Abstract
Background
Kaposi sarcoma (KS)–associated herpesvirus (KSHV) is etiologically linked to all KS forms, but mechanisms underlying KS development are unclear. The incidence of KS in human immunodeficiency virus type 1–infected (HIV-1+) individuals implicates immune dysregulation; however, the lack of characterization of KSHV immune responses in endemic KS makes the role of HIV-1 unclear. The study objective was to investigate the HIV-1 and KSHV roles in viral nucleic acid detection, antibody responses, and cytokine responses in polymerase chain reaction–confirmed epidemic KS and endemic KS patients and non-cancer controls from sub-Saharan Africa.
Methods
KSHV viral DNA (vDNA), total anti-KSHV antibody, KSHV neutralizing antibody (nAb), and cytokines were quantified.
Results
KSHV vDNA was detectable in tumors but variably in plasma and peripheral blood mononuclear cells. Consistent with elevated antibody-associated cytokines (interleukin [IL] 6, IL-5, and IL-10), nAb titers were higher in epidemic KS and endemic KS patients than in controls (P < .05). Despite HIV-1 coinfection in epidemic KS, nAb titers were similar between epidemic KS and endemic KS patients (P = 0.3).
Conclusions
Similarities in antibody and cytokine responses between epidemic and endemic KS patients suggest that KSHV drives KS pathogenesis, whereas HIV-1 exacerbates it.
Keywords: Kaposi, sarcoma, neutralizing antibody, KSHV, sub-Saharan Africa
Lack of immune response studies in African endemic Kaposi sarcoma (KS) makes the pathogenetic role of HIV-1 unclear. Similarities in antibody and cytokine responses between epidemic and endemic KS patients suggest that KSHV drives KS pathogenesis, whereas HIV-1 exacerbates it.
Kaposi sarcoma–associated herpesvirus (KSHV) is etiologically linked to all forms of Kaposi sarcoma (KS) [1, 2]. In sub-Saharan Africa, KSHV prevalence ranges from 30% to 90%, with higher levels in human immunodeficiency virus type 1 (HIV-1)–coinfected individuals [3–5]. Before the HIV-1/AIDS epidemic, African endemic KS comprised an estimated 4%–10% of African adult cancers [6–8]. However, with the HIV-1/AIDS epidemic, epidemic KS has become one of the most common tumors in sub-Saharan Africa [9–12].
The incidence of KS observed in HIV-1–coinfected individuals implicates immune dysregulation as an underlying mechanism [9–11]. The precise nature of this dysregulation and how it perpetrates KS remains poorly understood. During acute viral infections, production of neutralizing antibody (nAb) is often a correlate of protection or control, whereas viruses like Epstein-Barr virus and KSHV induce immune responses where high antibody (Ab) titers associate with disease [13, 14]. We previously reported higher prevalence and titers of KSHV nAb in Zambian epidemic KS patients vs asymptomatic controls [15]. Antibody responses in endemic KS patients are incompletely characterized. One study suggested higher levels of total anti-KSHV Ab in endemic KS patients vs HIV-1–seronegative patients with cancers other than KS [16]; however, nAb responses were not assessed.
Host cytokine alterations, particularly interleukin (IL) 6, have also been suggested to drive KS development in epidemic KS patients [17–19]. Our recent RNA-seq comparison of transcriptomes between KS tumors and normal tissues from the same individuals revealed elevated expression of CXCL-9, CXCL-10, CXCL-11, and transforming growth factor beta (TGF-β) in epidemic KS tumors [20]. HIV-1 coinfection and the presence of HIV-1 Tat protein in epidemic KS patients has also been proposed to contribute to cytokine dysregulations that promote KSHV reactivation and pathogenesis in vitro [21]; however, we did not detect tat transcripts in KS tumors [20]. Cytokine dysregulation and the role of innate immune mediators have not been studied in endemic KS patients.
We hypothesized that the prevalence and titer of nAbs and cytokines would correlate with high KSHV viral DNA (vDNA), but due to HIV-1 coinfection, the magnitude or breadth of responses would differ between the 2 types of KS. To test these hypotheses, Abs, nAbs, and cytokines were compared in 51 newly diagnosed KS patients and 80 noncancer controls from a region where both KSHV and HIV-1 are prevalent. The study highlights similarities between both types of KS while also revealing responses specific to each form. This comparison further refines our appreciation of the impact of KSHV and HIV-1 in the distinct clinical presentations of KS.
MATERIALS AND METHODS
Study Design, Subjects, and Samples
As part of larger ongoing projects, this cross-sectional study recruited 131 subjects (51 KS patients and 80 noncancer subjects) between 2016 and 2018 from Tanzania (n = 27) and Zambia (n = 104). Participants were aged >18 years and of both sexes. KS patients were newly diagnosed, and KS was histologically and polymerase chain reaction (PCR) confirmed. Due to reported 35% misdiagnosis of KS in the region following clinically based and/or histopathology-based (hematoxylin and eosin [H&E]) KS diagnosis [22, 23], we only included KS patients who were demonstrated to be vDNA positive by PCR from DNA extracted from tumor biopsies. KSHV and HIV-1 serology were the only criteria used to group the noncancer subjects.
Written consent was obtained from all study subjects. Peripheral blood samples (~10 mL) were collected in K2 ethylenediaminetetraacetic acid vacutainers, and peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (StemCell Technologies). Plasma and viable PBMCs were stored at –80°C. KS tumor biopsy samples (4 mm) were preserved in RNAlater solution (ThermoFisher) and frozen at –80°C. This study was approved by the review boards of the Tanzania National Institute for Medical Research, Ocean Road Cancer Institute, University of Zambia Biomedical Research Ethics Committee, and the University of Nebraska Medical Center and University of Nebraska–Lincoln.
