Abstract
Background
The association between respiratory syncytial virus (RSV) loads and clinical outcomes in children remains to be defined. In most studies, viral loads (VL) were evaluated in hospitalized children and at a single time-point. We investigated the relationship between VLs and disease severity in both outpatients and inpatients with RSV infection.
Methods
We enrolled previously healthy children with RSV infection. Disease severity was defined by level of care (outpatients vs ward vs pediatric intensive care unit [PICU]), and a clinical disease severity score (CDSS). Nasopharyngeal VLs by polymerase chain reaction and CDSS were measured at enrollment and daily in inpatients. VL decay according to disease severity was analyzed using linear mixed modeling.
Results
From February 2015 to March 2017, we enrolled 150 infants: 39 outpatients and 111 inpatients. VLs were higher in outpatients than in age-matched inpatients. Among inpatients, initial VLs were comparable in ward and PICU patients, and preceded the peak CDSS. However, after excluding infants treated with steroids, those hospitalized in the ward had higher VLs than infants requiring PICU care (P < .001). Dynamic analyses showed that VL decay was delayed in PICU patients, especially in those treated with steroids.
Conclusions
Higher VLs at presentation and a faster and consistent VL decline were both associated with less severe RSV disease in children.
Summary
Infants with less severe respiratory syncytial virus (RSV) disease had higher viral loads (VL) at presentation, and faster and consistent VL decline. Conversely, VL decay and overall viral exposure were prolonged and higher in infants severe RSV disease receiving steroids.
Keywords: respiratory syncytial virus; RSV, viral load, viral dynamics, clinical severity
Respiratory syncytial virus (RSV) is the number one cause of viral lower respiratory tract infection (LRTI) leading to hospitalization in infants worldwide, and the second cause of death in this age group [1, 2]. The burden of the disease, however, extends well beyond hospitalization, as it causes significant morbidity in the outpatient setting [3]. Despite RSV’s impact on child health, treatment remains supportive and there is no licensed vaccine, which is due in part to our incomplete understanding of the pathogenesis of the disease and its relation to disease severity.
The variability in clinical presentations is likely the result of a complex interplay between viral factors and the host immune response [4–9]. A number of studies with variable designs have examined the association between viral loads (VL) and disease severity, with conflicting results. While some authors found significant associations between RSV loads and clinical outcomes, others have not reproduced those findings [10–12]. Most of those studies, however, have been conducted in hospitalized infants, with RSV loads measured at a single time-point during the disease course, providing a snapshot of a dynamic process. A better understanding of the role of VL in children with mild RSV infection evaluated in the outpatient setting, and the interplay between viral dynamics and clinical disease severity would inform a targeted design for future intervention studies.
The aims of this study were (1) to define the differences in VLs between outpatients and hospitalized infants with RSV infection adjusted for age; and (2) to define the relationship between viral load dynamics and clinical disease severity in hospitalized infants with moderate (ward) and severe disease (pediatric intensive care unit [PICU]).
METHODS
Study Design
Healthy children <2 years of age hospitalized or evaluated in the outpatient setting for RSV infection were prospectively enrolled as a convenience sample at Nationwide Children’s Hospital (NCH; Columbus, Ohio) from 2015 to 2017. Exclusion criteria included duration of illness >7 days, prematurity (<36 weeks’ gestation), underlying medical conditions (ie, congenital heart disease, chronic lung disease, congenital or acquired immunodeficiency), use of immunomodulatory drugs (including systemic steroids >5 days within 2 weeks of presentation), previous history of wheezing or hospitalization for RSV bronchiolitis, and RSV hospitalization for apnea or social reasons. Inpatients were enrolled and samples obtained within a median of 16 hours (interquartile range [IQR], 9–23 hours) of hospitalization. Outpatients were enrolled at the emergency department, urgent care, and associated primary care clinics. Hospitalization was defined as a hospital admission for >24 hours. Viral diagnosis was established by rapid antigen testing (Sofia RSV Fluorescent Immunoassay; Quidel) or a multiplex polymerase chain reaction (PCR) panel (FilmArray Respiratory Viral Panel; BioFire) [13].
We collected in all study patients at enrollment: (1) demographic and clinical information including a standardized Clinical Disease Severity Score (CDSS) [14]; and (2) a midturbinate swab for RSV typing and quantitation by real-time PCR. In addition, hospitalized patients underwent daily sampling for RSV loads and disease severity evaluation using the CDSS, until discharge or for up to 7 days, whichever was first. Other parameters of disease severity were also collected in inpatients, including oxygen administration, PICU admission, need and duration of noninvasive and invasive positive pressure ventilation, and duration of hospitalization. The CDSS comprised 5 parameters, each ranking from 0 to 3, and included respiratory rate, auscultation, transcutaneous oxygen saturation, retractions, and level of activity (mainly assessed by feeding difficulty). The total score ranked from 0 (normal) to 15 (most abnormal) (Supplementary Table 1) [6, 7, 14]. Demographic and clinical information, including follow-up telephone calls at 2 and 4 weeks after discharge, were collected using standardized questionnaires designed for portable tablets, and information was automatically transferred to a secure database (REDCap) [15].
Initial analyses were performed with samples and clinical data obtained at enrollment from both outpatients and inpatients. Longitudinal analyses included sequential data from inpatients according to the admission unit (ward or PICU).
