Abstract
The durability of host resistance is challenged by the ability of pathogens to escape the defence of their hosts. Understanding the variability in the durability of host resistance is of paramount importance for designing more effective control strategies against infectious diseases. Here, we study the durability of various clustered regularly interspaced short palindromic repeats-Cas (CRISPR-Cas) alleles of the bacteria Streptococcus thermophilus against lytic phages. We found substantial variability in durability among different resistant bacteria. Since the escape of the phage is driven by a mutation in the phage sequence targeted by CRISPR-Cas, we explored the fitness costs associated with these escape mutations. We found that, on average, escape mutations decrease the fitness of the phage. Yet, the magnitude of this fitness cost does not predict the durability of CRISPR-Cas immunity. We contend that this variability in the durability of resistance may be because of variations in phage mutation rate or in the proportion of lethal mutations across the phage genome. These results have important implications on the coevolutionary dynamics between bacteria and phages and for the optimal deployment of resistance strategies against pathogens and pests. Understanding the durability of CRISPR-Cas immunity may also help develop more effective gene-drive strategies based on CRISPR-Cas9 technology.
This article is part of a discussion meeting issue ‘The ecology and evolution of prokaryotic CRISPR-Cas adaptive immune systems’.
Keywords: durability of resistance, pathogen evolution, clustered regularly interspaced short palindromic repeats-Cas, mutation rates
1. Introduction
Public health and agriculture are constantly challenged by the spread of infectious diseases. An arsenal of various prophylactic and therapeutic strategies has been developed to limit the circulation of pathogens (e.g. introgression of resistance genes in plant varieties, use of antimicrobial drugs). Yet, the efficacy of those interventions can be rapidly eroded by the evolution of pathogen populations [1–4]. It is important to note that distinct defence strategies may lead to very different evolutionary outcomes. For instance, imperfect immunity is known to select for more aggressiveness and virulence in pathogens [5,6]. In addition, distinct defence strategies may differ in their level of durability. Why are some host defence strategies overcome very rapidly while others remain effective for a long period of time [4,7,8]? A better understanding of the durability of host defences (defined as the inverse of the speed of pathogen adaptation to those defences) is key for the development of sustainable management strategies of pathogens and pests [7,9].
Empirical and experimental studies in plant pathosystems have played key roles in the identification of major factors acting on the durability of host resistance [4,7,9–11]. For instance, the type of plant resistance is known to have a significant impact on the speed of pathogen adaptation. Qualitative resistance, an all-or-nothing response, is often considered to be less durable than quantitative resistance, which reduces disease progression in the plant. This effect is usually attributed to the simpler genetic determinism of pathogen adaptation to qualitative resistance which involves a few (or even a single) major virulence genes [12]. By contrast, adaptation to the polygenic determinism of quantitative resistance requires multiple pathogen mutations [13,14]. Yet, qualitative resistance exhibits much variation in durability [4]. A classical explanation for this variation in durability involves selective constraints acting on the pathogen population. More specifically, host defence is likely to be more durable if the mutations (virulence alleles) that allow the pathogen to escape qualitative resistance are associated with fitness costs [4,12]. Understanding the selective constraints acting on the sites targeted by different resistance mechanisms may help predict the durability of resistance and limit the speed of pathogen adaptation [4,15]. Testing this hypothesis, however, is often difficult in plant pathosystems where measuring the durability of specific resistance mechanisms in controlled experiments raises practical difficulties [16,17].
Here, we use the interaction between bacteria and their lytic bacteriophages (or phages) to study the factors that modulate the durability of host resistance. Bacteria have access to a wide range of defence systems to defend themselves against phages [18–21]. Among these distinct defence systems, clustered regularly interspaced short palindromic repeats—CRISPR associated genes CRISPR-Cas has the unique ability to generate hundreds of different alleles of resistance targeting different sites in the phage genome [22]. Here, we exploit this unique property to explore the variability in durability among distinct CRISPR-Cas resistance alleles targeting the same phage. CRISPR-Cas is an adaptive prokaryotic immune defence which integrates into the CRISPR locus (integration of a spacer) a small phage-DNA sequence (the protospacer, here 30 bp long) from an invading genome and uses this memory to target and degrade subsequent invading matching DNA (interference) [23]. To select and integrate a specific protospacer from a foreign nucleic acid into its CRISPR array, many CRISPR-Cas systems rely on a 2–5 bp sequence, the protospacer adjacent motif (PAM) [24], flanking one side of the protospacer sequence and mandatory for spacer integration and interference. Given its size, the PAM is present numerous times on the phage genome, leading potentially to hundreds of different resistances targeting various protospacers [22].
