Abstract
T lymphocyte-mediated immune responses in the heart are potentially dangerous because they can interfere with the electromechanical function. Furthermore, the myocardium has limited regenerative capacity to repair damage caused by effector T cells. Myocardial T cell responses are normally suppressed by multiple mechanisms of central and peripheral tolerance. T cell inhibitory molecules, so called immune checkpoints, limit the activation and effector function of heart antigen-reactive T cells that escape deletion during development in the thymus. Programmed cell protein death-1 (PD-1) and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) are checkpoint molecules homologous to the costimulatory receptor CD28, and they work to block activating signals from the T cell antigen receptor and CD28. Nonetheless, PD-1 and CTLA-4 function in different ways and at different steps in a T cell response to antigen. Studies in mice have established that genetic deficiencies of checkpoint molecules, including PD-1, PD-L1, CTLA-4, and lymphocyte activation gene-3, result in enhanced risk of autoimmune T cell-mediated myocarditis and increased pathogenicity of heart antigen-specific effector T cells. The PD-1/PD-L1 pathway appears to be particularly important in cardiac protection from T cells. PD-L1 is markedly up-regulated on myocardial cells by interferon-gamma secreted by T cells and PD-1 or PD-L1 deficiency synergizes with other defects in immune regulation in promoting myocarditis. Consistent with these studies, myocarditis has emerged as a serious adverse reaction of cancer therapies that target checkpoint molecules to enhance anti-tumour T cell responses. Histopathology and immunohistochemical analyses of myocardial tissue from immune checkpoint blockade (ICB)-treated patients echoes findings in checkpoint-deficient mice. Many questions about myocarditis in the setting of cancer immunotherapy still need to be answered, including the nature of the target antigens, genetic risk factors, and variations in the disease with combined therapies. Addressing these questions will require further immunological analyses of blood and heart tissue from patients treated with ICB.
Keywords: Immunotherapy, Myocarditis, Checkpoint, T lymphocyte
This article is part of the Spotlight Issue on Cardio-oncology.
1. Introduction
Although the immune system has evolved to protect against microbial pathogens, its powerful effector mechanisms have the potential to injure and impair function of normal tissues and organs. In fact, innate and adaptive immune responses induced by infections often cause tissue injury, but many regulatory mechanisms have co-evolved to limit this collateral damage. Furthermore, the adaptive immune system randomly generates millions of B and T cell clones, each with different antigen receptor specificities. Many of these receptors recognize self-antigens, and we rely on mechanisms of self-tolerance that delete or inactivate the self-reactive B and T cells in order to prevent autoimmune disease. Overall, there is a critical balance that must be maintained between effective antimicrobial immunity and protection of normal cells and tissues. However, the set points for this balancing act differ in different places in the body. Certain tissues, such as the eye, brain, and heart whose continuous functions are critical for survival but are readily impaired by immune inflammatory events, have regulatory mechanisms that discourage the low-level immune responses that are continuously taking place in barrier tissues and organs.
There are very few T cells found in healthy human myocardium, consistent with a landscape in a relative state of immune privilege, which apparently discourages T cell recruitment, activation, and presence of tissue resident memory T cells. However, there are significant numbers of tissue resident macrophages and dendritic cells (DCs). Some of these subsets have phenotypes predicted to favour tissue repair, immune regulation, and tolerance. Nonetheless, other subsets appear capable of initiating effector innate and T cell responses.1 Furthermore, the dense microvascular network in the myocardium, required for sufficient oxygen and nutrient delivery to myocytes, provides ample opportunity for circulating T cells to migrate into the heart. This raises the question of how this hyper-vascularized organ maintains immune quiescence.
2. Inhibitory pathways that regulate T cell activation (immune checkpoint molecules)
Mature naïve CD4+ and CD8+ T lymphocytes emerge from the thymus and then recirculate through secondary lymphoid organs (SLOs) where protein antigens from blood or tissues are collected and displayed to the T cells as peptide-major histocompatibility complex (MHC) complexes on the surface of antigen-presenting cells (APCs). DCs are the type of APC that is critical for naïve T cell activation, a process that occurs in SLOs such as draining lymph nodes. T cell receptor (TCR) recognition of a peptide-MHC antigen is necessary, but not by itself sufficient, for activation of the naïve T cells. In addition, the presence of pathogens or necrotic tissue injury, collectively called danger signals, transform DCs to express surface ligands called costimulators, which induce signalling by costimulatory receptors on the naïve T cells. Both antigen recognition and costimulation are required to permit productive activation of naïve T cells, resulting in clonal expansion and differentiation of the T cells to effector CD4+ helper T (Th) cells or CD8+ cytotoxic T lymphocytes (CTL). Effector T cells must recognize antigen again at sites of infection or tissue injury in order to activate their functional responses, but many cell types other than DCs may display the peptide-MHC antigen to effector T cells, and costimulation may not be required for activation of these effectors. The best defined costimulators for naïve T cells are the two homologous immunoglobulin superfamily (IgSF) proteins B7-1 (CD80) and B7-2 (CD86), which are highly expressed on DCs that have been exposed to microbes or other innate immune stimuli.2 Both B7 proteins bind to CD28, which is ubiquitously expressed on naïve T cells and the resulting signalling by CD28 synergizes with antigen-TCR generated signals to initiate optimal T cell activation. Thus, the requirement for costimulation effectively limits T cell activation only to situations where there is danger. In addition to the B7-CD28 costimulatory pathways, there are other costimulatory pathways which contribute to optimal T cell responses. Inducible T cell costimulator (ICOS) ligand (CD275) is a B7 family member expressed on B cells, which binds to ICOS (CD278) on CD4+ Th cells, thereby promoting Th-B cell collaboration in antibody responses to protein antigens. There are also costimulatory pathways involving tumour necrosis factor superfamily proteins on APCs that bind the tumour necrosis factor receptor superfamily proteins on T cells. Two examples are 4-1BB ligand (4-1BBL) on APCs, which binds to CD137 (4-1BB) on T cells, considered an important costimulatory pathway for CD8+ T cell responses, and CD252 (OX40 ligand) on APCs, which binds CD134 (OX40) on activated T cells. Agonist monoclonal antibodies (mAbs) specific for 4-1BB and OX40 are being developed as drugs for cancer immunotherapy.3,4