HIV-1 Serology and Plasma Viral Load Quantification by Real-time PCR
Initial HIV-1 diagnosis was made according to Tanzania HIV Rapid Test Algorithm [24] and Alere Determine HIV-1/2 Ag/Ab Combo test in Zambia. HIV-1 serology results were verified using HIV-1–2.0 First Response kit (Premier Medical Corporation Ltd). HIV-1 viral RNA was extracted from plasma according to the QIAamp viral RNA extraction protocol (Qiagen). To quantify plasma HIV-1 copies, the RNA Ultra-Sense One-Step quantitative real-time PCR (qPCR) system (Applied Biosystems) was used as previously published [25]. Reaction composition and cycling parameters are listed in the Supplementary Materials.
DNA Extraction and PCR Detection of KSHV
Thawed plasma samples (400 µL) were centrifuged at 8000g at room temperature for 10 minutes to remove residual cells. Fifteen microliters of DNase-I (Qiagen) was added and then incubated (2 hours at room temperature). DNase-I was inactivated for 20 minutes at 65°C and virion-associated DNA was extracted according to the QIAamp DNA mini kit (Qiagen). The completeness of DNase-I treatment was demonstrated through a negative PCR result for the human β-actin gene. Viral DNA was detected by nested PCR using an open reading frame 26 (ORF26) amplicon. The TaqMan Universal PCR master mix (Applied Biosystems) was used to quantify KSHV copies by qPCR using a KSHV-ORF26 plasmid standard under the same conditions. Reaction composition and cycling parameters are listed in the Supplementary Materials.
Frozen biopsy tissue was cryopulverized in liquid nitrogen using a mortar and a pestle, and then extracted using a DNA purification kit (Qiagen). Amplification of human β-actin gene from the extracted DNA verified genomic DNA quality. The DNA from 8E5, a human T-lymphoblastoid cell line containing a single defective HIV-1 proviral genome, was used as standard for β-globin copies in qPCR. KSHV and human β-globin copies were quantified from equivalent amount of genomic DNA template (50 ng) in triplicate under the same conditions. Copies of β-globin were used to estimate cellular genome equivalents for quantifying KSHV copies per cell. Detection of KSHV vDNA was as described above.
rKSHV.219 Production
Vero.219 cells stably expressing green fluorescent protein from the KSHV genome during latency were used to generate recombinant KSHV (rKSHV.219), as previously described [26]. The rKSHV.219 was titered on HEK293T, and 105 50% Tissue culture Infective Dose (TCID50)/mL were used to test plasma for neutralization on the same cell line.
KSHV Serological Assays
KSHV serological status and Ab titer was determined by immunofluorescence assay (IFA) as described [27]. Slides were independently assessed by 2 readers. Neutralization assays were performed in triplicate as previously described [15]. In brief, heat-inactivated plasma (56°C for 60 minutes) was incubated at 1:50 dilution with rKSHV.219 at 37°C for 1 hour. HEK293T cells in 96-well plates were infected with virus–plasma mixture, centrifuged (400g for 20 minutes) and then incubated (37°C for 72 hours). Infection was quantified by flow cytometry at 72 hours postinfection. Plasma samples demonstrating >50% inhibition of infection compared to a negative control plasma were categorized as neutralizing. Samples that were KSHV nAb positive at the 1:50 dilution were reassayed in 2-fold dilutions of plasma from 1:50 to 1:800 to define the 50% inhibitory concentration.
Multiplex Bead-Based Immunoassay
Cytokines/chemokines in plasma were quantified using Becton Dickinson Cytometric Bead Array Flex Set kits. The standard sensitivity array included IL-4, chemokine CXCL10 (IP-10), IL-5, and TGF-β in picograms/mL. The enhanced sensitivity array included interferon gamma (IFN-γ), IL-1β, IL-6, IL-12p70, IL-17A, IL-10, and tumor necrosis factor alpha (TNF-α) in femtograms/mL. Raw data were collected and quantified on an Accuri C6 Plus cytometer (BD Biosciences) and analyzed with FlowJo version 10 software (TreeStar).
Flow Cytometry
Due to incomplete clinical records of CD4+ T-cell counts, flow cytometry was performed to calculate CD4+:CD8+ T-cell ratios and gauge immunosuppression in HIV-1–infected subjects. Ratios of 2 ± 0.8 are normal, and lower ratios indicate CD4+ T-cell suppression. Viable PBMCs were thawed, washed, and stained (10 minutes, at room temperature in the dark) with the following antibodies: hCD3-Percp.Cy5.5, hCD4-PE, and hCD8-FITC (BD Biosciences). Raw data were quantified by DxP10 (Cytek Biosciences) and analyzed with FlowJo version 10 software.
Statistical Analysis
We have compared viral nucleic acid detection, antibody, and cytokine responses between epidemic and endemic KS patients. To determine cancer-specific changes, we compared HIV-1+KSHV+ vs epidemic KS patients and HIV-1–KSHV+ vs endemic KS patients. To determine whether there are statistically significant differences between groups, we used one-way analysis of variance. A nonparametric Mann–Whitney U test was used to assess differences between comparison groups. Correlation of biological parameters between groups was done by nonparametric Spearman correlation analysis. GraphPad Prism 5 and SAS version 9.2 (SAS Institute) software was used for statistical analyses. All tests were 2-tailed, and P values < .05 were considered significant.
RESULTS
Characteristics of the Study Cohort
The study cohort was comprised of 131 subjects: 51 KS patients and 80 noncancer control subjects. KS patients included 34 epidemic KS and 17 endemic KS patients. Noncancer controls were comprised of 20 HIV-1–KSHV–, 27 HIV-1–KSHV+, 11 HIV-1+KSHV–, and 22 HIV-1+KSHV+ patients (Table 1). While the age distributions of the noncancer control groups and epidemic KS patients were similar (Table 1), the endemic KS patients were significantly older than both (P = .05 and P = .0009, respectively). The majority of the endemic KS patients (94.1%) were male, consistent with previously published reports of male predominance [28]. A greater proportion of epidemic KS patients were on antiretroviral therapy (ART) at KS diagnosis compared with HIV-1+KSHV+ controls (88.2% vs 59.1%, respectively; P = .01). Despite the high rate of ART use in epidemic KS patients, CD4+ T-cell reconstitution was suboptimal as evidenced by a significantly lower median CD4+:CD8+ T-cell ratio vs endemic KS patients or HIV-1–KSHV– controls (P < .0001). Median CD4+:CD8+ T-cell ratios were not significantly different between epidemic KS patients and HIV-1+KSHV+ controls (P = .3) or between endemic KS patients, and HIV-1–KSHV+ or HIV-1–KSHV– controls (P = .4). Epidemic KS patients self-reported shorter KS duration prior to seeking medical attention than endemic KS patients (5 vs 12 months, respectively; P = .003; Table 1). No statistically significant difference in any of the studied parameters was evident between subjects from the 2 countries.