To study the variability in the durability of CRISPR resistance, we monitored the evolution of the virulent phage 2972 against the CRISPR1 array of Streptococcus thermophilus DGCC7710. Streptococcus thermophilus is a Gram-positive bacterium, widely used in the dairy industry for the making of yogurts and cheese. The immunity of this bacteria against phages relies mainly on two active type II-A CRISPR-Cas systems, CRISPR1 being the more active [24]. When a spacer targeting phage 2972 is acquired, CRISPR immunity blocks the lytic cycle of the targeted phage [23,25] and can thus be viewed as a very specific form of qualitative resistance. In this system, phages can only escape CRISPR-Cas by mutating their PAM or seed sequence (i.e. the proximal part of the protospacer) [26]. In the following, we first quantified the ability of phage 2972 to escape a set of resistant bacteria, each of them having in their CRISPR1 array a distinct new spacer targeting a unique single protospacer site in the phage genome. Second, we isolated escape phage mutants on each of the resistant bacteria (e.g. each phage escape is mutated at a specific and different protospacer region) and we characterized their relative fitness during the infection of a population of phage-sensitive bacteria. This experimental protocol allowed us to discuss the potential link between the fitness effects of escape mutations in the phage and the durability of different resistance alleles in the bacteria.
2. Material and methods
(a). Bacterial strains and phages
The bacterium S. thermophilus DGCC 7710 (WT) and its virulent phage 2972 were obtained from the Félix d’Hérelle Reference Center for Bacterial Viruses (www.phage.ulaval.ca) [27]. Bacteria were grown in LM17 broth (M17 Oxoid (37 g l−1) with 5 g l−1 of lactose) and incubated at 40°C. For phage amplification 10 mM of sterile CaCl2 were added to the broth. Using a standardized protocol described in [28], a culture of S. thermophilus DGCC 7710 was challenged with the virulent phage 2972, and the surviving colonies/cells (bacteriophage insensitive mutants (BIMs)) were screened by polymerase chain reaction (PCR) for expansion of their CRISPR array, followed by a 2% agarose electrophoresis. Primer sequences and PCR protocol can be found in the electronic supplementary material, S1. To confirm that each BIM possesses a different spacer, we sequenced the newly acquired spacers (Sanger sequencing by Eurofins Genomics MWG). A total of 17 different BIMs, each with a single and distinct spacer acquired into the active CRISPR1 locus (we checked that no other spacer was acquired on the other active CRISPR array), were kept and used in this study. Spacer sequences are provided in the electronic supplementary material, S2. Finally, protospacers were positioned on the genome of phage 2972 that is published in [27].
(b). Phage detection and titration
Bacterial lawns were produced by plating 6 ml of soft agar (LM17 + CaCl2 with 0.8% agar and 400 μl of bacteria in mid-exponential phase) on top of plates previously poured with 30 ml of hard agar (LM17 + CaCl2 with 1.5% agar). For phage titration, 50 μl of diluted phages were added to soft agar. For phage detection, 5 μl of phage solution were spotted directly on the solidified soft agar. When needed, phages were diluted in phage buffer (50 mM Tris–HCl pH 7.5 + 100 mM NaCl + 8 mM MgSO4). Plates were incubated overnight at 40°C and plaques were counted (titration) or recorded (detection).
(c). Durability of resistance
The durability of host resistance is defined as the time during which it remains effective [7]. In the absence of a pre-existing escape parasite, the durability of a resistance depends on two factors: (i) the rate at which escape mutants are generated, and (ii) their spread into the host population. In the case of an homogeneous resistant host population, any escape mutant will spread quickly into the population. In this simple case, the durability of a resistance depends mainly on the rate at which viable escape mutants appear by mutation.
We used a three-step Luria–Delbrück protocol to measure the phage mutation rate for each targeted sequence by the 17 different BIM (see the electronic supplementary material, S3 for a graphic overview of the protocol). These measurements were replicated three times with three independent clonal lysates of phage 2972. To evaluate the potential influence of standing genetic variance on the rate of escape, we measured the initial frequency of escape mutants against each BIM. The frequency of pre-existing mutants to each of the 17 different BIMs was found to be below 2.9 × 10−5. Because we inoculated a small quantity of phage 2972 (see below), the impact of the standing genetic variance on the adaptation of the phage was assumed to be negligible.
In the first step of this protocol, for each BIM, wild-type (WT) phages were amplified in 96 independent replicates on the WT-phage-sensitive bacteria (i.e. in the absence of selection). In each replicate, 20 μl of LM17 + CaCl2 were inoculated with 0.2 μl of WT bacteria in mid-exponential phase, phages at a concentration of 300 plaque forming units (PFU)/20 μl and incubated at 40°C for 24 h. We confirmed by titrating four replicates before incubation that Ni ≈ 300 PFU/20 μl and we measured Nf by titrating 10 randomly chosen lysates. We found Nf ≈ 1.72 × 106 PFU/20 μl.
In the second step of the protocol, the bacteria from each replicate were pelleted down with a 5 min centrifugation (6189 g) (see the electronic supplementary material, S4) and 25% (5 μl) of the supernatant was inoculated into a 200 μl culture of the focal BIM and incubated for 24 h at 40°C. This second step ensured that even in replicates where the frequency of escape mutants was small at the end of the first step, the frequency of escape mutants would be sufficiently high to be detectable in the third step of the protocol.
