary hemostasis. Thrombocytopenia or a decrease in the level of platelets to less than 150×109/L may lead to bleeding complications. The causes of thrombocytopenia can be divided into two major groups, a decreased production of platelets or an underproliferative bone marrow (BM) and an over-destruction of platelets.1 Underproliferative BM defects include aplastic anemia (AA), acute leukemia, myelodysplastic syndrome (MDS), and chemotherapy-induced thrombocytopenia. On the contrary, peripheral destruction of platelets may be as a result of an immune-mediated process such as immune thrombocytopenia (ITP) or a nonimmune mediated one including disseminated intravascular coagulopathy (DIC), thrombotic thrombocytopenic purpura (TTP) or certain infections.1
A BM examination is considered as being a standard procedure in differentiating between these two pathogeneses of thrombocytopenia if the etiology is unclear.1 This procedure is important for the diagnosis of underproductive BM disorders. However, it is an invasive procedure and may be not necessary for establishing a clear diagnosis of peripheral destruction disorders such as ITP2 and DIC.3
Currently, automated blood analyzers are being developed and have many parameters which can be used to identify the causes of thrombocytopenia. Mean platelet volume (MPV) is one of automated platelet indices that has been investigated by many studies around the achievement of this objective due to its widespread availability.4 Previous earlier studies showed that patients with BM hypoplasia or thrombocytopenia resulting from cytotoxic drugs or chemotherapy had a low MPV.5-7 In contrast, disorders involving the peripheral destruction of platelets had a higher MPV compared to BM diseases.7 As a result, MPV may be the best non-invasive tool to use to differentiate between the two main pathogeneses of thrombocytopenia. However, the cut-off value, inclusion and exclusion criteria differed between trials, along with a lack of validation limit in the use of MPV in clinical practice.4
The primary objective of this study is to find the cut-off point of MPV for distinguishing the causes of thrombocytopenia. The secondary objective is to validate the cut-off value of MPV when using BM examination as a gold standard test.
Materials and Methods
Study overview
This was a cross-sectional study conducted at Chiang Mai University Hospital. The local Institutional Review Board approved the study stating it was in accordance with the guidelines given in the Declaration of Helsinki. Two cohorts of thrombocytopenic patients were enrolled onto the study, a training set and a validation set. The training set included patients who were admitted to hospital or attended out-patient clinics between 1st June 2013 and 31st May 2014. The data from this cohort was collected and analyzed from their medical records. The validation set included patients who were admitted or attended during the period 1st June 2014 to 31st June 2015.
The objective with the training set was to find a cut-off value of MPV that has appropriate sensitivity and specificity to differentiate between underproliferative and over-destructive causes of thrombocytopenia. The inclusion criteria were patients with thrombocytopenia with platelets <100×109/L who had MPV data. The diagnoses were established by hematologists and internists. The number of patients needed to ensure the study was statistically valid was calculated using the formula: N=Z2 [sensitivity (1-sensitivity)]/e2 where Z=95% confidence interval (95%CI) which was 1.96, sensitivity = 0.8, and e = error was set at 0.05. Using this, the population in the training set needed to be 240 with 120 patients being identified as those with underproduction and 120 patients with peripheral destruction as regards causes of thrombocytopenia.
The validation set aimed to validate the cut-off value of MPV derived from the training set. The inclusion criteria were thrombocytopenic patients (platelets <100×109/L) who had undergone a BM study which had been interpreted by hematologists. Exclusion criteria were patients who had received a platelet transfusion prior to MPV analysis.
Data pertinent to clinical characteristics including age, gender, and diagnosis as well as CBC and MPV results were collected from both cohorts. CBC and MPV were derived using an automated blood analyzer (Beckman Coulter LH780).