RSV Loads
Nasal midturbinate swabs were collected, transported in ice to the laboratory in viral transport media, centrifuged, and stored at –80°C [7, 14, 16]. In brief, RSV loads were measured by reverse-transcription (RT) quantitative PCR using known concentrations of RSV-A2 and RSV-B to derive a standard curve. Standards and negative controls were included and tested with each PCR assay. One-step RT-PCR (Qiagen QuantiTect) targeting the conserved region of the F gene was performed using described primers and probes [17, 18]. RSV quantification is reported as log10 copies/mL. All samples were also tested for other respiratory viruses by the FilmArray PCR panel [13].
Statistical Analysis
Continuous variables are presented as median (IQR) or mean ± standard deviation according to data distribution and compared using Mann–Whitney or t test, respectively. Categorical variables are presented as frequencies and compared using χ2 test. Mixed-effects linear regression models were built to assess the rate of VL and CDSS change over time, adjusted for duration of symptoms before enrollment. Two preplanned interaction effect models were constructed to determine whether the rate of change in VL differed by hospital unit or by duration of symptoms before enrollment. Sensitivity analyses were performed ad hoc to assess the potential effect of systemic steroids on VL decay and the CDSS. Raw data were used to calculate VL and CDSS area under the curve (AUC) using the midpoint rule, and group differences in AUC were assessed using 2-sample t tests, with a Satterthwaite correction for unequal group variance. Last, to determine which factors were predictive of worse clinical outcomes, we used multivariable logistic regression with Firth penalized likelihood when warranted to avoid small sample size bias. Analyses were conducted using SAS version 9.4 (SAS Institute) and GraphPad Prism version 6 (GraphPad Software), with a 2-sided P value < .05 considered statistically significant.
Ethical Considerations
This study was approved by the Institutional Review Board (IRB) at Nationwide Children’s Hospital (IRB number 14-00927), classified as a level 1 risk clinical study—no greater than minimal risk (pursuant under 45 Code of Federal Regulations [CFR] 46.404 and 21 CFR 50.51). Informed consent procedures followed in compliance with Nationwide Children’s Research Responsible Conduct Guidelines.
RESULTS
Characteristics of Study Patients
From February 2015 to March 2017, we enrolled 150 children <2 years of age with RSV infection. Of those, 39 (26%) were diagnosed with mild disease and managed as outpatients, while 111 required hospitalization for moderate/severe RSV LRTI.
Median age of outpatients was 6.1 months vs 2.2 months for inpatients, and 93% (140/150) were <12 months of age. Except for a greater proportion of black infants and a more frequent history of atopy in the outpatient cohort, no other differences in demographic information were observed between groups including history of breastfeeding, day care attendance, or secondhand smoke exposure (Table 1). CDSSs were higher in hospitalized children, and median duration of symptoms at enrollment was 4 days and comparable between cohorts. Families were contacted 2 and 4 weeks after enrollment and confirmed the lack of subsequent readmissions (inpatient group) or hospitalization (outpatient cohort).
Table 1.
Demographic, Clinical, and Virologic Data of All Children With Respiratory Syncytial Virus Infection
| Characteristic | Outpatients (n = 39) | Inpatients (n = 111) | P Value |
|---|---|---|---|
| Age, mo | 6.1 (3.7–8.6) | 2.2 (1.2–5.8) | < .001 |
| Female sex | 18 (46.6) | 56 (50.5) | .71 |
| Race | |||
| White | 23 (59) | 80 (72.1) | .02 |
| Black | 13 (33.3) | 13 (11.7) | |
| Other | 3 (7.7) | 17 (15) | |
| Gestational age, wk | 39 ± 1.4 | 39 ± 1.7 | .80 |
| Siblings/day care | 30 (76.9) | 97 (87.4) | .13 |
| Smoke exposure | 9 (23.1) | 37 (33.3) | .31 |
| Vaginal delivery | 31 (79.5) | 76 (68.5) | .22 |
| Breastfeda | 20 (51.3) | 40 (36) | .13 |
| Atopy history | 11 (28.2) | 14 (12.6) | .04 |
| Steroid useb | 5 (12.8) | 8 (7.2) | .32 |
| Vaccination status up-to-date | 33 (84.6) | 99 (89.2) | .39 |
| Days of symptoms | 4 (3–5) | 4 (3–5) | .76 |
| CDSS at enrollment | 4 (2–5) | 7 (5–9) | < .001 |
| Viral load, log10 copies/mL | 7.23 ± 1.2 | 6.48 ± 1.3 | .003 |
| Viral subtype | |||
| A | 26 (67) | 69 (62) | .72 |
| B | 13 (33) | 42 (38) | |
| Viral codetectionc | 11 (28) | 34 (31) | .84 |
| Rhinovirus/enterovirus | 5 | 18 | |
| Adenovirus | 0 | 5 | |
| Human metapneumovirus | 0 | 0 | |
| Influenza virus | 0 | 1 | |
| Parainfluenza virus | 2 | 2 | |
| Coronavirus | 4 | 11 |
Categorical data are expressed as frequencies (%) and analyzed using Fisher or χ2 test. Continuous data are expressed as median (interquartile range) or mean ± standard deviation and analyzed using Mann–Whitney rank test or Student t test accordingly Values in bold indicate significant 2-sided P values.