In the final and third step of the protocol, the presence of escape phages in each individual replicate was assessed using phage detection assays. PE, the probability of escape, was calculated as the fraction of replicates where phage escape was detectable. It is possible [29] to estimate the rate of escape mutations against each BIM using:
with μ the mutation rate per target sequence (seed and PAM sequences), z the fraction of lysate used for the second amplification (here 1/4), Nf the final number of phages per replicate and Ni the initial number of phages per replicate. Note that this method does not measure the frequency of lethal mutation.
(d). Relative fitness of phage escape mutants
For each of the 17 different BIMs, we selected at random five phage isolates that escaped bacterial resistance. A single plaque from each of these five isolates was amplified in liquid and re-isolated twice on plates, on the BIM on which they were isolated from. After amplification, phages and remaining bacteria were separated by filtration (0.2 μm) and phages were stored in 20% glycerol at −80°C. Genome sequencing (see the electronic supplementary material, S5 for the list of primers and S6 for their protospacer sequence) confirmed that all escape phages contained mutations in their PAM or their seed sequence. This protocol generated a collection of escape mutants for all BIMs.
The relative fitness of all the escape mutants was determined using triplicate competition experiments against a reference phage which contains a 37 bp deletion in its orf24 (see the electronic supplementary material, S7). This deletion allowed us to readily distinguish the reference strain from all the other escape mutants (see the electronic supplementary material, S7). Approximately 3000 phages (50% escape mutant and 50% reference phage) were inoculated in 10 ml LM17 + CaCl2 supplemented with 100 μl of WT bacteria in early stationary phase. After a 24 h incubation at 40°C, the remaining bacteria were removed by filtration and phages were stored at −80°C. Before and after amplification, the proportion of the tested phage was measured by quantitative PCR (qPCR) (see the electronic supplementary material, S7). The relative fitness of the escape mutant m was determined using
where rm and rWT refer to the Malthusian growth rates of the escape mutant and the WT phage, respectively, pi and pf (respectively, p′i and p′f) are the frequencies of the mutant phage before and after the competition (the prime refers to the frequency of the WT phage 2972).
(e). Statistical analyses
All statistical analyses were run using R software (v. 3.3.2, [30]), through RStudio (v. 1.0.136).
We performed an analysis of variance (ANOVA) to determine if the position of the protospacer on the phage genome impacts the durability of resistances.
Linear models were used for the analysis of relative fitness data. In the first model, we tested the effect of phage genotype on relative fitness. To control the false discovery rate, as all escape mutations were compared to the reference phage independently, the Benjamini-Hochberg procedure was applied to the derived p-values. In the second model, we tested the impact of mutation type (synonymous versus non-synonymous) on relative fitness of phages escaping the BIMs that target an orf (36 phage escape mutants). In the third model, we assessed the effect of the relative fitness of phage escape mutants on the durability of resistance of their respective BIM.
3. Results
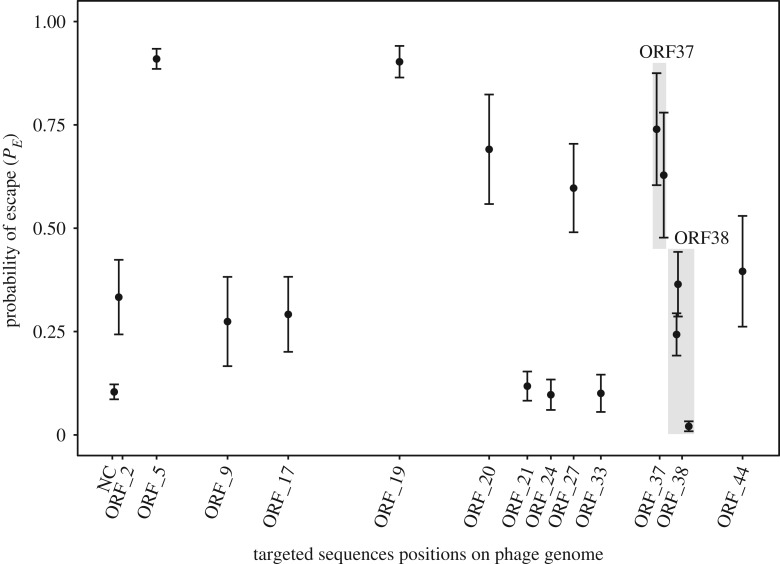
To study the durability of various CRISPR alleles, we generated 17 different resistant strains (BIMs) characterized by a new and unique spacer within the CRISPR1 array. Each spacer targets a different protospacer, i.e. a different part of the phage genome (electronic supplementary material, S2). In total, 13 of the 44 phage genes (as well as some non-coding regions) were targeted by at least one spacer, leading to a good coverage of the phage genome by these 17 BIMs (figure 1).
Figure 1.
Variability in the durability of CRISPR-Cas immunity. The mutation rate of 17 protospacers, whose positions are labelled on the x-axis, were measured using fluctuation tests. PE values, i.e. the number of replicates in which a phage escape mutant evolved, are reported and show heterogeneity among the targeted sequences, implying that there is heterogeneity in the durability of CRISPR-Cas resistances.
