Abstract
Inflammation participates in the pathogenesis of both cancer and cardiovascular disease. This review examines the mechanistic commonalities between these two scourges of humanity through the lens of inflammation biology. Inflammatory pathways contribute to the initiation, the progression, and the complication of both malignant tumours and atherosclerotic plaques. Modulation of inflammatory pathways have proven transformative in the treatment of cancers and have crossed the threshold of clinical reality as treatments to reduce the risk of cardiovascular events. The finding that clonal haematopoiesis drives both leukaemia and cardiovascular events provides yet another link between these two seemingly disparate diseases. The nascent specialty of cardio-oncology has initially focused on the cardiovascular complications of cancer therapies. The recognition of a more profound pathophysiologic connection between cancer and cardiovascular diseases should expand the concept of cardio-oncology. Embracing the mechanistic connection and transcending traditional barriers between disciplines offers immense opportunities for speeding innovative research that can address the growing burden of both cancer and cardiovascular disease.
Keywords: Inflammation, Cancer, Aging, Cardiovascular disease, Cardio-oncology
This article is part of the Spotlight Issue on Cardio-oncology.
As long ago as the mid-19th century Virchow1 and other keen observers linked inflammation to cancer and to cardiovascular disease. They drew these inferences from morphologic findings and careful deductive reasoning. Recently, the scientific bases supporting these conjectures has expanded markedly due to advances on several scientific fronts. The molecular characterization of inflammatory modulators, both protein (e.g. cytokines, chemokines, and haematopoietic growth factors) and lipid (e.g. pro-inflammatory or pro-resolving), provided tools for rigorous experimental work and evidence-based postulation of pathways. The elucidation of transcriptional programmes for the control of the expression of these modulators and their production [e.g. nuclear factor kappa B (NF-κB)] added a layer of mechanistic understanding to the inflammatory response. The elucidation of supramolecular intracellular structures such as the proteasome and the inflammasome yielded further insight into the regulation of immune and inflammatory responses.2,3 The explosion of knowledge of cellular immunity—both innate and adaptive—has enabled deep insight into the intricate network of host defenses and their operation in many diseases.4
These advances have provided a firm scientific foundation for inflammation biology, yet the application of these principles to human disease has remained fragmented. The field of inflammation and immunity in cardiovascular diseases has flourished. Tumour immunobiology has made enormous strides and the clinical translation of its findings has already transformed the practice of haematology and oncology. Indeed, many excellent contemporary publications refresh the venerable connections between cancer, cardiovascular disease, and inflammation.5–8 But these two edifices—cardiovascular and cancer medicine—stand in solitude to the detriment of the potential of freer interchange and more rapid progress in science and in harvesting its clinical benefits.
Here, we make the case that in very fundamental ways, the pathogenesis of these scourges of humanity share important pathophysiologic elements. The convergence of cytokine biology in these two diseases provides one type of important mechanistic connection. That blockade of the pro-inflammatory cytokine interleukin (IL)-1β can reduce both cardiovascular events and cancer incidence and mortality underscores the contribution of inflammation to both cardiovascular conditions and malignancy.9–11 The emergence of clonal haematopoiesis as a common and age-related risk factor for cardiovascular disease and for haematologic malignancies provides another novel link between these two categories of disease traditionally separated by disciplinary divisions, distinct literatures, and separate cultures. (See the contribution of Patel and Natarajan12,13 to this series for further details regarding clonal haematopoiesis.) The age dependence of clonal haematopoiesis may contribute to the strong relationship of age with both cardiovascular disease and cancer.