Statistical analysis
Clinical characteristics of patients and CBC results in both cohorts were analyzed as descriptive methods including mean ± standard deviation (SD). Chi-square test, student t-test, and Wilcoxon rank sum test were used to compare clinical characteristics and CBC between patients with underproductive BM disorders and over-destruction disorders depending on type and distribution of variables. A P-value of less than 0.05 was used to determine statistical significance. The sensitivity and specificity of each cutoff value of MPV were analyzed from the training set. A receiving operating characteristics (ROC) curve was subsequently plotted to obtain the cutoff value of MPV. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of the cut-off value of MPV derived from the training set were re-analyzed in the validation cohort. SPSS version 16.0 was used to analyze the data.
Results
Training set
There were 240 patients in the training set with 120 patients in the underproductive BM group and 120 patients in the overdestruction group as shown in Table 1. The majority of patients in the underproductive BM group were diagnosed with AML and AA whereas more than half the patients in the over-destruction group had DIC and about one-third suffered from ITP. The underproductive BM group had a predominance of females (60% vs. 47%, P=0.03) and was overall of younger age than the over-destruction group (mean age, 50.3 years vs. 55.9 years, P=0.01). The patients with underproductive BM also had lower Hb levels compared to the over-destruction group (mean ± SD, 8.3±1.6 g/dL vs. 9.6±2.5 g/dL, P=0.005). The MPV was significantly higher in the over-destruction group (mean ± SD, 9.6±2.4 vs. 9.0±1.8 fL, P=0.02).
Table 1.
Comparison of clinical characteristics between patients with underproductive bone marrow and those with over-destruction of platelets in the training set.
| Parameter | Underproductive bone marrow, N=120 (%) | Over-destruction of platelets, N=120 (%) | P-value |
|---|---|---|---|
| Gender | |||
| Male | 48(40) | 64(53) | 0.03 |
| Female | 72(60) | 56(47) | |
| Age (years), Mean±SD | 50.3±18.4 | 55.9±15.6 | 0.01 |
| Diagnosis (%) | AA, 38 (31.6) | ITP, 40 (33.3) | - |
| AML, 53 (44.2) | DIC, 65 (54.2) | ||
| ALL, 12 (10.0) | TTP, 4 (3.3) | ||
| CMT, 17 (14.2) | DHF, 11 (9.2) | ||
| Hemoglobin (g/dL), Mean±SD | 8.3±1.6 | 9.6±2.5 | 0.005 |
| White blood count (×109/L), Mean±SD | 4.5±145.8 | 6.6±8.2 | 0.46 |
| Platelet count (×109/L), Mean±SD | 33.5±27.2 | 38.2±24.3 | 0.37 |
| Mean platelet volume (fL), Mean±SD | 9.0±1.8 | 9.6±2.4 | 0.02 |
AA, aplastic anemia; AML, acute myeloid leukemia (pre-chemotherapy); ALL, acute lymphoblastic leukemia (pre-chemotherapy); CMT, chemotherapy-induced myelosuppression; ITP, immune thrombocytopenia; DIC, disseminated intravascular coagulation; TTP, thrombotic thrombocytopenia purpura; DHF, dengue hemorrhagic fever.
The ROC curve obtained from the MPV of patients in the training set had an area under the curve of 0.57 (Figure 1). The sensitivity MPV of 8.0, 8.8, and 9.0 fL in the differentiation between underproductive bone marrow and over-destruction causes of thrombocytopenia was 75%, 60%, and 50%, respectively whereas the specificity values of each cut-off were 30%, 50%, and 54%, respectively. Therefore, the cut-off of MPV at 8.8 fL that had a sensitivity of 60% and specificity of 50% was tested in the validation cohort.
Figure 1.
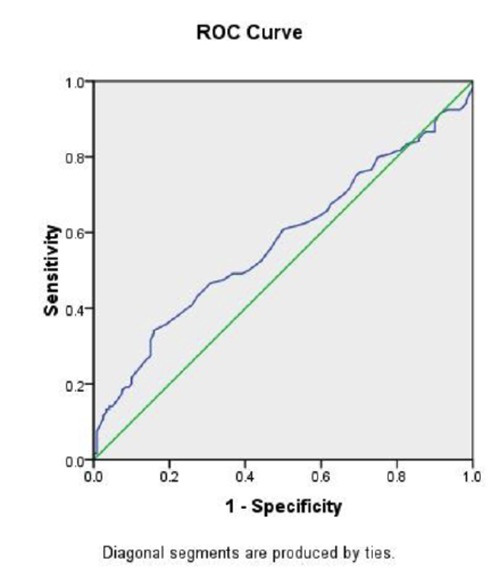
Receiving Operating Characteristics curve of mean platelet volume to differentiate between underproductive bone marrow and over-destruction causes of thrombocytopenia.