Our measures of BIMs’ durability using fluctuation tests, revealed considerable variation in the ability of the phage to escape different BIMs (figure 1; electronic supplementary material, S8, ANOVA, F16 = 10.89, p = < 0.001). We also used the probability of escape to estimate the mutation rates for each target sequence (seed and PAM sequences) (see the electronic supplementary material, S9). The average mutation rate was estimated to be 3.4 × 10−7 mutation/target sequence/replication and the rate of escape on the less durable BIM was 123 times higher than one of the most durable BIM.
One possible explanation for the observed variation in durability of resistances could result from differences in the fitness costs associated with their respective escape mutants. In principle, the use of the proportion of replicates with no escape mutants in the Luria–Delbrück protocol yields an estimation of the mutation rate that is unaffected by the selection on viable mutants [31]. To explore the validity of this hypothesis, we isolated 40 phage mutants escaping the 17 distinct single CRISPR-resistances. A total of 35 escape phages carry a single bp mutation in the targeted sequence, four of the remaining phages carry double bp mutations in the targeted sequence, one escape phage has a single bp deletion (see the electronic supplementary material, S6). Among the substitutions, 27 are transversions, 12 are purine transitions and four pyrimidine transitions. Ten escape mutants were characterized by synonymous mutations (see the electronic supplementary material, S10).
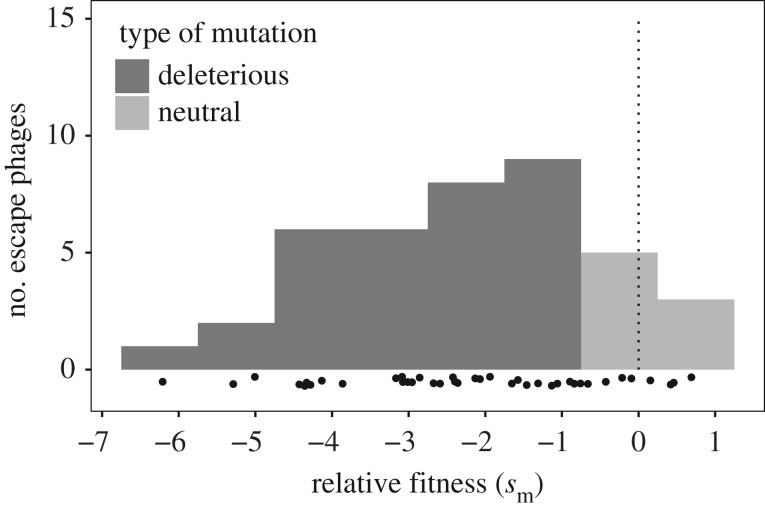
To measure fitness, we competed each of the escape phage mutants against a reference phage and measured their relative abundance before and after the experiment. From these data, we deduced relative fitness. We found that relative fitness was highly variable, ranging from −6.21 to 0.68 with an average of −2.22 and a standard deviation of 1.71 (figure 2; see the electronic supplementary material, S11). Although the majority of the phage escape mutants had a lower fitness than the WT phage (32 out of 40), some escape mutants were neutral (8 out of 40) (figure 2; electronic supplementary material, S11). The presence of non-synonymous mutations was not a good predictor of escape mutant fitness (t = −0.509, P(R > t) = 0.612) and all tested synonymous mutations but one lower phage fitness (see the electronic supplementary material, S12). We found that escape mutant fitness was not a good predictor of the durability of each BIM (figure 3, t = −0.423, P(R > t) = 0.673). Hence, the heterogeneity in the durability of CRISPR resistances is not caused by the heterogeneity of fitness costs associated with these escape mutations (figure 3).
Figure 2.
Distribution of fitness effects of escape mutations in the phage. Relative fitness was measured through competition experiments with a collection of 40 escape phages, mutated on their seed or PAM sequences. Phages that carry a neutral and deleterious mutations are represented in medium and dark grey, respectively. Black dots show the relative fitness of each escape phage. The dotted segment represents the fitness of WT phage 2972. Fitness value of each escape phage is also provided in the electronic supplementary material, S11.
Figure 3.

Relative fitness of phage escape mutants against durability (probability of escape PE) of their respective BIM. Each colour corresponds to a single BIM and each dot to a single escape phage. Error bars correspond to 95% confidence Intervals. Raw data are provided in the electronic supplementary material, S9 and S11.
4. Discussion
We studied the variation in the ability of the virulent phage 2972 to escape distinct resistance alleles at the CRISPR-Cas immune system of its host S. thermophilus DGCC7710. We found (i) considerable variation in the durability among these different resistant strains (and therefore in the apparent mutation rate of phage protospacers), and (ii) substantial variation in fitness among phages carrying escape mutations. Yet, the cost of those escape mutations was not associated with the durability of their respective resistance strains. If the fitness cost of escape mutations is not a good predictor of resistance durability what drives the variation in durability? We believe that two non-mutually exclusive processes could explain the observed patterns: (i) variation in the mutation rate along the phage genome, and (ii) variation in the probability of generating lethal mutations among different sequences targeted by the CRISPR-Cas system.