The nascent specialty of cardio-oncology has evolved as a concerted strategy to address the cardiovascular complications of cancer therapies. The emergence of cardio-oncology has already begun to ease the traditional boundaries. Here, we argue that recognition of a more profound pathophysiologic commonality between cancer and atherosclerosis should expand the concept of cardio-oncology, and contribute to the growing co-operation between practitioners of two traditionally disparate disciplines. Broadening the concept of cardio-oncology can certainly yield benefits to patient care in the short term. Moreover, embracing the mechanistic connections and transcending traditional barriers between disciplines offers immense opportunities for speeding innovative research that can address the growing burden of both cancer and cardiovascular disease, particularly as we face a global aging of the population. The commonalities between cancer and atherosclerosis operate on many levels: disease initiation, the growth and progression of lesions that likely involves a prolonged ebb and flow in both circumstances, and the invasion, propagation, and complications of the respective lesions, processes that share mechanistic similarities.
1. Disease initiation
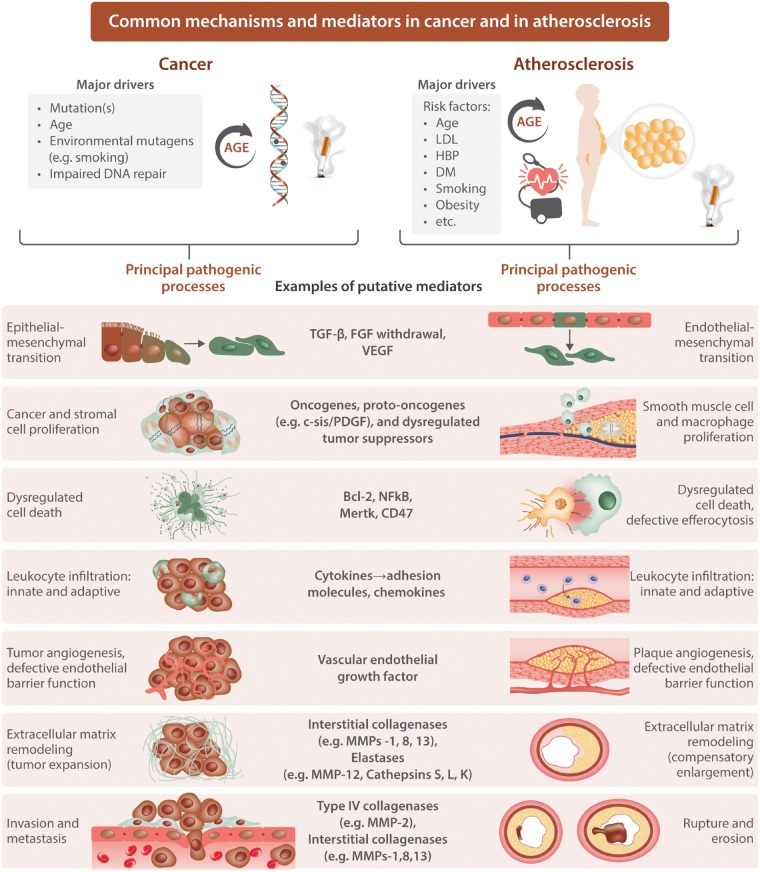
Current thinking attributes acquired genetic alterations as an initial pathogenic step in carcinogenesis (Figure 1, top left). Many mutations deemed causal for cancers evoke inflammatory responses. Examples include the oncogenes RET, Ras, Myc, and Src. Several authoritative reviews provide details regarding the ability of mutations in these oncogenes to elicit overproduction of pro-inflammatory mediators.6,14 Estimates in the literature attribute the origin of approximately a fifth of cancers directly to inflammatory stimuli. Vice versa, inflammation itself may trigger mutagenesis that can cause cancer. Notorious examples include overproduction of reactive oxygen species extracellularly (e.g. from hypochlorous acid produced by the plentiful granulocyte enzyme myeloperoxidase) or from within the cell due to mitochondrial overproduction.
Figure 1.
Common mechanisms and mediators in cancer and in atherosclerosis. Cancer and atherosclerosis share many common pathological mechanisms as depicted. Numerous mediators implicated in these two seemingly diverse diseases operate in both. FGF, fibroblast growth factor; MMP, matrix metalloproteinase; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor beta; VEGF, vascular endothelial growth factor.