Table 1.
Characteristics of the Study Cohort
| Noncancer Controls | Kaposi Sarcoma Patients | P Values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | HIV-1–KSHV– (n = 20) | HIV-1–KSHV+ (n = 27) | HIV-1+KSHV– (n = 11) | HIV-1+KSHV+ (n = 22) | Endemic KS (n = 17) | Epidemic KS (n = 34) | HIV-1–KSHV+ vs Endemic KS | HIV-1+KSHV+ vs Epidemic KS | Endemic KS vs Epidemic KS |
| Median age, y (range) | 35 (21–52) | 28 (21–60) | 40 (24–50) | 38 (29–68) | 51 (24–87) | 37 (21–60) | .0009 | .5 | .05 |
| Male sex, No. (%) | 7 (35) | 13 (48.1) | 5 (45.5) | 11 (50) | 16 (94.1) | 19 (55.9) | .002 | .7 | .006 |
| ART treatment, No. (%) | 9 (81.8) | 13 (59.1) | 30 (88.2) | .01 | |||||
| Median ART duration, mo (range) | 4 (0.25–144) | 9 (0.1–48) | 5 (0.1–120) | .8 | |||||
| Median CD4+:CD8+ T-cell ratio | 1.58 | 1.56 | 0.23 | 0.20 | 1.27 | 0.18 | .4 | .2 | < .0001 |
| Individuals with plasma HIV-1 detection (range in copies/mL) | 3 (<50–1.7 × 106) | 10 (<50–1.4 × 107) | 14 (<50–1.7 × 106) | .8 | |||||
| Median KS duration, mo (range) | 12 (3–108) | 5 (5–96) | .003 | ||||||
| Median self-reported HIV-1 duration, mo (range) | 12 (1–140) | 12 (0.5–120) | 4 (0.3–120) | .8 | |||||
–“–” denotes negative and “+” denotes positive. Values in bold type indicate significant differences.
Abbreviations: ART, antiretroviral therapy; HIV-1, human immunodeficiency virus type 1; KS, Kaposi sarcoma; KSHV, Kaposi sarcoma–associated herpesvirus.
Comparison of KSHV Viral DNA Detection in Epidemic KS and Endemic KS Patients
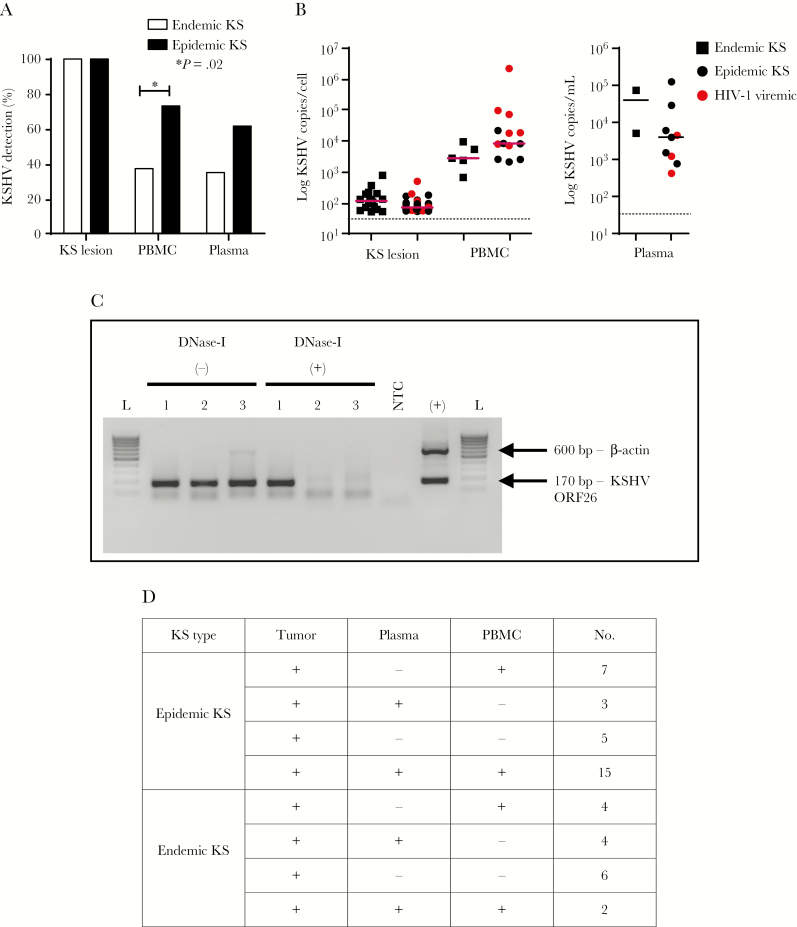
To investigate whether KSHV was being differentially produced or distinctly compartmentalized between epidemic KS and endemic KS patients, KSHV vDNA levels were compared in KS lesions, plasma, and PBMCs by PCR for KSHV-ORF26. As anticipated, vDNA was detected in all tumor tissues from both epidemic KS and endemic KS patients (Figure 1A). However, vDNA detection was significantly higher in PBMCs from epidemic KS vs endemic KS patients (P = .02), with a similar trend in plasma (P = .08). Surprisingly, HIV-1 plasma viremia in epidemic KS patients was not associated with KSHV-vDNA detection in any sampled compartment/tissue. Despite detectable vDNA in PBMCs and in plasma by nested PCR, KSHV copies were below the qPCR detection limit of 50 copies for several of the samples (Figure 1B). To ensure detection of authentic virion-associated DNA, we treated plasma with DNase I to remove vDNA from degraded infected cells before extraction. We verified this treatment by showing the extracted plasma to be devoid of amplifiable cellular DNA (Figure 1C). Consistent with ART treatment, most epidemic KS patients had undetectable plasma HIV-1 viral load. Overall, we observed a variable pattern of KSHV vDNA detection in plasma and PBMCs from both epidemic KS and endemic KS patients, highlighting inconsistent vDNA detection in these compartments (Figure 1D). Our results suggest that sampling KS tumor is the most reliable method for KSHV vDNA detection.