Abbreviation: CDSS, Clinical Disease Severity Score.
aBreastfed at any time since birth.
bSteroids received ≤5 days within 2 weeks of enrollment.
cNo bacterial coinfections were identified (FilmArray includes Bordetella pertussis, Chlamydia pneumoniae, and Mycoplasma pneumoniae).
Viral Load Comparisons Between Inpatients and Outpatients
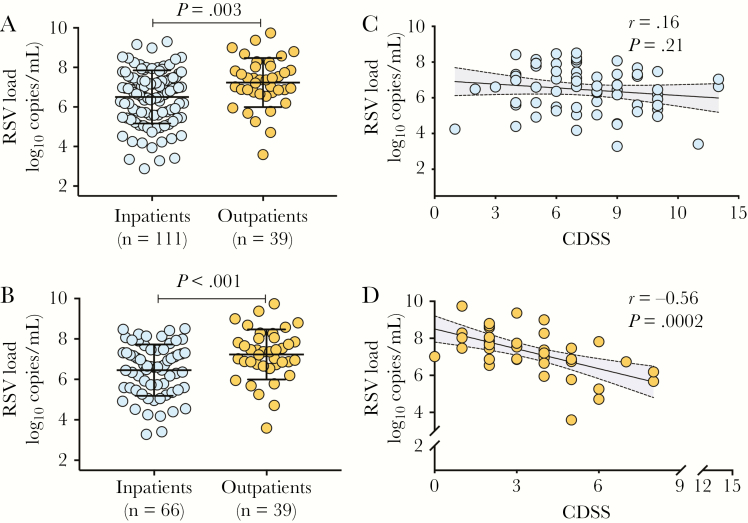
First, we conducted cross-sectional analyses and compared VL at enrollment between inpatients (moderate/severe disease), who were younger, and outpatients (mild disease). RSV loads were higher in outpatients vs inpatients (7.23 ± 1.2 vs 6.48 ± 1.3 log10 copies/mL, respectively; P = .003; Figure 1A), with no associations between VL and age (r = 0.04; P = .61). Overall, RSV-A was more frequently detected than RSV-B. Other respiratory viruses were identified in approximately 30% of infants, and at similar rates in outpatients and inpatients (Table 1).
Figure 1.
Comparison of respiratory syncytial virus (RSV) loads in children with mild or severe RSV infection. A, Nasopharyngeal swabs were obtained at enrollment, and RSV loads quantitated by polymerase chain reaction were compared between children requiring hospitalization (n = 111, blue dots) and those evaluated in the outpatient setting (n = 39; orange dots). B, Comparative analyses in RSV loads between inpatients and outpatients were adjusted for age, and 2 inpatients were matched for each outpatient. Graphs represent mean ± standard deviation and comparisons were made using Student t test (P < .05 considered significant). C, Correlations between the Clinical Disease Severity Score (CDSS) and RSV loads at enrollment in the age-matched inpatient cohort (n = 66). D, Correlation between CDSS and RSV loads at enrollment in outpatients. Each dot represents an individual patient. Abbreviations: CDSS, Clinical Disease Severity Score; RSV, respiratory syncytial virus.
To account for the possible influence of age on VL, we performed sensitivity analyses and selected approximately 2 age-matched inpatients (n = 66; median, 4.4 [IQR, 2.4–9.0] months) for each outpatient (n = 39; median, 6.1 [IQR, 3.7–8.6] months; Supplementary Table 2). These analyses confirmed that independent of age, RSV loads were almost 1 log higher in outpatients vs inpatients (Figure 1B). VLs were not associated with the CDSS in the inpatient cohort (Figure 1C), but correlated inversely with the CDSS in outpatients (r = –0.56; P = .0002; Figure 1D), and in the age-matched cohort of inpatients and outpatients (r = –0.4; P < .001).
Sequential Viral Loads and Clinical Dynamics in Hospitalized Children
Next, we explored differences in RSV loads and clinical parameters in the inpatient cohort separately. Of the 111 inpatients, 32 (29%) were severely ill requiring PICU care, while 79 (71%) were hospitalized in the ward with moderate disease. No differences in age (2.1 vs 2.3 months) or other demographic factors were identified between ward and PICU infants (Table 2). Chest radiographs were performed per standard of care in all PICU patients and in 58% of ward infants, with no differences in radiologic findings between groups. As expected, PICU infants had worse disease severity in all parameters evaluated including the CDSS, need and duration of supplemental oxygen, and total duration of hospitalization (1.9 vs 4 days for ward and PICU, respectively; P < .001). RSV types and initial RSV loads were similar in ward and PICU patients, who also had comparable duration of illness at enrollment.
Table 2.