First, a variation in the mutation rate along the phage genome can result from a heterogeneity of the replication machinery. Such a variation in mutation rates has previously been described in yeast [32], RNA viruses [33] and bacteria [34] but to our knowledge not yet in bacteriophages. The precise mechanism used by phage 2972 to replicate and repair its genome is unknown, limiting our ability to test this hypothesis. However, since phage 2972 encodes and expresses its own replication machinery and does not possess any repair mechanism [27,35], it is tempting to hypothesize that no repair mechanisms are involved and that the entire replication is made by its replication machinery. This machinery could yield substantial variation among different parts of the phage genome. Note, however, that most escape mutants we isolated were owing to transversions instead of transitions (see the electronic supplementary material, S10), whereas most replication machineries show a biased pattern to transition [32,36]. If a heterogeneous fidelity rate was at the origin of the observed heterogeneity in the durability of resistances, 2972 machinery would have an unconventional mutation bias.
Second, variation in the frequency of lethal mutations along the phage genome could also contribute to the observed variation in BIM durability. Lethal mutations are very common and can reach up to 40% of viruses total mutations [37–39], but, to our knowledge, the heterogeneity of the probability of lethal mutation along the genome has not been studied. Because some genes are known to be essential while others are accessory (e.g. orf39 and orf41 are not expressed during an infection by phage 2972 [35]), we can expect that mutations in different genes should result in different fractions of lethal mutations and, consequently, in variations in the durability among BIMs targeting these different genes.
Additional experiments are required to evaluate the relative importance of the variations in (i) mutation rate and (ii) the proportion of lethals along the phage genome on the durability of CRISPR resistance. The heterogeneity in the mutation rate could be assessed by measuring the durability of several spacers that target different non-functional coding regions of the phage genome. Phage 2972 carry such a sequence in the form of an incomplete lysogeny module that is not expressed [27,35]. If we could create different BIMs targeting this module, any heterogeneity in durability among those BIMs would only result from an heterogeneity in the mutation rates among the different target sequences. To evaluate the alternative hypothesis that the variation in durability results from variation in the fraction of lethal mutants, one could measure directly this fraction of lethal mutants through the systematic introduction of point mutations in the target sequence of BIMs with contrasted levels of durability [37–39]. Thanks to recent progress in molecular biology, a range of mutants can be produced by systematically changing each of the nucleotides of the target sequence [40,41]. The comparison of the number of lethal mutations for a durable and non-durable resistance would allow one to evaluate directly the impact of this factor on the variation of the durability.
CRISPR-Cas immunity is known to generate and maintain a high diversity of resistance alleles against the same phage [22,42] and this diversity in resistance is known to limit the growth of the phage population [29,42]. Theoretical models and experimental tests indicate that such diversity limits the evolutionary emergence of the pathogens [29]. Yet, those studies ignore the heterogeneity in the durability of resistance among different alleles. Our results indicate that another potential benefit of generating this diversity is to explore a range of durability of resistance. The most durable alleles will outcompete the other BIMs and this may provide a very robust way to hamper the evolution of the phage. In addition to this inter-host diversity, a single cell can acquire more than one spacer against the same parasite. The acquisition of multiple spacers targeting different parts of the phage genome implies that the phage needs multiple mutations before it can infect this multiply resistant bacteria [25]. As most escape mutations are costly (figure 2), carrying multiple escape mutations is likely to reduce dramatically the fitness of the phage. By contrast, the acquisition of multiple spacers does not alter the fitness of the bacteria [43]. This asymmetry may help in explaining the ultimate extinction of phage populations coevolving with CRISPR-Cas immunity [22,44]. It is also important to note that some phages have evolved the ability to defeat CRISPR immunity using anti-CRISPR proteins that inhibit the defence conferred by CRISPR-Cas [45,46] (note that to our knowledge, phage 2972 does not carry any anti-CRISPR against S. thermophilus CRISPR systems). Even though anti-CRISPR can be partially efficient against CRISPR-Cas, the cooperation between phages ensures that, above a minimal concentration, phages can invade a resistant host population without acquiring escape mutations in the sequences targeted by CRISPR-Cas [47,48].
Streptococcus thermophilus is widely used by the dairy industry for the manufacture of several fermented milk products (yoghurt, cheese) and the identification of BIMs with particularly durable resistance could have very practical implications. The use and/or the combination of these BIMs is likely to protect the starter cultures against phage infection. In addition, it would be particularly useful to identify durable spacers that target related phages. Such generalist spacers have been observed before [23]. The use of a durable generalist spacer could massively improve the resistance of S. thermophilus strains. Our biological model also provides a unique opportunity to evaluate experimentally the effectiveness of different intervention strategies on the long-term efficacy of resistance to pathogens. It may thus provide important insights for the implementation of sustainable management of pathogens and pests [4,9,29].