Figure 1.
A, Kaposi sarcoma (KS)–associated herpesvirus (KSHV) prevalence in KS tumor biopsies, plasma, and peripheral blood mononuclear cells (PBMCs) of KS patients (34 epidemic KS [human immunodeficiency virus type 1 {HIV-1}–positive Kaposi sarcoma] and 17 endemic KS [HIV-1–negative Kaposi sarcoma]), as determined by nested polymerase chain reaction (PCR) of KSHV open reading frame 26 (ORF26) DNA. B, Viral DNA (vDNA) copies in KS tumors, PBMCs, and plasma of KS patients as determined by real-time quantitative PCR (qPCR) of KSHV-ORF26. In some of the vDNA detectable PBMCs and plasma samples, vDNA copies were below the qPCR detection limit of 50 copies. Dotted line indicates qPCR detection limit. C, Human β-actin and KSHV-ORF26 (600 bp and 170 bp, respectively) duplex PCR products in 1.5% agarose gel. Forty nanograms of DNA extracted with or without DNase-I treatment of plasma was used as template for PCR reactions. Lane 1, sample number 3122 (40 ng); lane 2, sample number 3129 (40 ng); lane 3, sample number 3136; (+), positive control, BC-3 DNA (40 ng); L, 100 bp DNA ladder. D, vDNA detection patterns in KS tumor biopsies, plasma, and PBMCs of KS patients (34 epidemic KS and 17 endemic KS), as determined by nested PCR of KSHV-ORF26 DNA. Four epidemic KS samples and 1 endemic KS PBMC sample were not available. *P = .02. Abbreviations: HIV-1, human immunodeficiency virus type 1; KS, Kaposi sarcoma; KSHV, Kaposi sarcoma–associated herpesvirus; NTC, no template control; ORF26, open reading frame 26; PBMC, peripheral blood mononuclear cell.
Comparison of Total Anti-KSHV and nAb Responses in Epidemic KS and Endemic KS Patients
Because humoral responses correlate with levels of viral antigen in many viral infections, we examined whether vDNA correlated with total and KSHV nAb and whether HIV-1 coinfection led to differentials in the magnitude of responses. Total anti-KSHV Abs were titrated by IFA on KSHV chronically infected BC-3 cells. Both epidemic KS and endemic KS patients had significantly higher anti-KSHV Ab titers than noncancer controls (P < .05; Figure 2A). As anticipated, higher anti-KSHV Ab titers were detected in both KS groups as compared to their cognate noncancer controls (P < .05; Figure 2A). Interestingly, despite HIV-1 coinfection in epidemic KS patients, the levels of anti-KSHV Ab titers were comparable between epidemic KS and endemic KS patients (P = .8) (Figure 2A). Nevertheless, among the epidemic KS patients, those with detectable plasma HIV-1 viremia presented significantly lower anti-KSHV Ab titers compared to HIV-1 aviremic patients (P = .0004) (Figure 2B). However, anti-KSHV Ab titer did not correlate with KSHV vDNA detection in plasma or in PBMCs of KS patients. We could not establish whether anti-KSHV Ab titer correlated with KSHV vDNA copies in plasma or in PBMCs as the vDNA quantity was below the qPCR detection limit for most samples.
Figure 2.
Immunofluorescence assay for total anti– Kaposi sarcoma (KS)–associated herpesvirus (KSHV) antibody from plasma of KS patients and noncancer control subjects (reciprocal endpoint plasma dilution). A, Anti-KSHV antibody titer in plasma of KS patients and noncancer control subjects. B, Anti-KSHV antibody titer in human immunodeficiency virus type 1 plasma viremic and aviremic epidemic KS patients. Abbreviations: HIV-1, human immunodeficiency virus type 1; KSHV, Kaposi sarcoma–associated herpesvirus.