Additional mechanisms of regulation of T cell response have evolved, which function both to limit the extent of appropriate T cell activation, and to prevent inappropriate responses to self-antigens and to non-harmful environmental antigens. The two best characterized and currently most clinically relevant of these mechanisms involve the T cell molecules CTLA-4 (CD152) and PD-1 (CD279). Both are IgSF proteins structurally homologous to CD28, but unlike CD28 actually function to inhibit T cell activation.5 CTLA-4 is expressed on the surface of most naïve T cells shortly after activation and is constitutively expressed on regulatory T cells (Treg) and memory T cells with an exhausted phenotype seen in conditions of chronic antigen exposure. CTLA-4 binds to both B7-1 and B7-2 with significantly higher affinity than CD28 does, thus CTLA-4 competitively inhibits B7-dependent T cell costimulation. There are also data suggesting T cell-intrinsic inhibition by CTLA-4 signalling,6 but robust evidence for the relevance of inhibitory signalling in vivo is lacking. PD-1 expression on T cells is induced by antigen activation and like CTLA-4 is highly expressed on exhausted T cells.7 PD-1 binds programmed death ligand-1 (PD-L1/CD274) and programmed death ligand-2 (PD-L2/CD273), both IgSF proteins homologous to the B7 proteins. The cytokines interferon-gamma (IFN-γ) and type 1 IFNs can induce PD-L1 expression on many cell types, including DCs, macrophages, epithelial parenchymal cells of various organs, endothelial cells (EC), and many tumour cells. PD-L2 expression is largely restricted to bone marrow-derived APCs.8,9 Upon binding PD-L1 or PD-L2, PD-1 initiates biochemical events in the T cell that inhibit CD28 and TCR signalling. This inhibitory signalling involves recruitment of tyrosine phosphatases which counteract the action of protein tyrosine kinases that are activated by CD28 and TCR signalling. Overall, both CTLA-4 and PD-1 block antigen and costimulator activation of T cells, and both are often called co-inhibitors or immune checkpoints. Nonetheless, their mechanisms of action are distinct and their main physiological roles are likely to play out at different stages of a T cell response. B7-mediated costimulation is most critical for naïve T cell activation in SLOs, and therefore this is when and where CTLA-4 will have its greatest impact. PD-L1 is expressed in many non-lymphoid tissues and thus, PD-1 is important for inhibition of effector T cell activation in these tissues.
In addition to CTLA-4 and PD-1, other T cell inhibitory checkpoint molecules have been discovered which appear to be necessary for T cell immune regulation, as indicated by the immunopathological consequences of their genetic deficiency or blockade in mice. These are also being investigated as targets for tumour immunotherapy.10 Lymphocyte activation gene-3 (LAG-3 and CD223) is an IgSF protein expressed by T cells, B cells, DCs, and macrophages. LAG-3 binds to Class II MHC molecules and may block antigen presentation to or generate inhibitory cells within CD4+ T cells.11,12 T cell immunoglobulin-3 (TIM-3) is another IgSF protein expressed by T cells, natural killer (NK) cells, and macrophages. TIM-3 binds to galectin-9, phosphatidyl serine, and carcinoembryonic antigen-related cell adhesion molecule-1 (CEACAM-1), and has cell-intrinsic inhibitory effects on T cell activation. T cell immunoreceptor with Ig and ITIM domains (TIGIT) is a CD28 family protein expressed by T cells and NK cells, which binds poliovirus receptor family molecules CD155 and CD112 and has intrinsic inhibitory effects on the T cells on which it expressed. LAG-3, TIM-3, and TIGIT are all up-regulated in exhausted T cells.
Treg are essential for maintenance of self-tolerance, as well as tolerance to commensal organisms and non-pathogenic environmental antigens. The most abundant and well-characterized Treg are CD4+CD25+FoxP3+ T cells, and FoxP3 mutations in mice and humans results in deficiency of Treg leading to fatal systemic autoimmune disease.13 In addition to expression on conventional T cells (effector T cells and their naïve precursors), checkpoint molecules including CTLA-4, PD-1, and LAG-3 are expressed on Treg. CTLA-4 is essential for Treg function, as shown by autoimmune phenotype of mice with selective deletion of CTLA-4 only in FoxP3+ cells.14 The functions of PD-1 on Treg are not yet well described but given the cell-intrinsic inhibitory function of PD-1, blockade or deficiency of PD-1 on Treg may enhance suppressive function. Studies of Treg-restricted loss of LAG-3 in mice show that LAG-3 impairs Treg function at autoimmune inflammatory sites.15 These heterogenous functions of checkpoint molecules on Treg add to the complexity of the effects of immune checkpoint blockade (ICB) therapies, which are likely to impair functions of the checkpoint molecules on both conventional T cells and Treg. Thus, the effects of anti-CTLA-4 on both cell types should result in enhanced T cell responses, while the effects of anti-PD-1 and anti-LAG-3 on Treg might work in opposition to the activating effects on conventional T cells.
The discoveries of the CTLA-4 and PD-1 T cell inhibitory pathways stimulated research into the potential targeting of these molecules to enhance anti-tumour T cell responses. Studies in rodent cancer models using anti-CTLA-4 or anti-PD-1 and anti-PD-L1 mAbs indicated the potential for mAb-mediated ICB to treat cancer patients.16 These studies lead to clinical trials with remarkable benefit in a significant fraction of patients with metastatic melanoma.17,18 Anti-CTLA-4 mAb (ipilimumab) treatment of metastatic melanoma was approved in 2011, and anti-PD-1 mAbs (pembrolizumab and nivolumab) were approved for melanoma in 2014. Since then a very large number of clinical trials have been started with many now completed. The ICB therapies with anti-PD-1, anti-PD-L1, some in combination with anti-CTLA-4, have been approved and adopted in clinics for the treatment of a wide variety of malignancies.19 Trials of combinations of these ICBs with blockers of other inhibitory molecules, including LAG-3, TIM-3, and TIGIT are also underway. A predicted complication of these therapies is immune-related adverse events (irAEs) reflecting the loss of the immune regulatory functions of the molecules being blocked. In fact, irAEs of varying severity, affecting one or more of most tissue types and organ system in the body, occur in over 50% of ICB-treated cancer patients.20,21 The majority of irAEs can be clinically managed such that the lifesaving potential of the ICB therapy can be realized. However, lethal or potentially lethal complications do arise in a minority of patients. Furthermore, combination therapies now in development are likely to enhance incidence of irAEs. Among the most serious, albeit infrequent, irAEs are those affecting the heart,22 also reviewed in other articles in this issue. In the remainder of this review, we will discuss the underlying mechanisms of myocardial toxicity of ICBs. We will focus on the evidence that immune homeostasis of the myocardium depends on various mechanisms of self-tolerance, including T cell immune checkpoints such as the PD-1/PD-L1 pathway (Figure 1).