In addition to these applications in the dairy industry and in agriculture, the CRISPR-Cas9 technology can be used as a driving endonuclease, ie. a genetic tool that makes an engineered allele spread into natural populations by non-mendalian heredity [49]. Indeed, in a heterozygote carrying a CRISPR-Cas9 and its guide, the endonuclease will target and cleave the homologous allele. As repair mechanisms usually involve homologous DNA sequences, they will usually add a copy of the CRISPR-Cas9 and its guide at the place of the former allele, leading to the rapid spread of the CRISPR-Cas9/guide in the population [49,50]. However, if the presence of CRISPR-Cas9 is costly for its host, it is likely that escape mutation will emerge and break the spread of the gene-drive [50,51]. Our results indicate that the durability of gene-drive strategies targeting distinct genome regions is likely to be very variable. Understanding the ultimate source of the variation of durability is particularly important for the effectiveness of gene-drive based on CRISPR-Cas9.
Supplementary Material
Supplementary Material
Acknowledgements
The authors thank Alison Feder, Peter Grant, Olivier Tenaillon, Stéphanie Bedhomme, Oliver Kaltz, Elze Hesse, Matthias Grenié and Hélène Pidon for helpful discussions. The authors thank the qPHD, qPCR (head: Philippe Clair) and the GenSeq (head: Frédérique Cerqueira and Erick Desmarais) platforms of the University of Montpellier for their help.
Data accessibility
Raw data are accessible as the electronic supplementary material.
Authors' contributions
H.C., S.L. and S.G. designed the study. H.C., A.N., D.M.T., S.G., S.L. and S.Mo. developed the protocols. H.C., A.N. and L.P. performed the experiments. S.Me., H.C. and S.G. performed the statistical analyses. H.C., E.R.W., S.L., S.Mo. and S.G. wrote the manuscript. Editions of the manuscripts were carried by all authors. S.G. funds the study.
Competing interests
We declare we have no competing interests.
Funding
H.C. acknowledges funding from the EMBO(EMBO Short Term Fellowship 7191). S.G. acknowledges funding from the Leverhulme Trust (Visiting Professorships) and the CNRS (PEPS MPI grant). E.R.W. further acknowledges the Natural Environment Research Council (https://nerc.ukri.org) (NE/M018350/1), the BBSRC (BB/N017412/1) and the European Research Council (https://erc.europa.eu) (ERC-STG-2016-714478 - EVOIMMECH) for funding. S.Mo. acknowledges funding from the Natural Sciences and Engineering Research Council of Canada (Discovery Program). S.Mo. holds the Tier 1 Canada Research Chair in Bacteriophages.
References
- 1.O’Neill (chair) J. 2016. Tackling drug-resistant infections globally: final report and recommendations. The review on antimicrobial resistance. Commissioned by the UK Prime Minister. See http://amr-review.org/Publications.
- 2.Feder AF, Rhee SY, Holmes SP, Shafer RW, Petrov DA, Pennings PS. 2016. More effective drugs lead to harder selective sweeps in the evolution of drug resistance in HIV-1. eLife 5, e10670 ( 10.7554/eLife.10670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghany MG, Doo EC. 2009. Antiviral resistance and hepatitis B therapy. Hepatology 49, S174–S184. ( 10.1002/hep.v49.5s) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leach JE, Cruz CMV, Bai J, Leung H. 2001. Pathogen fitness penalty as a predictor of durability of disease resistance genes. Annu. Rev. Phytopathol. 39, 187–224. ( 10.1146/annurev.phyto.39.1.187) [DOI] [PubMed] [Google Scholar]
- 5.Gandon S, Michalakis Y. 2000. Evolution of parasite virulence against qualitative or quantitative host resistance. Proc. R. Soc. Lond. B 267, 985–990. ( 10.1098/rspb.2000.1100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandon S, Mackinnon MJ, Nee S, Read AF. 2001. Imperfect vaccines and the evolution of pathogen virulence. Nature 414, 751 ( 10.1038/414751a) [DOI] [PubMed] [Google Scholar]
- 7.Johnson R. 1984. A critical analysis of durable resistance. Annu. Rev. Phytopathol. 22, 309–330. ( 10.1146/annurev.py.22.090184.001521) [DOI] [Google Scholar]
- 8.Kennedy DA, Read AF. 2017. Why does drug resistance readily evolve but vaccine resistance does not? Proc. R. Soc. B 284, 20162562 ( 10.1098/rspb.2016.2562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mundt CC. 2014. Durable resistance: a key to sustainable management of pathogens and pests. Infect. Genet. Evol. 27, 446–455. ( 10.1016/j.meegid.2014.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson R. 1981. Durable resistances: definition of, genetic control, and attainment in plant breeding. Phytopathology 71, 567–568. ( 10.1094/Phyto-71-567) [DOI] [Google Scholar]
- 11.McDonald BA, Linde C. 2002. Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 40, 349–379. ( 10.1146/annurev.phyto.40.120501.101443) [DOI] [PubMed] [Google Scholar]
- 12.Flor HH. 1971. Current status of the gene-for-gene concept. Annu. Rev. Phytopathol. 9, 275–296. ( 10.1146/annurev.py.09.090171.001423) [DOI] [Google Scholar]
- 13.Stuthman DD, Leonard KJ, Miller-Garvin J. 2007. Breeding crops for durable resistance to disease. In Advances in agronomy, vol. 95 (ed. D Sparks), pp. 319–367. New York, NY: Academic Press. See http://www.sciencedirect.com/science/article/pii/S006521130795004X.