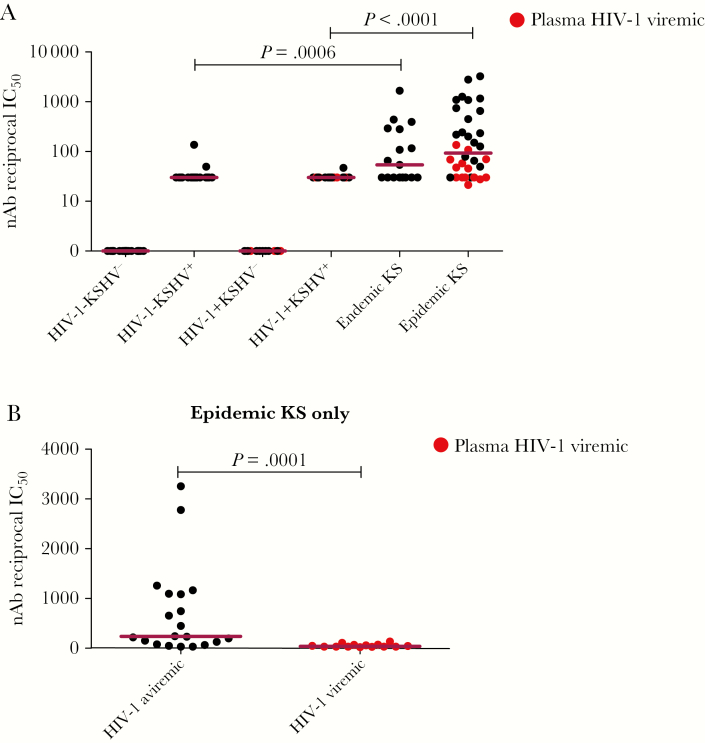
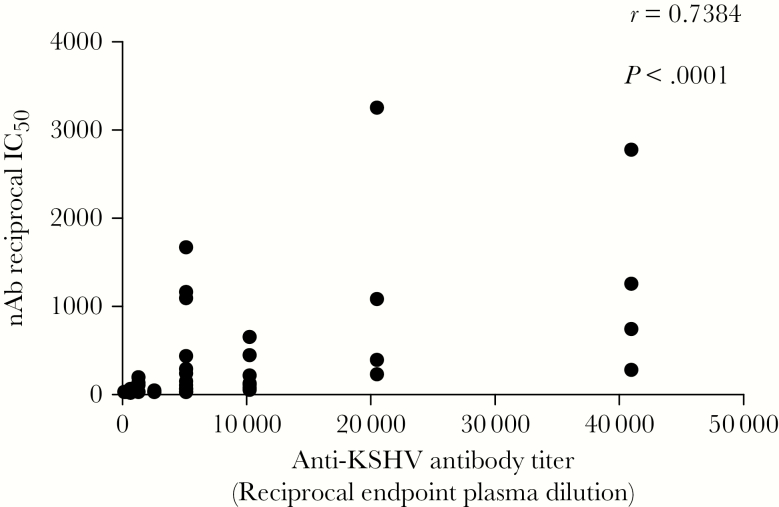
A KSHV neutralization assay with flow cytometric read-out was used to quantify KSHV nAb responses and determine whether differences between epidemic KS and endemic KS patients exist. Endemic KS patients mounted high KSHV nAb responses similar to those in epidemic KS patients (P = .3). KS patient responses were higher than those in their cognate noncancer controls (P < .05; Figure 3A). The majority of KSHV-seropositive noncancer controls showed <50% KSHV neutralization at a 50-fold dilution. Importantly, epidemic KS patients with plasma HIV-1 viremia had significantly lower KSHV nAb titers compared with HIV-1 aviremic cases (P = .0001; Figure 3B). However, KSHV nAb titer did not correlate with plasma or PBMC vDNA detection. There was a significant correlation between total KSHV Ab titer and KSHV nAb titer in both epidemic KS and endemic KS patients (r = 0.7384, P < .0001; Figure 4). The concordance between KSHV nAb titer and total KSHV Ab suggests that the latter may be a prerequisite for the development or maturation of nAb. Together, our data suggest that endemic KS and epidemic KS patients mount similar anti-KSHV and KSHV nAb responses, despite HIV-1 coinfection in epidemic KS.
Figure 3.
Kaposi sarcoma (KS)–associated herpesvirus (KSHV) neutralizing antibody (nAb) titer in plasma of KS patients and noncancer control subjects, presented as reciprocal of 50% inhibitory concentration (IC50). A, KSHV nAb titer in plasma of KS patients and noncancer control subjects. Plasma samples that were nAb positive at 1:50 dilution were reassayed in 2-fold dilutions of plasma from 1:50 to 1:800 to define the IC50. The IC50 values in reciprocal were used for plotting. All of the KSHV-seronegative samples were assigned a value of 1 in reciprocal IC50 in graphical depictions, and KSHV-seropositive samples with <50% KSHV neutralization at 1:50 dilution were assigned a value of 30 in reciprocal IC50 plots. B, KSHV nAb titer in human immunodeficiency virus type 1 plasma viremic and aviremic epidemic KS patients. Abbreviations: HIV-1, human immunodeficiency virus type 1; IC50, 50% inhibitory concentration; KSHV, Kaposi sarcoma–associated herpesvirus; nAb, neutralizing antibody.
Figure 4.
Correlation of plasma anti– Kaposi sarcoma (KS)–associated herpesvirus (KSHV) antibody and KSHV neutralizing antibody in epidemic KS, endemic KS, and noncancer controls by nonparametric Spearman correlation analysis. Abbreviations: IC50, 50% inhibitory plasma concentration; KSHV, Kaposi sarcoma–associated herpesvirus; nAb, neutralizing antibody.
Comparison of Cytokine Expression in Epidemic KS and Endemic KS Patients
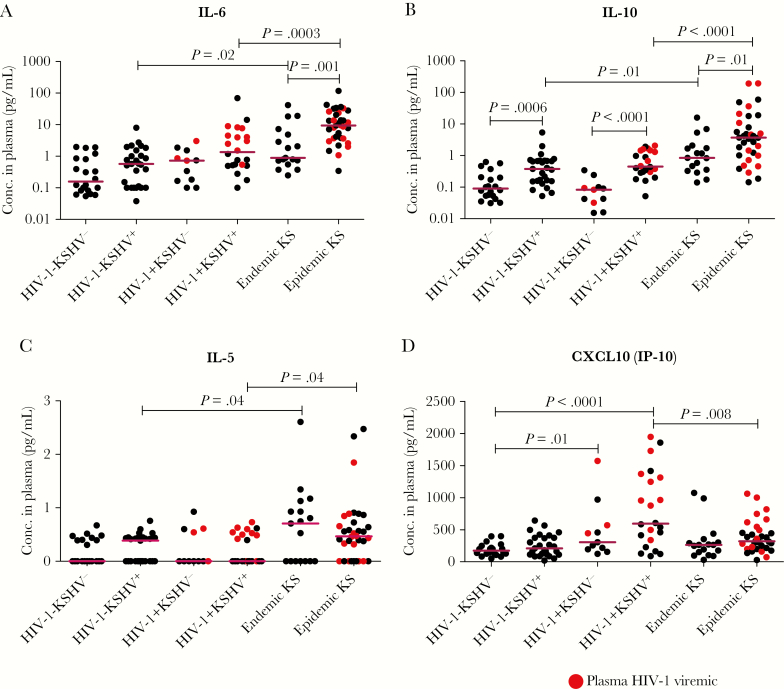
A dysregulated cytokine milieu has been suggested in epidemic KS development [17–19, 29, 30], but is also a hallmark of HIV-1 disease [31–33]. As their role in endemic KS has not been evaluated, we sought to quantify several cytokines and chemokines and compare those responses vs epidemic KS in an effort to differentiate effects due to HIV-1 from those associated with KS malignancy. A diverse panel of 11 cytokines/chemokines was selected based on reported association with KS, KSHV, HIV-1, and general viral infection (IL-17A, IFN-γ, IL-12p70, IL-1β, and TNF-α) [18, 34]. We included CXCL10 and TGF-β as they were reported in other cancer conditions and were highly expressed in our recent RNA-seq analysis of epidemic KS tumors [20, 35]. Also, due to observed high antibody responses in both forms of KS, antibody-associated cytokines were also included (IL-5, IL-4, IL-6, and IL-10) [36–39]. Consistent with previous reports, our data showed that IL-6 was elevated in both forms of KS compared to noncancer controls (P < .05; Figure 5A). This elevation was also significantly higher in epidemic KS than endemic KS patients (P = .001). Consistent with a role for IL-6 in B-cell proliferation and differentiation [39], we observed elevated anti-KSHV Ab responses in epidemic KS and endemic KS patients. We also observed a near-significant trend toward increased IL-6 in KSHV-seropositive noncancer controls, suggesting a KSHV-specific response (P = .05) (Figure 5A). Similarly, IL-10, an immunoregulatory cytokine that also associates with increased Ab responses [36, 37], was elevated in both epidemic KS and endemic KS patients compared with noncancer controls (P < .05; Figure 5B). However, despite similar KSHV Ab responses between epidemic KS and endemic KS patients, IL-10 was significantly higher in epidemic KS than endemic KS patients (P = .01). Like IL-6, IL-10 levels appeared to be KSHV associated, as they were also significantly elevated in KSHV-seropositive noncancer controls (P < .05; Figure 5B).