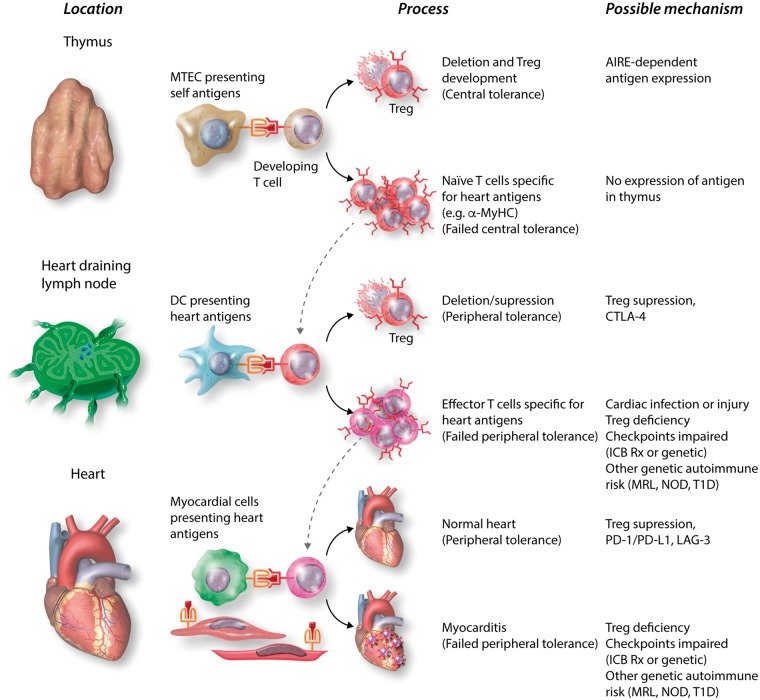
Figure 1.
T cell tolerance to heart antigens and its failure. The figure shows the likely sequence of steps in regulation of autoreactive T cells, which normally prevents autoimmune myocarditis. Central T tolerance to heart antigens occurs during thymic development of T cells, through autoimmune regulator (AIRE)-dependent expression of tissue-restricted antigens by medullary thymic epithelial cells (MTEC) and their presentation to immature T cells, which causes deletion of self-reactive clones and/or development of Treg specific for these antigens. Some heart antigen-specific T cells, such as those specific for α-MyHC are not deleted by this central tolerance mechanism, perhaps because they are not expressed by MTECs, and thus mature into naïve T cells, which recirculate through SLOs. If these naïve T cells encounter heart antigens presented by DCs in lymph nodes draining the heart, their activation may be blocked by peripheral tolerance mechanisms, such as Treg and/or CTLA-4-mediated block in costimulation. Naïve heart antigen-specific T cells may escape SLO-based tolerance mechanisms because of infections or injury that upregulate costimulators on DCs and/or genetic deficiencies in one or more regulatory pathways, and these T cells can be activated to clonally expand and differentiate into effector T cells. Some of the effector T cells may enter the heart, where their activation by antigens and their capacity to cause damage can be blocked by tissue-based tolerance mechanisms, such as PD-L1 expression on myocytes and endothelium. Genetic deficiencies in pathways that regulate effector T cell activation may increase the risk that these cells cause myocardial damage. In some ICB-treated patients, these peripheral tolerance mechanisms are sufficiently impaired so that autoreactive T cell myocarditis occurs. Anti-CTLA-4 is most likely to impair SLO-based tolerance, because this is where CTLA-4 normally blocks B7-costimulation, which is most critical for naïve T cell activation. Anti-PD-1 or anti-PD-L1 most likely impair regulation of effector T cell activation in the heart. The mechanisms where other checkpoint regulators work, including LAG-3 and TIM-4, are not yet clearly established. The hypothesis that LAG-3 impairment (blockade or genetic deficiency) contributes to loss of T cell tolerance to heart antigen is based on mouse studies discussed in the text. MRL, Murphy Roths Large; NOD, non-obese diabetic; T1D, type 1 diabetes.
3. PD-1/PD-L1 pathway in myocardial immune homeostasis
In the first published description of the PD-L1 molecule, a northern blot analysis of Pdl1 messenger RNA expression in different mouse tissues indicated high levels in the heart.23 Subsequent studies determined that PD-L1 expression could be induced on mouse cardiac EC in vitro by IFN-γ treatment.24 Although IFN-γ also up-regulated EC Class I MHC expression, the induced PD-L1 expression resulted decreased ability of the EC to activate cytotoxic T lymphocytes (CTLs) through Class I MHC-restricted antigen presentation, and an increased EC resistance to antigen-specific CTL killing. Work with an in vivo model of myocarditis mediated by adoptively transferred TCR-transgenic heart antigen-specific CTL25 established that PD-L1 expression, including expression on EC, is markedly up-regulated in the myocardium after infiltration and activation of CTL in the heart.26 Furthermore, PD-L1 upregulation in the heart was dependent on IFN-γ produced by the transferred CTLs and on IFN-γ receptor expression in the recipient mice tissues. Studies with mice genetically deficient in PD-L1 showed that PD-L1 serves a protective role in limiting T cell-mediated damage to the myocardium, and at least in part this is dependent on PD-L1 expression by non-haematopoietic tissue cells, such as endothelium or myocytes.26 These studies indicate that the heart maintains a state of resistance to local effector T cell-mediated responses by a negative feedback loop, sensing T cell IFN-γ production and upregulating PD-L1, thereby suppressing activation of other T cells (Figure 2). This scenario has more recently been shown to play out in the context of tumour resistance to effector T cells.27 The upregulation of PD-L1 in human myocardium, both on myocytes and endothelium, is evident in hearts of patients with idiopathic lymphocytic myocarditis (Andrew Lichtman’s unpublished data) and in hearts of cancer patients treated with ICBs (Figure 3, discussed below). Upregulation of PD-L1 can also be seen on EC extracted from hearts of IFN-treated mice and analysed by flow cytometry (Andrew Lichtman’s unpublished data).