Demographic, Clinical, and Virologic Data in Hospitalized Children With Respiratory Syncytial Virus Infection, by Admission Unit
| Characteristic | Ward (n = 79) | PICU (n = 32) | P Value |
|---|---|---|---|
| Age, mo | 2.1 (1–6.6) | 2.3 (1.6–4) | .94 |
| Female sex | 41 (52) | 15 (47) | .63 |
| Race | |||
| White | 55 (70) | 25 (78) | .66 |
| Black | 10 (12) | 3 (9) | … |
| Other | 14 (18) | 4 (13) | … |
| Days of symptoms | 4 (3–5) | 4 (2.5–5) | .18 |
| CDSS | 6 (4–7) | 10 (8.3–11) | < .001 |
| Chest radiograph | |||
| Normal | 3 (7) | 1 (3) | … |
| Bronchial wall thickening | 23 (51) | 10 (32) | .40 |
| Atelectasis/interstitial | 11 (24) | 15 (47) | … |
| Lobar consolidation | 8 (18) | 6 (18) | … |
| Total LOS, d | 1.9 (1.6–2.9) | 4 (3–7.6) | < .001 |
| Oxygen administration | 43 (52) | 32 (100) | < .001 |
| Duration of O2, d | 1 (1–2) | 3 (2.1–5) | < .001 |
| Intubation | 0 (0) | 5 (16) | NA |
| Viral load, log10 copies/mL | 6.6 ± 1.3 | 6.2 ± 1.4 | .24 |
| Viral subtype | |||
| A | 50 (63) | 19 (59) | .81 |
| B | 29 (37) | 13 (41) | … |
Categorical data are expressed as frequency (%) and analyzed using Fisher exact or χ2 test. Continuous data are expressed as median (interquartile range) or mean ± standard deviation and analyzed using Mann–Whitney rank test or Student t test accordingly. Values in bold indicate significant 2-sided P values.
Abbreviations: CDSS, Clinical Disease Severity Score; NA, not applicable; PICU, pediatric intensive care unit; LOS, length of stay; O2, supplemental oxygen.
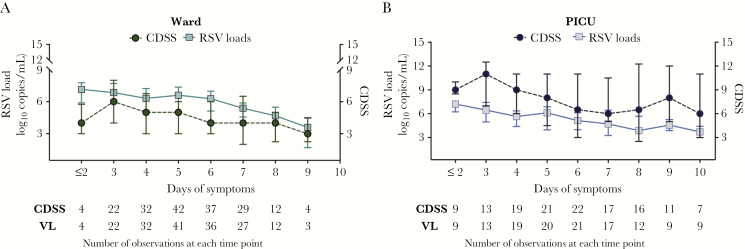
In hospitalized infants, VLs and the CDSS were measured daily during the hospitalization. First, the dynamics of these 2 variables were analyzed in parallel according to the admission unit (ward or PICU) and indexed to symptoms duration (Figure 2). In both ward and PICU infants, peak VLs were documented at the beginning of the illness (≤2 days of symptoms), whereas the peak of disease severity (worst CDSS) occurred on day 3 of symptoms. Data for the initial time point were limited as the number of patients with ≤2 days of symptoms at enrollment was small.
Figure 2.
Respiratory syncytial virus (RSV) dynamics and disease severity in hospitalized patients according to duration of illness and admission unit. Graphs represent the natural course of RSV bronchiolitis (viral load [VL] and Clinical Disease Severity Score [CDSS] dynamics) according to days of symptoms at the time of sampling, and not adjusted mathematically; ie, VLs measured on day 2 of hospitalization for a patient with 4 days of symptoms at the time of hospital admission would be plotted as day 6 of illness (4 days before hospitalization + 2 days of hospitalization). Children hospitalized in the inpatient ward are represented in green (A) and in the pediatric intensive care unit (PICU) in blue (B). Values for the CDSS and RSV loads represent median (interquartile range). Abbreviations: CDSS, Clinical Disease Severity Score; PICU, pediatric intensive care unit; RSV, respiratory syncytial virus; VL, viral load.
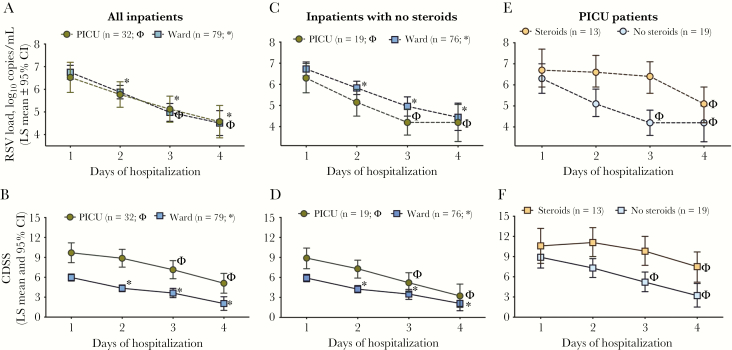
Next, to assess the change in VL over time according to day of hospitalization, as an objective reference value, we used linear mixed-effects models adjusted for duration of symptoms. We found a significant decline on VL of approximately 0.79 logs per day (P < .001) when including all hospitalized infants; this observation did not change significantly when the model was adjusted for duration of symptoms. Both unadjusted and adjusted models showed significantly lower VL on days 2–4 compared to day 1 (6.7 to 4.6 vs log10 copies/mL; Supplementary Table 3). When comparing viral dynamics according to the admission unit, we found that, compared to day 1 of hospitalization (baseline), VL significantly decreased by day 2 among ward patients and by day 3 in PICU infants, with no significant differences in the rate of change between units (P = .28; Figure 3A; Supplementary Table 4). The CDSS decreased approximately 1.4 units per day from baseline (P < .0001) when all inpatients were analyzed together. Similar to VL dynamics, the CDSS was significantly lower than baseline by day 2 of hospitalization in ward patients, and by day 3 in PICU infants (Figure 3B; Supplementary Table 4).
Figure 3.