- 14.Poland JA, Balint-Kurti PJ, Wisser RJ, Pratt RC, Nelson RJ. 2009. Shades of gray: the world of quantitative disease resistance. Trends Plant Sci. 14, 21–29. ( 10.1016/j.tplants.2008.10.006) [DOI] [PubMed] [Google Scholar]
- 15.Lafforgue G, Martínez F, Niu QW, Chua NH, Darós JA, Elena SF. 2013. Improving the effectiveness of artificial microRNA (amiR)-mediated resistance against turnip mosaic virus by combining two amiRs or by targeting highly conserved viral genomic regions. J. Virol. 87, 8254–8256. ( 10.1128/JVI.00914-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kousik CS, Ritchie DF. 1999. Development of bacterial spot on near-isogenic lines of bell pepper carrying gene pyramids composed of defeated major resistance genes. Phytopathology 89, 1066–1072. ( 10.1094/PHYTO.1999.89.11.1066) [DOI] [PubMed] [Google Scholar]
- 17.Brun H, Levivier S, Somda I, Ruer D, Renard M, Chèvre AM. 2000. A field method for evaluating the potential durability of new resistance sources: application to the Leptosphaeria maculans-Brassica napus pathosystem. Phytopathology 90, 961–966. ( 10.1094/PHYTO.2000.90.9.961) [DOI] [PubMed] [Google Scholar]
- 18.Labrie SJ, Samson JE, Moineau S. 2010. Bacteriophage resistance mechanisms. Nat. Rev. Micro. 8, 317–327. ( 10.1038/nrmicro2315) [DOI] [PubMed] [Google Scholar]
- 19.Goldfarb T, Sberro H, Weinstock E, Cohen O, Doron S, Charpak-Amikam Y, Afik S, Ofir G, Sorek R. 2015. BREX is a novel phage resistance system widespread in microbial genomes. EMBO J. 34, 169–183. ( 10.15252/embj.201489455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ofir G, Melamed S, Sberro H, Mukamel Z, Silverman S, Yaakov G, Doron S, Sorek R. 2018. DISARM is a widespread bacterial defence system with broad anti-phage activities. Nat. Microbiol. 3, 90–98. ( 10.1038/s41564-017-0051-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doron S, Melamed S, Ofir G, Leavitt A, Lopatina A, Keren M, Amitai G, Sorek R. 2018. Systematic discovery of antiphage defense systems in the microbial pangenome. Science 359, eaar4120 ( 10.1126/science.aar4120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paez-Espino D, Morovic W, Sun CL, Thomas BC, Ueda Ki, Stahl B, Barrangou R, Banfield JF. 2013. Strong bias in the bacterial CRISPR elements that confer immunity to phage. Nat. Commun. 4, 1430 ( 10.1038/ncomms2440) [DOI] [PubMed] [Google Scholar]
- 23.Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. 2007. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712. ( 10.1126/science.1138140) [DOI] [PubMed] [Google Scholar]
- 24.Horvath P, Romero DA, Coûté-Monvoisin AC, Richards M, Deveau H, Moineau S, Boyavel P, Fremaux C, Barrangou R. 2008. Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus. J. Bacteriol. 190, 1401–1412. ( 10.1128/JB.01415-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deveau H, Barrangou R, Garneau JE, Labonté J, Fremaux C, Boyaval P, Romero DA, Horvath P, Moineau S. 2008. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J. Bacteriol. 190, 1390–1400. ( 10.1128/JB.01412-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun CL, Barrangou R, Thomas BC, Horvath P, Fremaux C, Banfield JF. 2013. Phage mutations in response to CRISPR diversification in a bacterial population. Environ. Microbiol. 15, 463–470. ( 10.1111/emi.2013.15.issue-2) [DOI] [PubMed] [Google Scholar]
- 27.Lévesque C, Duplessis M, Labonté J, Labrie S, Fremaux C, Tremblay D, Moineau S. 2005. Genomic organization and molecular analysis of virulent bacteriophage 2972 infecting an exopolysaccharide-producing Streptococcus thermophilus strain. Appl. Environ. Microbiol. 71, 4057–4068. ( 10.1128/AEM.71.7.4057-4068.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hynes AP, Lemay ML, Trudel L, Deveau H, Frenette M, Tremblay DM, Moineau S. 2017. Detecting natural adaptation of the Streptococcus thermophilus CRISPR-Cas systems in research and classroom settings. Nat. Protoc. 12, 547–565. ( 10.1038/nprot.2016.186) [DOI] [PubMed] [Google Scholar]
- 29.Chabas H, Lion S, Nicot A, Meaden S, van-Houte S, Moineau S, Wahl LM, Westra ER, Gandon S. 2018. Evolutionary emergence of infectious diseases in heterogeneous host populations. PLoS Biol. 16, 1–20. ( 10.1371/journal.pbio.2006738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org/.