Figure 5.
Levels of significantly different cytokines/chemokines in plasma of Kaposi sarcoma (KS) patients and noncancer control subjects. A, Interleukin (IL) 6. B, IL-10. C, IL-5. D, CXCL10. Abbreviations: Conc., concentration; HIV-1, human immunodeficiency virus type 1; IL, interleukin; CXCL10 (IP-10), C-X-C motif chemokine 10 (Interferon gamma-induced protein 10); KSHV, Kaposi sarcoma–associated herpesvirus.
Furthermore, IL-5, a hallmark of antibody-associated Th2 effector cell function [38], was similarly elevated in both epidemic KS and endemic KS patients compared with noncancer controls (P < .05; Figure 5C). However, IL-5 exhibited dichotomous expression within KS patient and noncancer control groups, suggesting the possibility of other stimuli, such as parasitic infections. Unlike other analytes, the levels of chemokine CXCL10 were found to associate with HIV-1 infection. CXCL10 was elevated specifically in HIV-1–infected noncancer controls and epidemic KS patients (Figure 5D), and particularly in patients with detectable HIV-1 plasma viremia, consistent with previous reports [33]. Levels of CXCL10 in endemic KS patients were similar to those of noncancer controls. Similar to our recent RNA-seq analysis of epidemic KS tumor tissues, CXCL-10 and TGF-β were significantly elevated in plasma of epidemic KS [20] (Figure 5D and Supplementary Figure 1A). As some of the cytokines in the panel were known to associate with increased Ab responses, we performed correlation analyses to test for associations between cytokine responses and Ab production. IL-6, IL-10, and IL-5 were found to significantly correlate with KSHV nAb titers in KS patients (r = 0.4707, P < .0001; r = 0.6193, P < .0001; and r = 0.2386, P = .007, respectively; Figure 6A–C). Other tested analytes such as IL-4, IL-17A, IFN-γ, and TNF were below the detection limit for most samples, and IL-1β and IL-12p70 demonstrated no significant difference between groups (Supplementary Figure 1B and 1C). Overall, our data suggest the existence of a similarly dysregulated circulating cytokine/chemokine environment in epidemic KS and endemic KS patients despite the presence of HIV-1 coinfection in epidemic KS patients.
Figure 6.
Correlation of plasma cytokine levels and Kaposi sarcoma (KS)–associated herpesvirus (KSHV) neutralizing antibody (nAb) titers in KS patients and noncancer control subjects by nonparametric Spearman correlation analysis. Interleukin 6 (A), interleukin 10 (B), and interleukin 5 (C) vs KSHV nAb. Abbreviations: Conc., concentration; IC50, 50% inhibitory plasma concentration; IL, interleukin; nAb, neutralizing antibody.
DISCUSSION
We report here the first in-depth comparison of KSHV immune responses between epidemic KS, endemic KS patients, and noncancer controls from sub-Saharan Africa. Importantly, this comparative approach offered an opportunity to dissect the roles of KSHV and HIV-1 coinfection in KS pathogenesis in a way that could not be accomplished by studying epidemic KS alone. Our findings revealed remarkable similarities in KSHV vDNA detection and immune parameters between the epidemic and endemic forms of KS, but also highlighted some intriguing distinctions in cytokine responses that require further investigation.
In this study, KSHV was detectable in all KS tumors with comparable vDNA load despite longer self-reported duration of KS in endemic KS compared with epidemic KS patients (P = .003). This shorter KS duration in epidemic KS patients suggested increased awareness of opportunistic conditions in HIV-1 by clinicians and by individuals infected with HIV-1 visiting care and treatment centers. It is possible that a more aggressive disease course in epidemic KS led the patients to seek medical attention earlier compared to a more indolent disease progression in endemic KS patients. Furthermore, the observed inconsistent KSHV-vDNA detection pattern in comparison to the 100% detection rate in KS tumor suggests that directly sampling of KS tumors for the presence of vDNA by PCR is the most reliable method. From the reported high rates of KS misdiagnosis from the standard diagnostic (clinical and H&E) methods in the region [22, 23], PCR detection of KSHV vDNA from the tumors should be considered as a complement to histological and immunohistochemical diagnosis of KS. On a cautionary note, we have found that most of the PCR-amplifiable KSHV vDNA in plasma is from degraded infected cells and not virion associated.