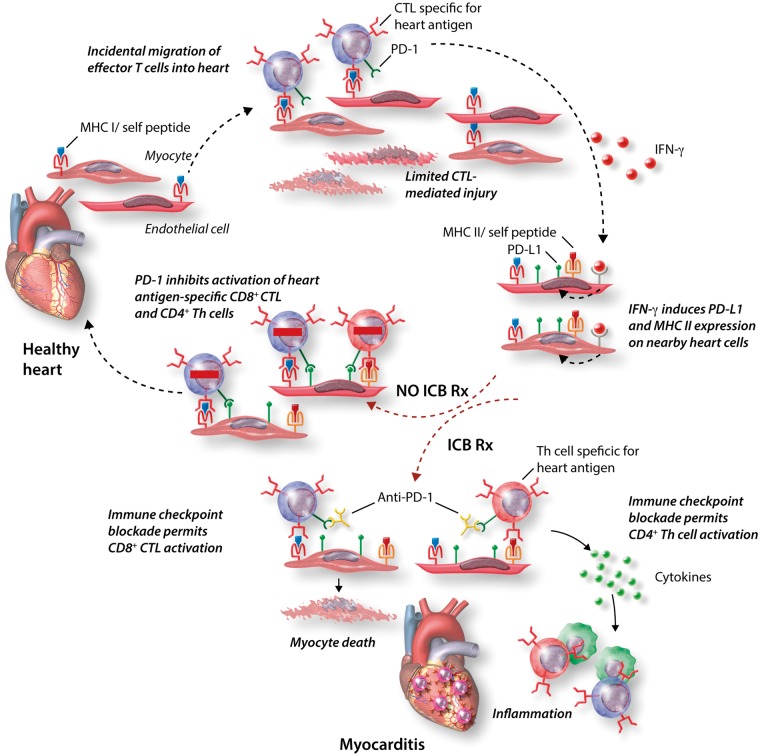
Figure 2.
The protective roles of IFN-γ and PD-L1 in the heart and the consequences of ICB. Normal myocardial cells in the healthy heart, including myocytes and EC, express only low levels of PD-L1 or Class II MHC (MHCII), but do express Class I MHC (MHCI). Given the high density of myocardial microvasculature, it is likely that effector T cells sporadically enter the uninflamed heart. If effector CD8+ CTL specific for heart antigens enter the myocardium, they can be activated by myocardial cells expressing the relevant peptide-MHC I antigens, and the CTL can kill those cells, as well as secrete IFN-γ. Myocardial cells nearby will respond to IFN-γ by upregulating expression of PD-L1 and Class II MHC. If additional heart antigen-specific CTLs see antigen presented by these PD-L1 expressing cells, their activation will be suppressed by PD-1 signalling, and damage by the CTL will be limited. In cancer patients treated with anti-PD-1 or anti-PD-L1 mAbs, CTL activation in the heart may not be suppressed, and CTL-mediated damage will be enhanced. This is despite more IFN-γ production by the CTL and induced PD-L1 on the heart cells. Furthermore, because MHC II is up-regulated, CD4+ Th cells specific for heart antigens that may enter the now inflamed myocardium can also be activated and will not be suppressed by the mAb-blocked PD-1 pathway. Th cells will promote inflammation and macrophage activation which appears to contribute significantly to the immunopathology of ICB-associated myocarditis.
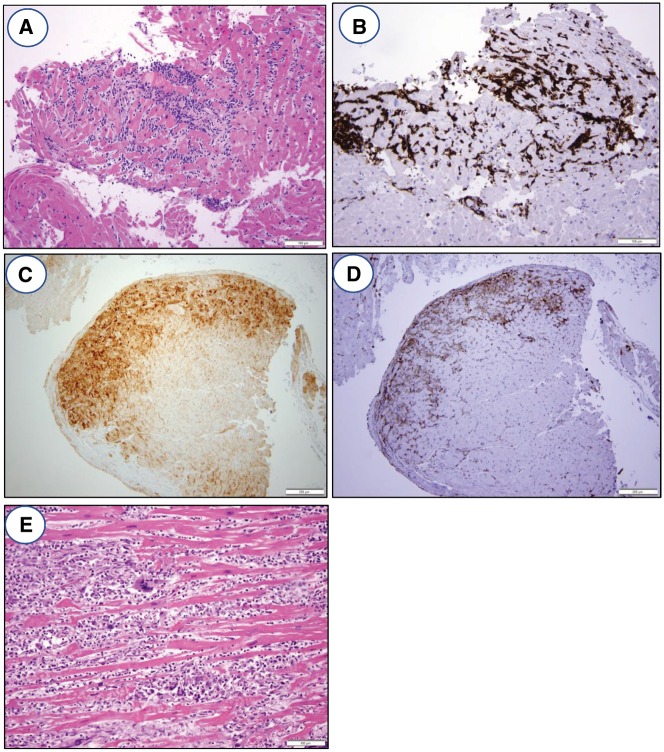
Figure 3.
Histology and immunohistochemistry of ICB myocarditis. (A) Photomicrograph of endomyocardial biopsy showing lymphocytic myocarditis with a dense mononuclear cell infiltrate, associated myocyte damage, and oedema. H&E stained section, ×200 original magnification. (B) Immunohistochemistry for CD3 demonstrating that the infiltrate is predominantly composed of T lymphocytes, ×200 original magnification. (C) Immunohistochemistry for PD-L1 shows positive staining of myocytes only in the areas of lymphocytic myocarditis, ×100 original magnification. (D) Immunohistochemistry for HLA-DR, a surrogate marker for IFN-γ activity, shows positive staining in the areas of lymphocytic myocarditis, ×100 original magnification. (E) Photomicrograph of endomyocardial biopsy showing giant cell myocarditis with an inflammatory infiltrate, extensive myocyte damage, and the presence of foreign body giant cells. This type of myocarditis can be seen in patients on ICB therapy. H&E stained section, ×200 original magnification.
4. Adverse cardiac consequences of deficiencies of PD-1/PD-L1 and CTLA-4 in mice
Several murine studies indicate that impairment of the PD-1/PD-L1 pathway enhances risk for autoimmune cardiac pathology. A common theme of these studies is that the risk is restricted to particular genetic backgrounds, including mice with known propensity to develop autoimmune disease. The first published study implicating a role of PD-1 pathway deficiency in cardiac disease showed that BALB/c mice lacking a functional PD-1 gene (Pcd1−/−) spontaneously developed a dilated cardiomyopathy with congestive heart failure. This mouse disease was associated with autoantibodies specific for cardiac troponin I (cTnI).28,29 The antibodies bound to TnI on the surface of cardiomyocytes, thereby causing stimulation of voltage-dependent L-type Ca++ currents; the disease phenotype could be reproduced by injecting wild-type BALB/c mice with monoclonal anti-TnI antibody. However, there was no evidence of myocardial inflammation or of T cell-mediated cardiac injury, distinguishing this mouse model from later reports of myocardial disease associated with PD-1 deficiency or blockade in mice or humans. Furthermore, spontaneous dilated cardiomyopathy is not seen in PD-1-deficient BALB/c mice derived in another laboratory30 but those mice do show enhanced T cell inflammation compared to control BALB/c mice in experimental autoimmune myocarditis induced by α-myosin heavy chain (α-MyHC) peptide immunization.31 Immunization of A/J or BALB/c mice with murine cTnI and adjuvant causes an inflammatory myocarditis progressing to fibrosis and heart failure, in which anti-TnI antibodies may play a role in the phenotype.32 However, the effect of PD-1 deficiency in this immunization model has not been reported.