- 31.Sanjuán R, Nebot MR, Chirico N, Mansky LM, Belshaw R. 2010. Viral mutation rates. J. Virol. 84, 9733–9748. ( 10.1128/JVI.00694-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lujan SA. et al. 2014. Heterogeneous polymerase fidelity and mismatch repair bias genome variation and composition. Genome Res. 24, 1751–1764. ( 10.1101/gr.178335.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geller R, et al. 2016. Highly heterogeneous mutation rates in the hepatitis C virus genome. Nat. Microbiol. 1, 16045 ( 10.1038/nmicrobiol.2016.45) [DOI] [PubMed] [Google Scholar]
- 34.Dillon MM, Sung W, Lynch M, Cooper VS. 2018. Periodic variation of mutation rates in bacterial genomes associated with replication timing. mBio 9, e01371 ( 10.1128/mBio.01371-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young JC. et al. 2012. Phage-induced expression of CRISPR-associated proteins is revealed by shotgun proteomics in Streptococcus thermophilus. PLoS ONE 7, 1–12. ( 10.1371/journal.pone.0038077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hestand MS, Houdt JV, Cristofoli F, Vermeesch JR. 2016. Polymerase specific error rates and profiles identified by single molecule sequencing. Mutat. Res./Fundam. Mol. Mech. Mutagen. 784–785, 39–45. ( 10.1016/j.mrfmmm.2016.01.003) [DOI] [PubMed] [Google Scholar]
- 37.Sanjuán R, Moya A, Elena SF. 2004. The distribution of fitness effects caused by single-nucleotide substitutions in an RNA virus. Proc. Natl Acad. Sci. USA 101, 8396–8401. ( 10.1073/pnas.0400146101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Domingo-Calap P, Cuevas JM, Sanjuán R. 2009. The fitness effects of random mutations in single-stranded DNA and RNA bacteriophages. PLoS Genet. 5, 1–7. ( 10.1371/journal.pgen.1000742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peris JB, Davis P, Cuevas JM, Nebot MR, Sanjuán R. 2010. Distribution of fitness effects caused by single-nucleotide substitutions in bacteriophage f1. Genetics 185, 603–609. ( 10.1534/genetics.110.115162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martel B, Moineau S. 2014. CRISPR-Cas: an efficient tool for genome engineering of virulent bacteriophages. Nucleic Acids Res. 42, 9504–9513. ( 10.1093/nar/gku628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemay ML, Tremblay DM, Moineau S. 2017. Genome engineering of virulent lactococcal phages using CRISPR-Cas9. ACS Synth. Biol. 6, 1351–1358. ( 10.1021/acssynbio.6b00388) [DOI] [PubMed] [Google Scholar]
- 42.van Houte S. et al. 2016. The diversity-generating benefits of a prokaryotic adaptive immune system. Nature 532, 385–388. ( 10.1038/nature17436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vale PF, Lafforgue G, Gatchitch F, Gardan R, Moineau S, Gandon S. 2015. Costs of CRISPR-Cas-mediated resistance in Streptococcus thermophilus. Proc. R. Soc. B 282, 20151270 ( 10.1098/rspb.2015.1270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weissman JL, Holmes R, Barrangou R, Moineau S, Fagan WF, Levin B, Johnson PL. 2018. Immune loss as a driver of coexistence during host-phage coevolution. ISME J. 12, 585 ( 10.1038/ismej.2017.194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bondy-Denomy J, Pawluk A, Maxwell KL, Davidson AR. 2012. Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system. Nature 493, 429–432. ( 10.1038/nature11723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hynes AP. et al. 2018. Widespread anti-CRISPR proteins in virulent bacteriophages inhibit a range of Cas9 proteins. Nat. Commun. 9, 2919 ( 10.1038/s41467-018-05092-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landsberger M, Gandon S, Meaden S, Rollie C, Chevallereau A, Chabas H, Buckling A, Westra ER, van Houte S. 2018. Anti-CRISPR phages cooperate to overcome CRISPR-Cas immunity. Cell 174, 908–916. ( 10.1016/j.cell.2018.05.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borges AL, Zhang JY, Rollins MF, Osuna BA, Wiedenheft B, Bondy-Denomy J. 2018. Bacteriophage cooperation suppresses CRISPR-Cas3 and Cas9 immunity. Cell 174, 917–925. ( 10.1016/j.cell.2018.06.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Godfray HCJ, North A, Burt A. 2017. How driving endonuclease genes can be used to combat pests and disease vectors. BMC Biol. 15, 81 ( 10.1186/s12915-017-0420-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noble C, Adlam B, Church GM, Esvelt KM, Nowak MA. 2018. Current CRISPR gene drive systems are likely to be highly invasive in wild populations. eLife 7, e33423 ( 10.7554/eLife.33423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Unckless RL, Clark AG, Messer PW. 2017. Evolution of resistance against CRISPR/Cas9 gene drive. Genetics 205, 827–841. ( 10.1534/genetics.116.197285) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are accessible as the electronic supplementary material.