Our study is the first to quantify KSHV nAb responses in African endemic KS patients. We found that both epidemic KS and endemic KS patients have similar KSHV nAb prevalence and titers and were higher than noncancer controls. Importantly, epidemic KS patients with detectable HIV-1 viremia and low median CD4+:CD8+ T-cell ratio had significantly lower anti-KSHV Ab and KSHV nAb titers compared with HIV-1 aviremic patients, suggesting a possible role of T-cell help in KSHV nAb maturation that needs to be further investigated. Also, as we have previously suggested for epidemic KS [15], it appeared that KSHV viremia was necessary to drive maturation of KSHV nAb–producing B cells specific to KSHV in both forms of KS patients. Nevertheless, humoral immune responses appear to lack protective efficacy against KS. Whether such responses, especially KSHV nAb, could be protective against KS if present prior to KSHV infection needs to be investigated.
Similar to several reports of elevated IL-6 in epidemic KS patients, we found that IL-6 was also elevated in endemic KS patients compared with cognate controls (P = .02). Since IL-6 is known to enhance proliferation of KSHV-infected cells, it could contribute to development and maintenance of KS tumors. Our analyses of the immune responses in both KS groups suggest the presence of a robust humoral response that is induced or maintained by a plasma cytokine environment that appears to be skewed toward Ab production. This is supported by the elevation of other Ab-associated cytokines like IL-10 and IL-5 in both epidemic KS and endemic KS patients. Nevertheless, the cytokine environment does not seem to favor profound inflammation or cytotoxic T-lymphocyte responses. Unfortunately, it appears that generation of KSHV nAb, much like in the case of HIV-1 infection, appears too late in disease to be efficacious in preventing or limiting tumorigenesis. Furthermore, HIV-1 infection induces chronic inflammatory response with dysregulation of cytokines despite ART [40]. For instance, HIV-1 induces inflammation through increased CXCL10 production [33]. In epidemic KS patients and HIV-1+ noncancer controls, CXCL10 was elevated, particularly in plasma of HIV-1 viremic individuals, suggesting chronic immune activation by low level of HIV-1 replication in latent reservoirs [41, 42]. Together, our findings suggest that KS is largely due to chronic immune activation derived from KSHV infection or from KS tumorigenesis and that HIV-1 coinfection exacerbates the disease.
The unique strength of our study is the comparative investigation of immune responses in sub-Saharan Africa epidemic KS and endemic KS patients and noncancer controls. Previous studies have focused almost exclusively on epidemic KS or on classical KS. In a few instances, comparisons have been made between epidemic KS and classical KS, though typically in geographical regions with different KSHV and HIV-1 prevalence patterns than sub-Saharan Africa. Our study has begun the assessment of immune responses in endemic KS patients, but the limited recognition of endemic KS patients and the late disease presentation when they were identified have limited the depth of our investigations. Studies on KSHV cell-mediated responses, especially on endemic KS patients, are warranted to fully characterize the immune responses. Longitudinal analyses of the current cohort as they undergo KS- and HIV-1–specific treatment will help to illuminate additional potential immune markers of treatment response and long-term control.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all study participants for agreeing to participate in this study, as well as all workers at Ocean Road Cancer Institute (Tanzania) and the Dermatology and Venereology Section, University Teaching Hospital (Zambia) for recruiting participants, procuring initial samples, and managing data.
Financial support. This work was supported by the National Cancer Institute, US National Institutes of Health (grant numbers RO1 CA75903, U54 CA190155, and U54 CA221204); the Fogarty International Center (grant number D43 TW010354); and the National Institute of General Medical Sciences (grant number P30 GM103509 to C. W.). O. N. is a Fogarty fellow.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: International Conference on EBV and KSHV, Madison, Wisconsin, 28 July–1 August 2018.
References
- 1. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994; 266:1865–9. [DOI] [PubMed] [Google Scholar]
- 2. Moore PS, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and those without HIV infection. N Engl J Med 1995; 332:1181–5. [DOI] [PubMed] [Google Scholar]
- 3. Dollard SC, Butler LM, Graves Jones AM, et al. Substantial regional differences in human herpesvirus 8 seroprevalence in sub-Saharan Africa: insights on the origin of the “KS Belt”. Int J Cancer 2010; 127:2395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nalwoga A, Cose S, Wakeham K, et al. Association between malaria exposure and Kaposi’s sarcoma-associated herpes virus seropositivity in Uganda. Trop Med Int Heal 2015; 20:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Newton R, Labo N, Wakeham K, et al. Kaposi sarcoma–associated herpesvirus in a rural Ugandan cohort, 1992–2008. J Infect Dis 2018; 217:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McHardy J, Williams EH, Geser A, de-Thé G, Beth E, Giraldo G. Endemic Kaposi’s sarcoma: incidence and risk factors in the West Nile district of Uganda. Int J Cancer 1984; 33:203–12. [DOI] [PubMed] [Google Scholar]
- 7. Slavin G, Cameron HM, Singh H. Kaposi’s sarcoma in mainland Tanzania: a report of 117 cases. Br J Cancer 1969; 23:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cook-Mozaffari P, Newton R, Beral V, Burkitt DP. The geographical distribution of Kaposi’s sarcoma and of lymphomas in Africa before the AIDS epidemic. Br J Cancer 1998; 78:1521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bohlius J, Valeri F, Maskew M, et al. Kaposi’s sarcoma in HIV-infected patients in South Africa: multi-cohort study in the antiretroviral therapy era HHS Public Access. Int J Cancer 2014; 135:2644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rohner E, Valeri F, Maskew M, et al. Incidence rate of Kaposi sarcoma in HIV-infected patients on antiretroviral therapy in southern Africa: a prospective multi-cohort study. J Acquir Immune Defic Syndr 2014; 67:547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ngalamika O, Minhas V, Wood C. Kaposi’s sarcoma at the University Teaching Hospital, Lusaka, Zambia in the antiretroviral therapy era. Int