Spontaneous T cell-mediated myocarditis (i.e. without adoptive transfer of T cells or immunization) does arise in mice with a genetic deficiency of the PD-1 pathway, combined with a second autoimmune risk factor. For example, the MRL strain of mice is prone to develop a systemic lupus-like autoimmune disease, but not myocarditis, and this propensity is greatly enhanced in MRL mice lacking functional FAS (MRL-lpr−/−) background. However, MRL mice with a genetic deletion of the PD-L1 gene, with or without functional FAS genes, spontaneously develop lethal myocarditis and pneumonitis, characterized by CD8+ greater than CD4+ T cell infiltrates.33 The results of studies with bone marrow chimeric mice suggested that PD-L1 deficiency only on haematopoietic cells was sufficient to cause autoimmune myocarditis in the MRL background. A similar spontaneous lymphocytic myocarditis was observed in PD-1-deficient MRL mice.34 Another example of combined genetic risk for myocarditis in mice is spontaneous lethal T cell-mediated myocarditis in mice lacking both PD-1 and LAG-3, but not PD-1 or LAG-3 deficiency alone, which has been described in both BALB/c and C57BL/6 backgrounds.35,36 The myocarditis in the Pdcd1−/−Lag3−/− mice consisted of CD8+ and CD4+ IFN-γ producing T cells. In the C57BL/6 background, the Pdcd1−/−Lag3−/− mice also developed pancreatitis. Overall, these studies highlight the fact that deficiency of the PD-1 pathway alone is not sufficient to trigger myocarditis but synergizes with additional genetically determined autoimmune susceptibilities resulting in T cell-mediated myocarditis. These insights may be relevant to understanding which patients receiving anti-PD-1 immunotherapy are at most risk for cardiac complications.
There are fewer studies which have explored an association of isolated CTLA-4 deficiency with myocarditis, in part because of the difficulty in breeding CTLA-4 deficient mice, and the less frequent use of anti-CTLA-4 compared to anti-PD-1 in humans. In mice, deletions of the CTLA-4 gene results in a severe lymphoproliferative disease that is lethal within a few weeks.37,38 Among the organs affected is the heart and myocarditis contributes significantly to the lethality of this condition. CTLA-4 deficiency in TCR-transgenic mice in which the T cell repertoire is restricted to a non-self-protein and do not display the lymphocytic inflammatory phenotype. This indicates that the disease in mice with a normal T cell repertoire is likely autoimmune,39 and analysis of tissue infiltrating T cell in CTLA-4-deficient mice showed specificity for self-antigens.40 There is strong evidence that CTLA-4 functions to block B7-mediated costimulation of naïve T cells,41 and therefore, myocarditis associated with genetically or pharmacologically impaired CTLA-4 function most likely reflects enhanced priming of naïve autoreactive heart antigen-specific T cells in SLOs. However, in certain cases memory T cell activation in tissues may be enhanced by B7-CD28 costimulation,42 and thus blocking CTLA-4 could enhance reactivation of previously generated heart antigen-specific memory T cells. CTLA-4 may also have T cell-intrinsic inhibitory signalling functions, which protect the myocardium and other tissues from effector T cell activation. In this regard, adoptive transfer of CTLA-4-deficient ovalbumin-specific CTLs into mice that express ovalbumin in the heart induced more severe myocarditis than control CTLs.43
5. Mechanisms of adverse cardiac consequences of blockade of PD-1/PD-L1 and CTLA-4 in humans
Based on the mouse studies reviewed here and the incidence of non-cardiac irAEs that occurred during ICB clinical trials, it is not surprising that myocarditis has emerged as an immune complication of ICB therapy as more patients are being treated.22,44 Myocarditis has occurred in patients treated with anti-CTLA-4 alone, anti-PD-1 or anti-PD-L1 alone, or in patients receiving combination therapy, and the clinical features are reviewed in more detail elsewhere.45 Our understanding of the immunological mechanisms underlying these cases of myocarditis is extremely limited at this point, because of the infrequency of obtaining myocardial tissue from patients with active disease, and limited interrogation of immune cells from blood.
A fundamental, albeit often unanswered, question about any autoimmune disease is what self-antigens are being targeted by antibodies or T cells. In the case of autoimmune myocarditis, there are several antigens that have been implicated by the presence of circulating autoantibodies specific for those antigens in patients. These antigens include cardiac troponins and myosins, and cardiac β1 adrenergic receptors.46 However, there is no conclusive evidence that these antibodies, or antibody-mediated immune reactions in general, contribute to the acute inflammatory phase of human (or mouse) myocarditis, although some of these antibodies may be biomarkers of patients at risk for Th cell-mediated myocarditis.47 Likewise, there is limited knowledge of the relevant target antigens for human T cell-mediated myocarditis but one interesting candidate is α-MyHC.48,49 Healthy people have low frequencies of α-MyHC-specific CD4+ T cells in their blood, indicating a lack of central T cell tolerance to this protein.50 The not uncommon presence of IgG antibodies specific for α-MyHC in people indicates a role for α-MyHC-specific Th cells. Patients with autoimmune or type 1 diabetes (T1D) mellitus have increased risk of developing myocarditis after a myocardial infarction (MI) compared to MI patients without T1D, and they have a higher frequency of α-MyHC-specific IFN-γ producing T cells than controls. The frequency of these T cells increases markedly if myocarditis develops.49,51 Thus T cells specific for α-MyHC are central to autoimmune myocarditis in certain strains of mice, and in some people with an underlying genetic risk for autoimmunity.