Validation set
One hundred and nineteen patients who had undergone BM investigation were included in the validation set. Eighty-four patients were in the underproductive BM group and 35 patients in the over-destruction group (Table 2). Similar to the findings from the training set, most patients in the underproductive BM group were diagnosed with AML and AA. However, all patients in the over-destruction group had ITP. In both groups, males predominated with a mean age ± SD of 43.5±18.4 and 50.0±20.0 years, respectively. The patients with underproductive BM had lower Hb (mean ± SD, 8.4±1.8 g/dL vs. 9.9±2.5 g/dL, P=0.03) and lower WBC counts (mean ± SD, 2.6±36 ×109/L vs. 6.7±6.3×109/L, P=0.03) compared to those recorded in the over-destruction group. The over-destruction group had a higher MPV (mean ± SD, 10.4±2.1 vs. 7.2±1.2 fL, P=0.03). By applying the cutoff value of 8.8 fL in this cohort, the patients can be divided into 4 groups according to causes of thrombocytopenia based on the BM study results and MPV. In the over-destruction of platelet group, 27 out of 35 patients (77.1%) had MPV ≥8.8 fL whereas only 8 patients out of 84 patients (9.5%) from the underproductive bone marrow group had an MPV over the same cutoff value. As a consequence, sensitivity, specificity, PPV, NPV, and accuracy of MPV ≥8.8 fL indicating over-destruction causes of thrombocytopenia were 77%, 89%, 89%, 77%, and 86%, respectively.
Table 2.
Comparison of clinical characteristics between patients with underproductive bone marrow and those with over-destruction of platelets in the validation set.
| Parameter | Underproductive bone marrow, N=84 (%) | Over-destruction of platelets, N=35 (%) | P-value |
|---|---|---|---|
| Gender | |||
| Male | 56 (66.7) | 22 (62.9) | 0.69 |
| Female | 28 (33.3) | 13 (37.1) | |
| Age (years), Mean±SD | 43.5±18.4 | 50.0±20.0 | 0.84 |
| Diagnosis (%) | AA 22 (26.2) | ITP, 35(100) | - |
| AML 40 (47.6) | |||
| ALL 12 (14.3) | |||
| CMT 10 (11.9) | |||
| Hemoglobin (g/dL), Mean±SD | 8.4±1.8 | 9.9±2.5 | 0.03 |
| White blood count (×109/L), Mean±SD | 2.6±36 | 6.7±6.3 | 0.03 |
| Platelet count (×109/L), Mean±SD | 2.79±27.6 | 41.1±26.6 | 0.45 |
| Mean platelet volume (fL), Mean±SD | 7.2±1.2 | 10.4±2.1 | 0.03 |
AA, aplastic anemia; AML, acute myeloid leukemia (pre-chemotherapy); ALL, acute lymphoblastic leukemia (pre-chemotherapy); CMT, chemotherapy-induced myelosuppression; ITP, immune thrombocytopenia.