J Cancer 2015; 136:1241–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Semeere A, Wenger M, Busakhala N, et al. A prospective ascertainment of cancer incidence in sub-Saharan Africa: the case of Kaposi sarcoma cancer medicine. Cancer Med 2016; 5:914–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coghill AE, Hildesheim A. Epstein-Barr virus antibodies and the risk of associated malignancies: review of the literature. Am J Epidemiol 2014; 180:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wakeham K, Johnston WT, Nalwoga A, et al. Trends in Kaposi’s sarcoma-associated herpesvirus antibodies prior to the development of HIV-associated Kaposi’s sarcoma: a nested case-control study. Int J Cancer 2015; 136:2822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar P, Kuwa NY, Minhas V, et al. Higher levels of neutralizing antibodies against KSHV in KS patients compared to asymptomatic individuals from Zambia. PLoS One 2013; 8:e71254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ziegler J, Newton R, Bourboulia D, et al. Uganda Kaposi’s Sarcoma Study Group Risk factors for Kaposi’s sarcoma: a case-control study of HIV-seronegative people in Uganda. Int J Cancer 2003; 103:233–40. [DOI] [PubMed] [Google Scholar]
- 17. Giffin L, West JA, Damania B, Biron CA. Kaposi’s sarcoma-associated herpesvirus interleukin-6 modulates endothelial cell movement by upregulating cellular genes involved in migration. MBio 2015; 6:e01499-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Host KM, Jacobs SR, West JA, et al. Kaposi’s sarcoma-associated herpesvirus increases PD-L1 and proinflammatory cytokine expression in human monocytes. MBio 2017; 8:e00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uldrick TS, Wang V, O’Mahony D, et al. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without multicentric Castleman disease. Clin Infect Dis 2010; 51:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tso FY, Kossenkov AV, Lidenge SJ, et al. RNA-Seq of Kaposi’s sarcoma reveals alterations in glucose and lipid metabolism. PLoS Pathog 2018; 14:e1006844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou F, Xue M, Qin D, et al. HIV-1 tat promotes Kaposi’s sarcoma-associated herpesvirus (KSHV) vIL-6-induced angiogenesis and tumorigenesis by regulating PI3K/PTEN/AKT/GSK-3b signaling pathway. PLoS One 2013; 8:e53145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Bogaert L-J. Clinicopathological proficiency in the diagnosis of Kaposi’s sarcoma. ISRN AIDS 2012; 2012:565463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amerson E, Woodruff CM, Forrestel A, et al. Accuracy of clinical suspicion and pathologic diagnosis of Kaposi sarcoma in East Africa. J Acquir Immune Defic Syndr 2016; 71:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. United Republic of Tanzania, Ministry of Health and Social Welfare, National AIDS Control Program. Guidelines on HIV testing and counseling in clinical settings. 2007. http://www.who.int/hiv/topics/vct/TZ_PITC-Guidelines_ final edit_July2007.pdf Accessed July 26, 2017. [Google Scholar]
- 25. Tso FY, Kang G, Kwon EH, et al. Brain is a potential sanctuary for subtype C HIV-1 irrespective of ART treatment outcome. PLoS One 2018; 13:e0201325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vieira J, O’Hearn PM. Use of the red fluorescent protein as a marker of Kaposi’s sarcoma-associated herpesvirus lytic gene expression. Virology 2004; 325:225–40. [DOI] [PubMed] [Google Scholar]
- 27. Minhas V, Crosby LN, Crabtree KL, et al. Development of an immunofluorescence assay using recombinant proteins expressed in insect cells to screen and confirm presence of human herpesvirus 8-specific antibodies. Clin Vaccine Immunol 2008; 15:1259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Host KM, Horner M-J, Van Der Gronde T, et al. Kaposi’s sarcoma in Malawi: a continued problem for HIV-positive and HIV-negative individuals. AIDS 2017; 31: 318–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dirk P. Dittmer and blossom Damania. Kaposi sarcoma–associated herpesvirus: immunobiology, oncogenesis, and therapy. J Clin Investig 2016; 126:3165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Purushothaman P, Uppal T, Sarkar R, Verma SC. KSHV-mediated angiogenesis in tumor progression. Viruses 2016; 8. doi:10.3390/v8070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kahle EM, Bolton M, Hughes JP, et al. Plasma cytokine levels and risk of HIV type 1 (HIV-1) transmission and acquisition: a nested case-control study among HIV-1-serodiscordant couples. J Infect Dis 2015; 211:1451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reuter MA, Pombo C, Betts MR. Cytokine production and dysregulation in HIV pathogenesis: lessons for development of therapeutics and vaccines. Cytokine Growth Factor Rev 2012; 23:181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simmons RP, Scully EP, Groden EE, et al. HIV-1 infection induces strong production of IP-10 through TLR7/9-dependent pathways. AIDS 2013; 27:2505–17. http://www.ncbi.nlm.nih.gov/pubmed/24096630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rojas JM, Avia M, Martín V, Sevilla N. IL-10: a multifunctional cytokine in viral infections. J Immunol Res 2017; 2017: 6104054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Landskron G, De Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Review article chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014; 2014:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001; 19:683–765. [DOI] [PubMed] [Google Scholar]
- 37. Mathers AR, Cuff CF. Role of interleukin-4 (IL-4) and IL-10 in serum immunoglobulin G antibody responses following mucosal or systemic reovirus infection. J Virol 2004; 78:3352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kouro T, Takatsu K. IL-5-and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol 2009; 21:1303–9. [DOI] [PubMed] [Google Scholar]
- 39. Dienz O, Eaton SM, Rey J, et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4 + T cells. J Exp Med 2009; 206:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Albino E, Godoy L, Hill M. Markers of chronic immune activation in HIV patients receiving antiretroviral therapy. J Immunol 2016; 196(1 Suppl):217–39.26582947 [Google Scholar]
- 41. Lorenzo-Redondo R, Fryer HR, Bedford T, et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 2016; 530:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Couturier J, Suliburk JW, Brown JM, et al. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. AIDS 2015; 29:667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.