Substantial findings from observational epidemiology link adiposity to cancer (reviewed in15). Numerous plausible mechanisms can explain how inflammatory mediators derived from adipose tissue may drive oncogenesis. Infections such as Helicobacter pylori, human papillomavirus, Epstein–Barr virus, and hepatitis viruses link infection to oncogenesis. Many environmental stimuli elicit inflammation and can prove mutagenic and hence carcinogenic. Examples include asbestos promoting mesothelioma, and particulates and cigarette smoke as aetiologic agents in lung cancer. Not only pathogen-associated molecular patterns but intrinsically derived damage-associated molecular patterns (DAMPs) can elicit inflammatory responses that can promote malignant transformation of cells.
Many of the stimuli listed above can activate members of the inflammasome family. The prototypical NLRP3 inflammasome generates active IL-1β and IL-18 from their inactive precursors. This mechanism provides insight into some of the molecular machinery that links carcinogenic conditions and agents with increased inflammation that may drive mutagenesis and other aspects of early carcinogenesis within the microenvironment of the nascent malignancy. Many pro-inflammatory cytokines act by activating NF-κB, which in turn can generate further cytokines providing an amplification loop for inflammatory processes. The role of NF-κB in combatting cell death also likely contributes to tumour progression as described below (Figures 1 and 2).
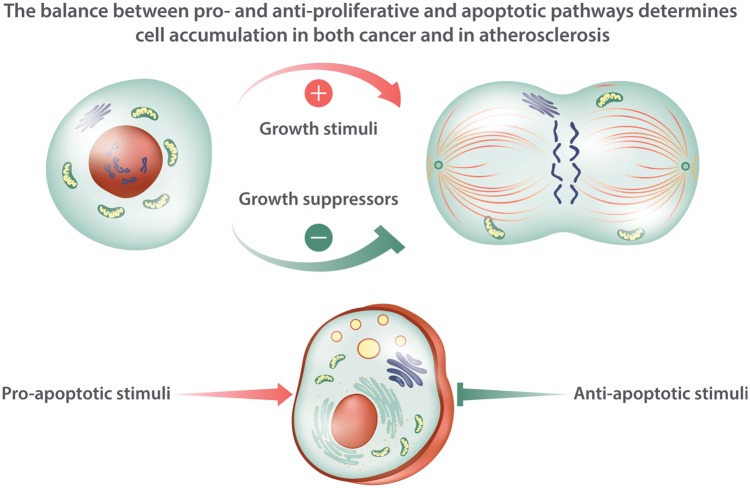
Figure 2.
Regulation of cell accumulation in cancer and in atherosclerosis. The accumulation of cells in both cancer and atherosclerosis depends on the balance of growth stimulators and growth suppressors. In addition, some mediators promote cell death including programme cell death by apoptosis, and other pathways oppose cell death. The text and Figure 1 mention some of the mediators involved in regulating this balance between proliferation, cytostasis, and cell death.
Recent work has highlighted the contribution of epithelial to mesenchymal transition as a key element malignant transformation and invasive behaviour of tumours16 (Figure 1). A number of inflammatory mediators can participate in epithelial to mesenchymal transition. Epigenetic alterations that can promote oncogenesis may also operate subject to modulation by inflammatory mediators. Thus, inflammation can participate in the earliest phases of carcinogenesis through several routes.
The initiation of atheroma formation resemble in many, but not all, circumstances outlined above for carcinogenesis. Somatic mutations probably play a minor role during human atherogenesis. (An exception, worthy of exploration might be accelerated athero- or arterio-sclerosis following radiation, e.g. for Hodgkin’s disease.) Yet, many of the factors that drive mutagenesis implicated in carcinogenesis overlap substantially with contributors to early malignant transformation. Situations that promote chronic inflammation associate with adverse cardiovascular events17 (Figure 1, top right). Drivers of the chronic inflammation associated with increased risk of atherosclerotic events include visceral adiposity, certain acute and chronic infections, oxidative stress, and diabetes. Cigarette smoking represents another common risk factor for cancer and cardiovascular disease.