Discussion and Conclusions
In peripheral destruction causes of thrombocytopenia production of platelets is stimulated to compensate for their destruction and leads to an increase in the immature form of platelets.8 Since the size of the immature platelets is larger than the mature form, MPV measured using an automated hematology analyzer in cases of peripheral destruction is usually higher than cases involving BM defects.7 Analytical findings of the data from both the training cohort and validation cohort from this study supported this hypothesis. In the validation cohort, where the causes of thrombocytopenia were confirmed by BM studies, the mean MPV in the over-destruction group was 10.4 fL compared to 7.2 fL in the underproductive BM group. The mean MPV in the overdestruction group was close to a previous study (9.8 fL)7 as well as those found in other studies into ITP patients (9.86-12.4 fL).9-11 To the contrary, MPV was demonstrated to be lower in patients with underproductive BM defects, a finding similar to the data reported in previous studies (6.2-8.1 fL).7,12 When using a cut-off value of 8.8 fL, the sensitivity, specificity, PPV and NPV were 77%, 89%, 89%, and 77%, respectively with 86% accuracy in the differentiation between the two pathogeneses of thrombocytopenia. A previous study involving 699 enrolled patients divided the causes of thrombocytopenia into two categories, patients with or without marrow diseases. In that study, the mean MPV measured using an MS-9 Automatic Full Digital Cell Counter in the marrow disease group was 7.3 fL whereas that in the normal BM group was 8.62 fL, the difference being statistically significant. However, the sensitivity and specificity of MPV at a cut-off point of 8.15 fL were only 67.7% and 65%, respectively and a BM study for accurate diagnosis of the causes of thrombocytopenia was still recommended following these results12. The lower sensitivity and specificity of previous cut-off MPV values when compared to the current study might be explained by a difference between the populations included in the two studies. The previous study had a greater variety in underlying BM defects including megaloblastic anemia, MDS, and multiple myeloma (MM) comprising 31.2%, 6.5%, and 4.6% respectively in the underproductive BM group. Since megaloblastic anemia and MM had a relatively high MPV (mean 7.8 and 7.6 fL) compared with AA, AML, ALL (mean MPV of 6.4, 7.1, and 6.2, respectively), these might influence the sensitivity and specificity of cut-off points when comparing them to the current study which included no patients with megaloblastic anemia and MM. Moreover, the previous study included thrombocytopenic patients with anemia of chronic disease, totaling one-third (31.9%) in the normal BM group. This disease ensures a relatively low MPV (mean 7.9 fL) when compared to “true” peripheral destruction causes of thrombocytopenia such as DIC, infections, and ITP (mean MPV of 10.1, 8.9, and 8.5 fL, respectively) and might also affect the MPV cut-off value.
The difference between sensitivity and specificity of the MPV cut-off of 8.8 fL in the training and validation cohorts in the current study might be also explained by the proportion of diseases included. This is bearing in mind that the sensitivity and specificity of this cut-off point were 60% and 50% in the training cohort but they increased to 77% and 89%, respectively in the validation cohort, more reliable findings, because the BM studies were performed in all patients to confirm the causes of thrombocytopenia. Since ITP accounted for 100% of the over-destruction group in the validation cohort but only one-third in training cohort, it might affect the mean MPV in the over-destruction group in these cohorts (10.4 fL in validation set vs. 9.6 fL in the training set) and consequently affected the sensitivity and specificity of this cutoff value.
The other factor that might affect the sensitivity and specificity of the MPV cutoff point is the degree of thrombocytopenia. A previous study reported a higher mean platelet count (63.1×109/L) compared to all groups in both the training and validation sets and might lead to less change in MPV.6
The previous studies from Thailand used a mean MPV of the Thai population as 7.9 fL,13 to distinguish between hypodestructive and hyperdestructive thrombocytopenia cases which were confirmed with BM studies. A Coulter counter STKS was used to measure MPV. The result was prospectively evaluated and revealed a sensitivity, specificity, PPV, and NPV of 82.3%, 92.5%, 94.4%, and 77.1%, respectively. 14 In that study, the mean MPV in the peripheral destruction group was 8.8 fL compared to the 10.4 fL found in the current study although ITP (both primary and secondary) accounted for the majority of patients, a statistic similar to this study. The variation of mean MPV in each study might be explained by the use of different automated blood analyzers. Standardized measurement of MPV may partly decrease this variation between studies in the future. Nevertheless, a mean MPV in cases of overdestruction of platelets was higher than that found in underproduction BM defects across all studies.7,12,14 Since there are variations in cut-off points as well as sensitivity and specificity in many studies, the MPV interpretation should be done together with careful history taking, and physical examination, which will lead to further appropriate investigations and management.