In the case of ICB-associated myocarditis, we still have only a few clues about relevant antigens, and we do not know if the antigens targeted are also target antigens in autoimmune myocarditis not related to ICB therapy. Analysis of clonal TCR beta chain CDR3 sequences in two cases of anti-PD-1 plus anti-CTLA-4-associated fatal myocarditis indicated there were several expanded T cell clones present in both the heart and tumour. The T cell clones in the tumour that expanded the most following ICB therapy were also found in the heart.52 Furthermore, whole transcriptome RNA sequencing indicated expression of some muscle-specific transcripts in the tumours. Interestingly, in both these cases, the patients had myositis as well as myocarditis. Myositis appears to be a more frequently diagnosed irAE than myocarditis ICB-treated patients, but up to 38% of reported myositis cases also had myocarditis.53 These observations suggest that some ICB-treated patients with myocarditis mount an autoreactive T cell response to striated muscle proteins expressed by the tumour. However, the connection between shared tumour and heart antigens remains tenuous. Furthermore, activated T cells specific for tumour antigens are found in the blood of cancer patients and these could migrate into inflamed myocardium, even if their specificity is not for any heart protein. The relative incidence of myocarditis in ICB-treated patients with different tumour types is not yet known, nor is there additional data on the correlation of tumour expression of muscle or myocardial antigens with myocarditis. To better understand the type and range relevant antigens that drive ICB-associated myocarditis, it will be important to collect data on titres of antibodies and frequency of T cells specific for cardiac and tumour antigens in patients with and without myocarditis.
The histologic findings in patients with checkpoint inhibitor myocarditis have been reported in a number of case reports54–60 and are fairly consistent. At the light microscopic level with standard haematoxylin and eosin (H&E) staining, there is a dense mononuclear inflammatory infiltrate associated with myocyte damage/necrosis, consistent with the definition of myocarditis set forth in the Dallas criteria (Figure 3A). Some fulminant cases have shown a mixed inflammatory infiltrate with abundant giant cells, consistent with the diagnosis of giant cell myocarditis. Eosinophilic myocarditis, which generally is a type 1 hypersensitivity (allergic) reaction rather than a T cell-mediated process, has not been reported with checkpoint inhibitors. In patients who have been treated for checkpoint inhibitor myocarditis with anti-inflammatory medications such as corticosteroids, the inflammatory infiltrate is reduced, and there is evidence of healing with granulation tissue and/or fibrosis in the previously damaged areas of myocardium.
Immunohistochemistry in many of these reports demonstrates a predominantly CD3-positive T cell infiltrate (Figure 3B) with an associated CD68-positive macrophage population. The T cell population is a mixture of CD8-positive cytotoxic T cells and CD4-positive Th cells, with the former more abundant than the latter. Occasional CD20-positive B cells and CD138-positive plasma cells can be found, but these are a minor component of the overall infiltrate. As mentioned earlier, cardiac myocytes have found to be positive for PD-L1 in areas affected by myocarditis52 (Figure 3C), and expressed human leucocyte antigens (HLA)-DR, which, as discussed above, is a surrogate marker for IFN-γ production (Figure 3D). While these immunohistochemistry studies have been helpful in defining the cell populations involved in the infiltrate for purposes of investigating the mechanism of disease, they are not required for the diagnosis of checkpoint inhibitor myocarditis in routine practice in our experience.
Pathologic evaluation of myocardial tissue obtained by endomyocardial biopsy is an important tool for the diagnosis of checkpoint inhibitor myocarditis. Many clinical reports have analysed this tissue both to establish the diagnosis of myocarditis and to carry out research investigations aimed at elucidating mechanisms of disease. While endomyocardial biopsy may not be clinically appropriate or safe in certain patients, it will allow for a definitive pathologic diagnosis. For patients who present with signs and symptoms of (cardiac) myocarditis along with (skeletal muscle) myositis, a less invasive skeletal muscle biopsy may be informative to establish an autoimmune inflammatory process; the histologic and immunophenotypic findings in skeletal muscle are similar to those in the myocardium.
6. Other clinical contexts in which cardiac PD-1/PD-L1 plays a mitigating role or if blocked may aggravate diseases
Patients with cardiac allografts must be maintained on chronic immunosuppression to prevent graft rejection. However, this places them at increased risk for developing malignancies, the most frequent of which are skin cancers. Extensive preclinical studies indicated that the PD-1 pathway contributes to allograft ‘tolerance’, including cardiac allografts.61,62 In some reported cases where ICB therapy was given to heart transplant patients to treat life-threatening tumours, the immunotherapy resulted in acute rejection episodes consistent with enhanced alloreactive T cell activation in the setting of checkpoint blockade.63,64 However, several patients with cardiac allografts tolerated ICB therapy. At this time, the numbers of reported cases are too small and the details of the cases too variable to draw conclusions about risks or mechanisms of ICB therapy in the setting of heart transplantation. This includes if cardiac transplant rejection is more likely compared to rejection of kidney or other solid organ transplants.
Myocardial T lymphocytes are implicated in the pathogenesis of heart failure associated with disorders not traditionally considered to be immunologic. These include remodelling after ischaemic injury65 and in response to chronic pressure overload.66 The contribution of T lymphocytes to these common myocardial pathologies have been studied in mouse models, but their role in human disease is still not clear. If in fact myocardial T cells do contribute to heart failure, then checkpoint blockade therapy may aggravate heart failure in cancer patients, without necessarily causing overt, clinically or pathologically diagnosable myocarditis.
7. Conclusions, unanswered questions, and future work
Studies in mice have established that immune checkpoint molecules that regulate T cell responses are required to prevent T cell-mediated damage to the heart. Predictably, clinical experience has demonstrated that checkpoint inhibitor drugs used to treat cancer increase risk of myocarditis. Nonetheless, many questions about mechanisms of ICB-associated myocarditis remain unanswered and mostly unstudied. These questions concern: the relevant antigens; the relative contributions of different T cell subsets; HLA alleles that associate with risk; the phase of the autoimmune responses that are dysregulated such as priming of naïve T cells in lymphoid organs or activation of effector T cell in the heart; the contribution of ICB effects on Treg function to the disease phenotype; and the contribution of underlying genetic susceptibilities to autoimmunity. Answers to these questions should lead to information of obvious clinical importance such as how to identify the most at risk patients, and how to tailor cancer treatment based on the nature of those risk factors. In order to advance understanding of human checkpoint inhibitor-associated cardiotoxicity, it will be necessary to obtain more information from blood cells of living patients and tissues at autopsy of ICB-treated patients who die of myocarditis or cancer. Importantly, rapid autopsies in which viable lymphocytes can be extracted from myocardium, and myocardial RNA degradation is minimized, will allow for more in depth immunophenotyping than is possible with standard immunohistochemistry of formalin fixed, paraffin imbedded tissues.