In parallel with epithelial to mesenchymal transition in carcinogenesis, many investigators accord a role for endothelial-mesenchymal transition in the generation of arterial diseases18 (Figure 1). Epigenetic alterations linked to inflammatory processes and oxidative stress also likely contribute to early atherogenesis.19 Indeed, cardiovascular events attributable to some of the mutations that cause clonal haematopoiesis likely arise from epigenetic regulation of pro-inflammatory gene expression.13 These parallels illustrate the pathways shared between many cancers and atherosclerotic cardiovascular disease even if the initial trigger events may vary with mutagenesis less prominent in atherosclerosis than in cancer.
2. Disease progression
Accumulation of bulk characterizes both advanced solid tumours and atherosclerotic plaques. Simplistic early formulations focused primarily on cellular proliferation as a mechanism contributing to the volume of both tumours and atheromata.20,21 A much more elaborate and subtle construction has replaced the simple focus on proliferation. The mass of both malignant tumours and atheromata depends not only on the malignant cells or arterial smooth muscle cells but also on a complex and malleable extracellular matrix (ECM). The proliferative stimuli for the cancer cell, tumour stromal cells, or the arterial smooth muscle cell may vary, but overlap considerably, particularly in the downstream signal transduction (often via kinases) and transcriptional control (cell-cycle control) mechanisms (Figure 1).
Malignant transformation implies autonomous growth. An oft-invoked stimulus for smooth muscle cell proliferation, platelet-derived growth factor, arises from the simian sarcoma virus proto-oncogene Sis. Yet, although smooth muscle cells can elaborate the c-Sis gene product,22 no consistent evidence suggests that a mutation in the oncogene confers autonomous growth upon human arterial smooth muscle cells during atherogenesis. In addition to the malignant cells or accumulating vascular smooth muscle cells and ECM, stromal cells contribute to the bulk of tumours and also participate actively in creating the tumour microenvironment that can foster or frustrate growth, evolution, and the invasive behaviour of tumours (Figure 2).
Leucocyte invaders also contribute decisively to the progression of tumours as well as of atherosclerotic lesions (Figure 1). Tumour-infiltrating mononuclear phagocytes of various subtypes have garnered much interest in this regard. These cells can engage in cross talk with other leucocyte classes such as dendritic cells or CD8+ cytotoxic lymphocytes.23 Many excellent reviews have pursued this topic in depth.6,14 A similar focus on mononuclear phagocytes dominated thinking about leucocyte participation in atherosclerosis for several decades. The heterogeneity of mononuclear phagocytes also contributes to the pathogenesis of cardiovascular diseases including atherosclerosis, myocardial infarction, and heart failure.24 In both tumour biology and atherosclerosis research, the role of the polymorphonuclear leucocyte has gained foothold as another leucocytic participant in the pathogenesis of both cancer and the complications of atherosclerosis. A large literature also supports the operation of other leucocyte lineages in both oncogenesis and atherogenesis and allied conditions.6,25
Beyond proliferation of cells within the tumour or atheroma, considerable defects in cell death or in clearance of dead cells (efferocytosis) may contribute to cell accumulation within the lesions of both diseases. (Figures1and2) As described above, pro-inflammatory cytokines generally activate NF-κB which can exert anti-apoptotic actions through augmenting expression of Bcl-2 and Bcl-xL (Figures1and2). The cellular accumulation depends not only on proliferation but also on impaired clearance of cells through apoptosis and other forms of cell death. Avoidance of immune attack and evading suppression through well-documented tumour suppressive mechanisms likely play a greater role in cancer than in atherosclerosis. Yet, regulatory T cells furnish one source in the atheroma for transforming growth factor-beta that can prove cytostatic under some circumstances (Figure 2).26