There were studies that used other automated platelet indices to discriminate between the causes of thrombocytopenia. The majority of them were differentiated from ITP. Firstly, the study by Kaito et al. used platelet distribution width (PDW) and platelet-to-large-cell ratio (P-LCR) as well as MPV to differentiate between thrombocytopenia resulting from AA and ITP. MPV was higher in patients with ITP compared to AA (mean 12.2 fL vs. 10.2 fL, respectively, P<0.0001) in agreement with PDW and PLCR. 9 Another two studies compared platelet indices between patients with ITP and hypoproliferative thrombocytopenia. The ITP group had a higher mean MPV (11.38 fL vs. 7.17 fL in one study and 12.4 fL vs. 9.7 fL in another),10,11 as well as a higher PDW, and P-LCR. In one study, the cut-off values of 9-10 fL for MPV and 15-17 fL for PDW led to a sensitivity, specificity, PPV and NPV of 100% in the discrimination between two groups which were better than P-LCR10. An immature platelet fraction (IPF) is another platelet index that was studied in cases of childhood ITP. This index not only can discriminate thrombocytopenia from ITP and hematological malignancies under chemotherapy (median IPF 11.8% vs. 7%) but can also distinguish acute and chronic ITP (median IPF 9.8% vs. 19.4%) as well as determine the active disease stage, response to treatment and relapsed disease. Although MPV did not differentiate between acute and chronic ITP in that study, it still had value in discriminating between ITP and hematological malignancies (median MPV 11.6 fL vs. 10.5 fL) as well as PDW, plateletcrit and P-LCR.15
The main strength of this study was the confirmation of a cut-off MPV value calculated from data collected from the training cohort in a validation cohort in which the causes of thrombocytopenia had been confirmed by BM studies in all patients. A limitation of the study was that the information from the training set was gathered from a review of medical records in which the BM investigation had only been completed in some patients. In addition, the training set was not well matched as regards age and sex as there were a predominance of females and younger patients in the underproductive BM group and this may well have impact on MPV. Another limitation was that no patients with MDS, MM or megaloblastic anemia were included in the cohort, as discussed previously, as well as diseases with sequestration of platelets such as hypersplenism and gestational thrombocytopenia. Nonetheless these diseases usually have clues from the patient history and physical examination and lead to specific investigations.16-18 This means the problem in the differentiation from hyperdestructive thrombocytopenia would be less. Some patients with diseases that are associated with both underproductive and overdestructive thrombocytopenia, for example chronic lymphocytic leukemia, were also not enrolled in this study but a BM study should be carried out in these cases. A final limitation was that patients with inherited thrombocytopenia were also not enrolled onto the study. A previous study showed an MPV at a cutoff value of 12.4 fL had a high specificity (91%) in distinguishing inherited macrothrombocytopenia from ITP.19 As a result, when using an MPV of more than 8.8 fL to indicate hyperdestructive thrombocytopenia, inherited macrothrombocytopenia is likely to be one exception and should be suspected if the MPV is very high. Future research into MPV in specific groups of patients that is more relevant in clinical practice such as patients with normal levels of hemoglobin, numbers of white blood cells, and no blasts is warranted.
In conclusion, MPV is useful as a screening test for differentiating the cause of thrombocytopenia. The value of MPV ≥8.8 fL has acceptable sensitivity and specificity for the diagnosis of over-destructive thrombocytopenia and may help to avoid the need for invasive bone marrow examination in these patients.
Funding Statement
Funding: this research had funding support from the Faculty of Medicine, Chiang Mai University, Thailand.