Respiratory syncytial virus (RSV) dynamics and disease severity in hospitalized patients according to days of hospitalization. A and B, Linear mixed-effects model analyses in hospitalized patients were used to analyze viral load (VL) and Clinical Disease Severity Score (CDSS) decline over time according to day of hospitalization and adjusted for days of symptoms. Symbols (* for ward and Φ for pediatric intensive care unit [PICU]) represent a significant decline (P < .05) for that specific day compared with day 1 of hospitalization (baseline). Rate of decline, exact values, and interactions are specified in Supplementary Table 4. C and D, VL and CDSS decline over time according to day of hospitalization and adjusted for days of symptoms among hospitalized patients in the ward or PICU not treated with steroids during admission. E and F, VL and CDSS decline over time according to day of hospitalization and adjusted for days of symptoms and steroid use only in patients admitted to the PICU. Details of the exact numbers and P values for each time point are specified in Supplementary Table 6. Abbreviations: CDSS, Clinical Disease Severity Score; CI, confidence interval; LS, length of stay; PICU, pediatric intensive care unit; RSV, respiratory syncytial virus.
Impact of Systemic Steroids on Viral Load and Clinical Dynamics
Eight infants (PICU, n = 3 [9%]; ward, n = 5 [6%]) had received systemic steroids for ≤5 days at enrollment (Table 1). During hospitalization, 13 (41%) PICU and 3 (4%) ward patients were treated with steroids. To exclude the confounding impact of steroids on VL [19] and clinical dynamics, sensitivity analyses were conducted ad hoc in infants not treated with steroids during hospitalization (ward, n = 76; PICU, n = 19). In these children, rates of VL decline adjusted for symptoms duration, were comparable between ward (–0.83 ± 0.16 logs per day) and PICU infants (–0.80 ± 0.36; P = .75; Figure 3C), as well as CDSS decline rates (–1.36 ± 0.21 vs –1.89 ± 0.52 units per day for ward and PICU; P = .22; Figure 3D). Similar to the whole cohort (Figure 3A and 3B), a significant decline in VL and CDSS was observed 1 day earlier in ward (day 2) vs PICU (day 3). In addition, among this cohort of 95 infants not treated with steroids, VLs were lower in those admitted to the PICU vs the ward across all time-points evaluated (P < .001; Figure 3C).
To further analyze the effect of steroids exclusively in PICU infants, we compared VL and CDSS dynamics in infants treated (n = 13) and not treated (n = 19) with steroids during hospitalization. PICU infants treated with steroids had higher CDSS at enrollment, received mechanical ventilation more frequently, and had a longer duration of hospitalization compared with those who did not (Supplementary Table 5). VLs at enrollment were 6.8 ± 1.5 vs 5.9 ± 1.5 log10 copies/mL in the steroid and nonsteroid group, respectively (P = .08). The overall rate of VL decline did not differ according to steroid use (P = .41). However, VLs in the nonsteroid group were significantly lower than baseline by day 3 (rate of change, –0.80 logs per day), whereas among those treated with steroids, VL did not decline significantly from baseline until day 4 (rate of change, –0.54 logs per day; Figure 3E; Supplementary Table 6). Similar findings were observed with the CDDS; infants not treated with steroids had a significant decline from baseline earlier (day 3) than infants treated with steroids (day 4; Figure 3F; Supplementary Table 6).
Area Under the Curve Analyses
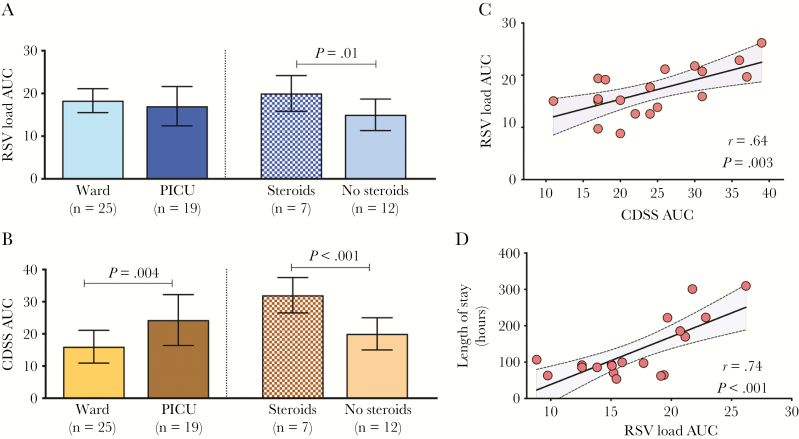
To quantify both the overall viral exposure and clinical disease severity, we calculated the AUC for VL and CDSS using raw sequential data. To limit the influence of patients with longer duration of hospitalization on AUC analyses, AUCs were calculated among 44 patients who had complete VL and CDSS data for the first 3 days of hospitalization (PICU = 19; ward = 25). Comparative analyses were also performed exclusively in PICU patients according to steroid treatment (steroids, n = 7; no steroids, n = 12), since only 3 of the 25 infants in the ward had received steroids. Rates of VL and CDSS decline in these 44 patients were almost identical to those reported in the complete cohort (Supplementary Table 7).