Conflict of interest: none declared.
Funding
This work was supported by the National Institutes of Health (HL121363 and HL131862 to A.H.L.).
References
- 1. Frodermann V, Nahrendorf M.. Macrophages and cardiovascular health. Physiol Rev 2018;98:2523–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharpe AH. Mechanisms of costimulation. Immunol Rev 2009;229:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumar P, Bhattacharya P, Prabhakar BS.. A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity. J Autoimmun 2018;95:77–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chester C, Sanmamed MF, Wang J, Melero I.. Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies. Blood 2018;131:49–57. [DOI] [PubMed] [Google Scholar]
- 5. Schildberg FA, Klein SR, Freeman GJ, Sharpe AH.. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity 2016;44:955–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Araki M, Chung D, Liu S, Rainbow DB, Chamberlain G, Garner V, Hunter KM, Vijayakrishnan L, Peterson LB, Oukka M, Sharpe AH, Sobel R, Kuchroo VK, Wicker LS.. Genetic evidence that the differential expression of the ligand-independent isoform of CTLA-4 is the molecular basis of the Idd5.1 type 1 diabetes region in nonobese diabetic mice. J Immunol 2009;183:5146–5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R.. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007;27:670–684. [DOI] [PubMed] [Google Scholar]
- 8. Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N, Honjo T.. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett 2002;84:57–62. [DOI] [PubMed] [Google Scholar]
- 9. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ.. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261–268. [DOI] [PubMed] [Google Scholar]
- 10. Anderson AC, Joller N, Kuchroo VK.. LAG-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 2016;44:989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andrews LP, Marciscano AE, Drake CG, Vignali DA.. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev 2017;276:80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maruhashi T, Okazaki IM, Sugiura D, Takahashi S, Maeda TK, Shimizu K, Okazaki T.. LAG-3 inhibits the activation of CD4(+) T cells that recognize stable pMHCII through its conformation-dependent recognition of pMHCII. Nat Immunol 2018;19:1415–1426. [DOI] [PubMed] [Google Scholar]
- 13. Plitas G, Rudensky AY.. Regulatory T cells: differentiation and function. Cancer Immunol Res 2016;4:721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wing K, Yamaguchi T, Sakaguchi S.. Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation. Trends Immunol 2011;32:428–433. [DOI] [PubMed] [Google Scholar]
- 15. Zhang JC, Chen WD, Alvarez JB, Jia K, Shi L, Wang Q, Zou N, He K, Zhu H.. Cancer immune checkpoint blockade therapy and its associated autoimmune cardiotoxicity. Acta Pharmacol Sin 2018;39:1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Intlekofer AM, Thompson CB.. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol 2013;94:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD.. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517–2526. [DOI] [PubMed] [Google Scholar]
- 19. Wei SC, Duffy CR, Allison JP.. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018;8:1069–1086. [DOI] [PubMed] [Google Scholar]
- 20. Postow MA, Sidlow R, Hellmann MD.. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–168. [DOI] [PubMed] [Google Scholar]
- 21. Young A, Quandt Z, Bluestone JA.. The balancing act between cancer immunity and autoimmunity in response to immunotherapy. Cancer Immunol Res 2018;6:1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB.. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018;391:933.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T.. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ.. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol 2003;33:3117–3126. [DOI] [PubMed] [Google Scholar]
- 25. Grabie N, Delfs MW, Westrich JR, Love VA, Stavrakis G, Ahmad F, Seidman CE, Seidman JG, Lichtman AH.. IL-12 is required for differentiation of pathogenic CD8+ T cell effectors that cause myocarditis. J Clin Invest 2003;111:671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, Keir ME, Freeman GJ, Sharpe AH, Lichtman AH.. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation 2007;116:2062–2071. [DOI] [PubMed] [Google Scholar]
- 27. Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, Parisi G, Saus CP, Torrejon DY, Graeber TG, Comin-Anduix B, Hu-Lieskovan S, Damoiseaux R, Lo RS, Ribas A.. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep 2017;19:1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T.. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med 2003;9:1477–1483. [DOI] [PubMed] [Google Scholar]
- 29. Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T.. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001;291:319–322. [DOI] [PubMed] [Google Scholar]
- 30. Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH.. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 2006;203:883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH.. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol 2012;188:4876–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goser S, Andrassy M, Buss SJ, Leuschner F, Volz CH, Ottl R, Zittrich S, Blaudeck N, Hardt SE, Pfitzer G, Rose NR, Katus HA, Kaya Z.. Cardiac troponin I but not cardiac troponin T induces severe autoimmune inflammation in the myocardium. Circulation 2006;114:1693–1702. [DOI] [PubMed] [Google Scholar]
- 33. Lucas JA, Menke J, Rabacal WA, Schoen FJ, Sharpe AH, Kelley VR.. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol 2008;181:2513–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang J, Okazaki IM, Yoshida T, Chikuma S, Kato Y, Nakaki F, Hiai H, Honjo T, Okazaki T.. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol 2010;22:443–452. [DOI] [PubMed] [Google Scholar]
- 35. Okazaki T, Okazaki IM, Wang J, Sugiura D, Nakaki F, Yoshida T, Kato Y, Fagarasan S, Muramatsu M, Eto T, Hioki K, Honjo T.. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med 2011;208:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DA.. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 2012;72:917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamada A, Kishimoto K, Dong VM, Sho M, Salama AD, Anosova NG, Benichou G, Mandelbrot DA, Sharpe AH, Turka LA, Auchincloss H Jr, Sayegh MH.. CD28-independent costimulation of T cells in alloimmune responses. J Immunol 2001;167:140–146. [DOI] [PubMed] [Google Scholar]
- 38. Tivol EA, Boyd SD, McKeon S, Borriello F, Nickerson P, Strom TB, Sharpe AH.. CTLA4Ig prevents lymphoproliferation and fatal multiorgan tissue destruction in CTLA-4-deficient mice. J Immunol 1997;158:5091–5094. [PubMed] [Google Scholar]