References
- 1.Stasi R. How to approach thrombocytopenia. Hematol Am Soc Hematol Educ Program 2012;2012:191-7. [DOI] [PubMed] [Google Scholar]
- 2.Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011;117:4190-207. [DOI] [PubMed] [Google Scholar]
- 3.Wada H, Thachil J, Di Nisio M, et al. Guidance for diagnosis and treatment of disseminated intravascular coagulation from harmonization of the recommendations from three guidelines. J Thromb Haemost 2013;11:761-7. [DOI] [PubMed] [Google Scholar]
- 4.Leader A, Pereg D, Lishner M. Are platelet volume indices of clinical use? A multidisciplinary review. Ann Med 2012;44:805-16. [DOI] [PubMed] [Google Scholar]
- 5.Bessman JD, Gilmer PR, Gardner FH. Use of mean platelet volume improves detection of platelet disorders. Blood Cell 1985;11:127-35. [PubMed] [Google Scholar]
- 6.Levin J, Bessman JD. The inverse relation between platelet volume and platelet number. Abnormalities in hematologic disease and evidence that platelet size does not correlate with platelet age. J Lab Clin Med 1983;101:295-307. [PubMed] [Google Scholar]
- 7.Bowles KM, Cooke LJ, Richards EM, Baglin TP. Platelet size has diagnostic predictive value in patients with thrombocytopenia. Clin Lab Haem 2005;27:370-3. [DOI] [PubMed] [Google Scholar]
- 8.Abe Y, Wada H, Tomatsu H, et al. A simple technique to determine thrombopoiesis level using immature platelet fraction (IPF). Thromb Res 2006;118:463-9. [DOI] [PubMed] [Google Scholar]
- 9.Kaito K, Otsubo H, Usui N. Platelet size deviation width, platelet large cell ratio, and mean platelet volume have sufficient sensitivity and specificity in the diagnosis of immune thrombocytopenia. Br J Haematol 2005;128:698-702. [DOI] [PubMed] [Google Scholar]
- 10.Ntaios G, Papadopoulos A, Chatzinikolaou A. Increased values of mean platelet volume and platelet size deviation width may provide a safe positive diagnosis of idiopathic thrombocytopenic purpura. Acta Haematol 2008;119:173-7. [DOI] [PubMed] [Google Scholar]
- 11.Negash M, Tsegaye A, Medhin AG. Diagnostic predictive value of platelet indices for discriminating hypo productive versus immune thrombocytopenia purpura in patients attending a tertiary care teaching hospital in Addis Ababa, Ethiopia. BMC Hematology 2016;16:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandra H, Chandra S, Rawat A, Verma SK. Role of mean platelet volume as discriminating guide for bone marrow disease in patients with thrombocytopenia. Int J Lab Hematol 2010;32:498-505. [DOI] [PubMed] [Google Scholar]
- 13.Pathepchotiwong K., Dhareruchta P, Adirojananon W. Platelet parameter in healthy subjects analyzed by automation analyzer. Thai J Hematol Transf Med 2001;11:93-100. [Google Scholar]
- 14.Numbenjapon T, Mahapo N, Pornvipawee R, et al. A prospective evaluation of normal mean platelet volume in discriminating hyperdestructive thrombocytopenia from hypoproductive thrombocytopenia. Int J Lab Hem 2008;30:408-14. [DOI] [PubMed] [Google Scholar]
- 15.Adly AAM, Ragab IA, Ismail EAR, Farahat MM. Evaluation of the immature platelet fraction in the diagnosis and prognosis of childhood immune thrombocytopenia. Platelets 2015;26:645-50. [DOI] [PubMed] [Google Scholar]
- 16.Fenaux P, Haase D, Sanz GF, et al. Myelodysplastic syndromes: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:iii57-69. [DOI] [PubMed] [Google Scholar]
- 17.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538-48. [DOI] [PubMed] [Google Scholar]
- 18.Green R, Datta Mitra A. Megaloblastic Anemias: Nutritional and Other Causes. Med Clin North Am 2017;101:297-317. [DOI] [PubMed] [Google Scholar]
- 19.Noris P, Klersy C, Gresele P, et al. Platelet size for distinguishing between inherited thrombocytopenias and immune thrombocytopenia: a multicentric, real life study. Br J Haematol 2013;162:112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