Viral load AUCs were similar in infants hospitalized in the ward and PICU. However, when PICU patients were stratified according to steroid treatment, those in the steroid group had higher VL AUC (P = .01; Figure 4A). As expected, CDSS AUCs were higher in PICU vs ward patients (P = .004), especially in children treated with steroids (P < .001; Figure 4B). We did not find significant associations between VL AUC and CDSS AUC or duration of hospitalization when including all 44 children (ward and PICU) or those hospitalized in the ward, irrespective of steroids treatment (data not shown). However, VL AUC in all PICU infants was significantly correlated with the CDSS AUC and duration of hospitalization (Figure 4C and 4D). These associations were lost when children treated with steroids were excluded from analyses.
Figure 4.
Respiratory syncytial virus (RSV) load and Clinical Disease Severity Score (CDSS) area under the curve (AUC) analyses. A and B, Representation of the differences in viral load (VL) and CDSS AUC between pediatric intensive care unit (PICU) (stratified by steroid use) and ward patients with RSV lower respiratory tract infection. Raw data were used for AUC analyses, and values calculated using the midpoint rule, for patients with complete data for the first 3 days of hospitalization. Differences in AUC were assessed using 2-samples t test (P < .05), with a Satterthwaite correction for unequal group variance. C and D, Correlations between VL AUC and CDSS AUC and VL AUC with length of stay exclusively in infants admitted to PICU; r represents the Pearson correlation coefficient. Dots represent the pair information for each patient. Abbreviations: AUC, area under the curve; CDSS, Clinical Disease Severity Score; PICU, pediatric intensive care unit; RSV, respiratory syncytial virus.
Multivariate Analysis
To further define the influence of VL and clinical parameters on outcomes of care in hospitalized infants, different multivariable models were constructed. The following covariates were selected based on significance in bivariate analyses or clinical grounds: age, steroid treatment, CDSS, and initial VL. We selected as clinical outcomes: supplemental oxygen administration, prolonged hospitalization (defined as >3 days, based on median length of stay), positive pressure ventilation, and PICU admission. While higher VLs were associated with lower need for supplemental oxygen, this was not the case for other outcomes. Higher CDSS was independently associated with all 4 clinical outcomes (Table 3).
Table 3.
Multivariable Analyses in All Hospitalized Patients
| Variable | Oxygen | LOS | PPVa | PICU | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age (mo) | 1.0 (.9–1.1) | .51 | 0.94 (.9–1.0) | .24 | 0.9 (.7–1.0) | .11 | 0.9 (.8–1.0) | .15 |
| CDSS | 1.7 (1.4–2.2) | < .001 | 1.7 (1.40–2.2) | < .001 | 2.1 (1.5–2.8) | < .001 | 1.9 (1.5–2.5) | < .001 |
| RSV loads | 0.7 (.5–1.0) | .04 | 1.0 (.7–1.4) | .88 | 1.3 (.8–2.1) | .34 | 0.8 (.5–1.3) | .37 |
| Steroid use | 0.4 (.1–3.0) | .38 | 1.5 (.2–9.9) | .66 | 0.5 (.05–5.2) | .56 | 0.99 (.1–9.1) | .99 |
For these models, we used RSV loads and the CDSS at enrollment. LOS was dichotomized as ≤3 vs >3 based on median LOS values. Oxygen need was defined as at least 12 hours of oxygen administration. Values in bold indicate significant 2-sided P values.
Abbreviations: CDSS, Clinical Disease Severity Score; CI, confidence interval; LOS, length of stay; PPV, positive pressure ventilation; OR: odds ratio; RSV, respiratory syncytial virus.
aPPV included noninvasive (high-flow nasal cannula), invasive ventilation (continuous positive airway pressure/synchronized inspiratory positive airway pressure), and endotracheal intubation.
DISCUSSION
In this prospective, single-center study, we showed that children with mild RSV infection evaluated in the outpatient setting had significantly higher VL than hospitalized patients. This was a consistent finding even after analyses were adjusted for age. Within the inpatient cohort, initial VLs were comparable between infants with moderate (ward) and severe (PICU) disease. However, when sequential data were analyzed, and adjusted for steroid use, higher VLs were also present in ward vs PICU infants at all time-points evaluated, and independently associated with lower supplemental oxygen needs. On the other hand, VL decay in PICU infants was delayed, especially in children treated with steroids, who also had evidence of worse disease at enrollment. Overall, these data suggest that both higher initial VL and faster and consistent viral clearance are associated with a less-severe clinical course in young children with RSV infection.
The mechanisms and directionality of the interaction between RSV loads and the host immune response are not well understood. In the outpatient population, there is limited information on the influence of RSV loads on disease severity, and the data derived from hospitalized children with severe disease are conflicting. It is possible that during severe disease some components of the inflammatory cascade elicit a more vigorous response, which could favor viral clearance and at the same time contribute to a more severe clinical disease phenotype. On the other hand, it could also be possible that higher RSV loads promote an early robust innate immune response that can protect from disease progression. Supporting the latter, and in agreement with our findings, a recent study conducted in older infants with RSV infection showed that those with milder disease not requiring hospitalization had higher RSV loads, and that VL was significantly correlated with mucosal concentrations of proinflammatory cytokines [5, 20].
Cross-sectional, large studies conducted in different settings and mostly among hospitalized patients have examined the association between VL and disease severity. While some of these studies found significant associations between VL and longer duration of hospitalization and PICU care [10, 11, 21–23], others did not [12, 24, 25]. These discrepancies could reflect differences in study design (ie, days of symptoms at sample collection), patients included (age, and presence of underlying comorbidities), different methods of sample collection, or the variability in the laboratory assays used for RSV quantification (plaque assay vs PCR), emphasizing the need to standardize these methods.