- 39. Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH.. CTLA-4 regulates induction of anergy in vivo. Immunity 2001;14:145–155. [DOI] [PubMed] [Google Scholar]
- 40. Ise W, Kohyama M, Nutsch KM, Lee HM, Suri A, Unanue ER, Murphy TL, Murphy KM.. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat Immunol 2010;11:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH.. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol 2016;34:539–573. [DOI] [PubMed] [Google Scholar]
- 42. Liu D, Badell IR, Ford ML.. Selective CD28 blockade attenuates CTLA-4-dependent CD8+ memory T cell effector function and prolongs graft survival. JCI Insight 2018;3:e96378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Love VA, Grabie N, Duramad P, Stavrakis G, Sharpe A, Lichtman A.. CTLA-4 ablation and interleukin-12 driven differentiation synergistically augment cardiac pathogenicity of cytotoxic T lymphocytes. Circ Res 2007;101:248–257. [DOI] [PubMed] [Google Scholar]
- 44. Varricchi G, Galdiero MR, Marone G, Criscuolo G, Triassi M, Bonaduce D, Marone G, Tocchetti CG.. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2017;2:e000247.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG.. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cihakova D, Rose NR.. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv Immunol 2008;99:95–114. [DOI] [PubMed] [Google Scholar]
- 47. Lipes MA, Galderisi A.. Cardiac autoimmunity as a novel biomarker, mediator, and therapeutic target of heart disease in type 1 diabetes. Curr Diab Rep 2015;15:30.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reddy J, Massilamany C, Buskiewicz I, Huber SA.. Autoimmunity in viral myocarditis. Curr Opin Rheumatol 2013;25:502–508. [DOI] [PubMed] [Google Scholar]
- 49. Lv H, Lipes MA.. Role of impaired central tolerance to alpha-myosin in inflammatory heart disease. Trends Cardiovasc Med 2012;22:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gottumukkala RV, Lv H, Cornivelli L, Wagers AJ, Kwong RY, Bronson R, Stewart GC, Schulze PC, Chutkow W, Wolpert HA, Lee RT, Lipes MA.. Myocardial infarction triggers chronic cardiac autoimmunity in type 1 diabetes. Sci Transl Med 2012;4:138ra180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lv H, Havari E, Pinto S, Gottumukkala RV, Cornivelli L, Raddassi K, Matsui T, Rosenzweig A, Bronson RT, Smith R, Fletcher AL, Turley SJ, Wucherpfennig K, Kyewski B, Lipes MA.. Impaired thymic tolerance to alpha-myosin directs autoimmunity to the heart in mice and humans. J Clin Invest 2011;121:1561–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA Jr, Anders RA, Sosman JA, Moslehi JJ.. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moreira A, Loquai C, Pföhler C, Kähler KC, Knauss S, Heppt MV, Gutzmer R, Dimitriou F, Meier F, Mitzel-Rink H, Schuler G, Terheyden P, Thoms K-M, Türk M, Dummer R, Zimmer L, Schröder R, Heinzerling L.. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur J Cancer 2019;106:12–23. [DOI] [PubMed] [Google Scholar]
- 54. Yamaguchi S, Morimoto R, Okumura T, Yamashita Y, Haga T, Kuwayama T, Yokoi T, Hiraiwa H, Kondo T, Sugiura Y, Watanabe N, Kano N, Kohno K, Fukaya K, Sawamura A, Yokota K, Ishii H, Nakaguro M, Akiyama M, Murohara T.. Late-onset fulminant myocarditis with immune checkpoint inhibitor nivolumab. Can J Cardiol 2018;34:812.e1–812.e3. [DOI] [PubMed] [Google Scholar]
- 55. Touat M, Maisonobe T, Knauss S, Ben Hadj Salem O, Hervier B, Auré K, Szwebel T-A, Kramkimel N, Lethrosne C, Bruch J-F, Laly P, Cadranel J, Weiss N, Béhin A, Allenbach Y, Benveniste O, Lenglet T, Psimaras D, Stenzel W, Léonard-Louis S.. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 2018;91:e985–e994. [DOI] [PubMed] [Google Scholar]
- 56. Mir H, Alhussein M, Alrashidi S, Alzayer H, Alshatti A, Valettas N, Mukherjee SD, Nair V, Leong DP.. Cardiac complications associated with checkpoint inhibition: a systematic review of the literature in an important emerging area. Can J Cardiol 2018;34:1059–1068. [DOI] [PubMed] [Google Scholar]
- 57. Imai R, Ono M, Nishimura N, Suzuki K, Komiyama N, Tamura T.. Fulminant myocarditis caused by an immune checkpoint inhibitor: a case report with pathological findings. J Thorac Oncol 2019;14:e36–e38. [DOI] [PubMed] [Google Scholar]
- 58. Koelzer VH, Rothschild SI, Zihler D, Wicki A, Willi B, Willi N, Voegeli M, Cathomas G, Zippelius A, Mertz KD.. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. J Immunother Cancer 2016;4:13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reuben A, Petaccia de Macedo M, McQuade J, Joon A, Ren Z, Calderone T, Conner B, Wani K, Cooper ZA, Tawbi H, Tetzlaff MT, Padera RF, Durand JB, Lazar AJ, Wargo JA, Davies MA.. Comparative immunologic characterization of autoimmune giant cell myocarditis with ipilimumab. Oncoimmunology 2017;6:e1361097.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Norwood TG, Westbrook BC, Johnson DB, Litovsky SH, Terry NL, McKee SB, Gertler AS, Moslehi JJ, Conry RM.. Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer 2017;5:91.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, Murayama T, Grimm M, Waaga AM, Ueno T, Padera RF, Yagita H, Azuma M, Shin T, Blazar BR, Rothstein DM, Sayegh MH, Najafian N.. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol 2007;179:5204–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang L, Han R, Hancock WW.. Programmed cell death 1 (PD-1) and its ligand PD-L1 are required for allograft tolerance. Eur J Immunol 2007;37:2983–2990. [DOI] [PubMed] [Google Scholar]
- 63. Grant MJ, DeVito N, Salama AKS.. Checkpoint inhibitor use in two heart transplant patients with metastatic melanoma and review of high-risk populations. Melanoma Manag 2018;5:MMT10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Owonikoko TK, Kumar M, Yang S, Kamphorst AO, Pillai RN, Akondy R, Nautiyal V, Chatwal MS, Book WM, Sahu A, Sica GL, Ahmed R, Ramalingam SS.. Cardiac allograft rejection as a complication of PD-1 checkpoint blockade for cancer immunotherapy: a case report. Cancer Immunol Immunother 2017;66:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bansal SS, Ismahil MA, Goel M, Patel B, Hamid T, Rokosh G, Prabhu SD.. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ Heart Fail 2017;10:e003688.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nevers T, Salvador AM, Grodecki-Pena A, Knapp A, Velazquez F, Aronovitz M, Kapur NK, Karas RH, Blanton RM, Alcaide P.. Left ventricular T-cell recruitment contributes to the pathogenesis of heart failure. Circ Heart Fail 2015;8:776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]