Information in regard to the dynamics of VL in infants with RSV infection is also limited, possibly due to the difficulty of performing daily sampling in these young children [10, 26, 27]. Two different studies of different sample sizes evaluated VL in children with RSV LRTI during the first 3 days of hospitalization or every other day during the first week of hospital stay. Those studies found that VL on day 3 of admission was associated with worse disease severity as defined by a clinical score [26], PICU admission, or respiratory failure [10], which differs from our findings. In those 2 studies, however, the duration of hospitalization was longer, from 6 to 7 days (compared with 2.3 days in our study), steroid use during hospitalization was not mentioned, and analyses were not adjusted for duration of symptoms, suggesting that the study population and inpatient management strategies differ between institutions. A recent study evaluated sequential VL and cytokine concentrations and mucosal gene expression profiling in 30 infants hospitalized with RSV LRTI. The study showed that intubated infants had lower VL and reduced interferon and CCL5/RANTES concentrations than children with moderate disease [27]. Although the study was small, not adjusted for steroid use, and included children with comorbidities, our findings agree with those of this latter study. Indeed, we found that higher RSV loads at enrollment were associated with a less-severe clinical course in both outpatients and inpatients, after adjusting for steroid use.
While the peak of VL (day 1) preceded the CDSS peak, which occurred on day 3 of symptom onset, the rate of RSV clearance in PICU patients was stagnant during the first 3 days, especially in infants treated with steroids. The limited data available in regard to the effect of systemic steroids on VL decay are derived from 2 small randomized, double-blind, placebo-controlled trials in children mechanically ventilated for RSV LRTI. While the first study (n = 22) did not show differences in viral decay according to steroid treatment [28], in the second study (n = 41), children treated with dexamethasone had a significantly slower decay in VL during the first 48 hours [19]. Our findings agree with the latter study, and although we measured viral loads by PCR, and not plaque assay, and in the upper respiratory tract, we also observed a slower viral decay during the first 72 hours of hospitalization in PICU infants treated with steroids.
Taken together, these data emphasize the complex interplay between VL dynamics and disease severity, and the possible influence of a variety of factors, which may help explain the varying results derived from different studies, where the proportion of mild and very severely ill patients varied, and daily sequential data were not evaluated. Moreover, steroid use is not mentioned in the majority of studies that have analyzed RSV load dynamics. Although with the current study design it not possible to determine whether steroids are responsible for the slower viral decay, indicative of sicker children, or a combination of both, our study suggests that this is an important cofounding factor [5, 6, 27] that may have contributed to the differences observed.
Our study has limitations. We did not perform sequential sampling in outpatients with milder RSV infection and thus the description of this cohort provides a snapshot of a continuous event. Nevertheless, these patients were contacted after enrollment, and none of them required hospitalization, confirming that their classification was appropriate. Another limitation is the small number of children enrolled with <3 days of symptoms. This is an intrinsic limitation of this disease and the current standard of care for children with mild respiratory infections. As mentioned before, there were significant age differences between outpatients and hospitalized infants. However, sensitivity analyses with age-matched inpatients and outpatients were conducted, confirming the results, and age was included as a key covariate in all multivariable models.
In conclusion, the peak of VL preceded the peak of clinical disease severity and was inversely related to clinical outcomes. While higher VL was associated with a milder clinical disease severity phenotype, a subset of patients with markedly severe disease and treated with steroids had a larger overall viral exposure and worst clinical outcomes. These are important observations that may help elucidate the optimal therapeutic window and target populations for future interventions for RSV LRTI.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all members of the clinical research team for their extraordinary efforts enrolling our patients; Dr Cohen’s team at the NCH emergency department, Dr Brian Bowden at NCH urgent care clinics, Dr Bill Long at Pediatric Associates in Pickerington, and Dr Darryl Robbins at Central Ohio Primary Care in Gahanna for their incredible support; and especially our patients and their families for their participation in the study.
Disclaimer. The funding agencies had no input into data analyses or interpretation of the study findings.
Funding. (1) Janssen (OBSERVERSV0006); National Institutes of Health: (2) National Institute of Allergy and Infectious Diseases (AI112524) to AM and OR; (3) National Center for Advancing Translational Sciencens (CCTS) UL1TR002733 to MMC.
Financial support. This work was supported by a research grant from Janssen. Some authors were supported by the National Institutes of Health (grant numbers AI112524 to A. M. and O. R. and UL1TR002733 to M. M. C.) at the Research Institute at Nationwide Children’s Hospital.
Potential conflicts of interest. A. M. and O. R. and have received research grants from Janssen. A. M. has received fees for participation in advisory boards from Janssen and lectures from AbbVie and Novartis. O. R. has received fees for participation in advisory boards from AbbVie, HuMabs, Janssen, Medimmune, Merck, and Regeneron, and lectures from Pfizer, Janssen, and Merck. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Pediatric Academic Societies Annual Meeting, San Francisco, California, May 2017.
References
- 1. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Russell CD, Unger SA, Walton M, Schwarze J. The human immune response to respiratory syncytial virus infection. Clin Microbiol Rev 2017; 30:481–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piedra FA, Mei M, Avadhanula V, et al. The interdependencies of viral load, the innate immune response, and clinical outcome in children presenting to the emergency department with respiratory syncytial virus-associated bronchiolitis. PLoS One 2017; 12:e0172953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mejias A, Dimo B, Suarez NM, et al. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med 2013; 10:e1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mella C, Suarez-Arrabal MC, Lopez S, et al. Innate immune dysfunction is associated with enhanced disease severity in infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis 2013; 207:564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodriguez-Fernandez R, Tapia LI, Yang CF, et al. Respiratory syncytial virus genotypes, host immune profiles, and disease severity in young children hospitalized with bronchiolitis. J Infect Dis 2017; 217:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brint ME, Hughes JM, Shah A, et al. Prolonged viral replication and longitudinal viral dynamic differences among respiratory syncytial virus infected infants. Pediatr Res 2017; 82:872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El Saleeby CM, Bush AJ, Harrison LM, Aitken JA, Devincenzo JP. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis 2011; 204:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hasegawa K, Jartti T, Mansbach JM, et al. Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: multicenter cohort studies in the United States and Finland. J Infect Dis 2015; 211:1550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mazur NI, Bont L, Cohen AL, et al. South African Severe Acute Respiratory Illness (SARI) Surveillance Group Severity of respiratory syncytial virus lower respiratory tract infection with viral coinfection in HIV-uninfected children. Clin Infect Dis 2017; 64:443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Babady NE, England MR, Jurcic Smith KL, et al. Multicenter evaluation of the ePlex respiratory pathogen panel for the detection of viral and bacterial respiratory tract pathogens in nasopharyngeal swabs. J Clin Microbiol 2018; 56:e01658–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suárez-Arrabal MC, Mella C, Lopez SM, et al. Nasopharyngeal bacterial burden and antibiotics: influence on inflammatory markers and disease severity in infants with respiratory syncytial virus bronchiolitis. J Infect 2015; 71:458–69. [DOI] [PubMed] [Google Scholar]
- 15. Melendi GA, Coviello S, Bhat N, Zea-Hernandez J, Ferolla FM, Polack FP. Breastfeeding is associated with the production of type I interferon in infants infected with influenza virus. Acta Paediatr 2010; 99:1517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Steenhuijsen Piters WA, Heinonen S, Hasrat R, et al. Nasopharyngeal microbiota, host transcriptome, and disease severity in children with respiratory syncytial virus infection. Am J Respir Crit Care Med 2016; 194:1104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ispas G, Koul A, Verbeeck J, et al. Antiviral activity of TMC353121, a respiratory syncytial virus (RSV) fusion inhibitor, in a non-human primate model. PLoS One 2015; 10:e0126959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bouzas ML, Oliveira JR, Queiroz A, et al. Acute Respiratory Infection and Wheeze Study Group Phase I and II Diagnostic accuracy of digital RNA quantification versus real-time PCR for the detection of respiratory syncytial virus in nasopharyngeal aspirates from children with acute respiratory infection. J Clin Virol 2018; 106:34–40. [DOI] [PubMed] [Google Scholar]
- 19. Buckingham SC, Jafri HS, Bush AJ, et al. A randomized, double-blind, placebo-controlled trial of dexamethasone in severe respiratory syncytial virus (RSV) infection: effects on RSV quantity and clinical outcome. J Infect Dis 2002; 185:1222–8. [DOI] [PubMed] [Google Scholar]
- 20. Nicholson EG, Schlegel C, Garofalo RP, et al. Robust cytokine and chemokine response in nasopharyngeal secretions: association with decreased severity in children with physician diagnosed bronchiolitis. J Infect Dis 2016; 214:649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis 2005; 191:1861–8. [DOI] [PubMed] [Google Scholar]
- 22. Fodha I, Vabret A, Ghedira L, et al. Respiratory syncytial virus infections in hospitalized infants: association between viral load, virus subgroup, and disease severity. J Med Virol 2007; 79:1951–8. [DOI] [PubMed] [Google Scholar]
- 23. Houben ML, Coenjaerts FE, Rossen JW, et al. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol 2010; 82:1266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin ET, Kuypers J, Heugel J, Englund JA. Clinical disease and viral load in children infected with respiratory syncytial virus or human metapneumovirus. Diagn Microbiol Infect Dis 2008; 62:382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wright PF, Gruber WC, Peters M, et al. Illness severity, viral shedding, and antibody responses in infants hospitalized with bronchiolitis caused by respiratory syncytial virus. J Infect Dis 2002; 185:1011–8. [DOI] [PubMed] [Google Scholar]
- 26. Zhou L, Xiao Q, Zhao Y, Huang A, Ren L, Liu E. The impact of viral dynamics on the clinical severity of infants with respiratory syncytial virus bronchiolitis. J Med Virol 2015; 87:1276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thwaites RS, Coates M, Ito K, et al. Reduced nasal viral load and IFN responses in infants with respiratory syncytial virus bronchiolitis and respiratory failure. Am J Respir Crit Care Med 2018; 198:1074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Woensel JB, Lutter R, Biezeveld M, et al. Effect of dexamethasone on tracheal viral load and interleukin-8 tracheal concentration in children with respiratory syncytial virus infection. Pediatr Infect Dis J 2003; 22:721–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.