The recruitment of leucocytes to tumours and to plaques share considerable commonality (Figure 1).27,28 Adhesion molecules for leucocytes contribute in both cases and the locomotion of adherent cells into the lesions depends on the action of chemokines. Once resident in the lesions, be they malignant tumours or atheromata, the cells can amplify local immune responses and modify the local microenvironment through release of growth factors, reactive oxygen species, proteinases, and angiogenic factors. Systemic inflammatory responses in both the malignant state and in atherosclerotic individuals can stimulate haematopoiesis in the bone marrow from whence cells that enter lesions derive.29
Not only innate but also adaptive immunity operate prominently during both oncogenesis and atherogenesis. T cells of various subtypes can promote or mute the development of both types of lesions.6,30–32 In cancer, immune checkpoints confer one mechanism for avoiding immune attack that can enhance survival of malignant cells. Cytokines can elicit the expression of Fas in human smooth muscle cells, heightening their sensitivity to death through apoptosis.33 Indeed, harnessing anti-cancer therapies to target T lymphocyte activation might prove useful in limiting atherogenesis.34,35
Remodelling of the ECM characterizes both oncogenesis and atherogenesis (Figure 1). The expansion of a tumour and of an atherosclerotic plaque requires an alteration in the ECM to accommodate to the expansion. In the case of atherosclerotic plaques, a compensatory outward remodelling of arteries maintains lumen caliber until the later stages of the disease. A multidimensional relationship between cancer cells, stromal cells, and the ECM contributes to the particular microenvironment of a given tumour. Similar interactions between the ECM and endothelial and smooth muscle cells also govern the remodelling of atherosclerotic lesions.
As championed by the late Judah Folkman, angiogenesis promotes tumour growth. Rich plexi of neovessels also occur in human atherosclerotic lesions (Figure 1).36 A number of mediators and mechanisms of angiogenesis have emerged. Some angiogenic factors such as vascular endothelial growth factor (VEGF) have become targets for anti-tumour therapy. Changes in metabolic programming arise in both cancer and atherosclerosis. Hypoxia characterizes the centre of both growing tumours and of atherosclerotic plaques alike.37 Regions of low oxygen tension through hypoxia inducible factor and inactivation of the tumour suppressor von Hippel Lindau augment glycolysis and drive the production of angiogenic factors such as VEGF. During the progression of tumours and of atherosclerotic plaques alike, local thrombosis as evidenced by accumulations of fibrin may contribute to the evolution of the respective lesions.38,39
During tumour progression and atherogenesis alike, defective resolution of inflammation due to chronic pro-inflammatory stimulation or ineffective of pro-resolving mediators including lipid mediators of resolution and anti-inflammatory cytokines such as IL-10 may occur.40,41 An increasing interest in the relationship of the microbiome with atherogenesis and cancer has emerged providing yet another connection between inflammation and these two major diseases.
As noted above, aging powerfully associates with both atherosclerosis and cancer. The age-dependence of clonal haematopoiesis may contribute to this association. Other aspects of senescence such as shortening of telomeres or acquisition of a senescence—associated secretary phenotype (SASP) can occur during cancer progression.8 Inflammatory mediators linked both to telomere shortening and aspects of cellular senescence. In their advanced stages, both cancer and heart disease can cause cachexia. The cachexia of cancer and of advanced heart disease (notably heart failure) links tightly to sarcopenia associated with overproduction and increased activity of pro-inflammatory cytokines such as tumour necrosis factor (TNF), also initially known as cachexin.42 In sum, as in the case of lesion initiation, the progression of malignant and atherosclerotic plaques share many common pathophysiologic mechanisms and the mediators associated with these inflammatory processes.
3. Commonalities in complications of cancer and atherosclerosis
In cancer, invasion and metastasis ultimately lead to most fatal complications. In contrast, atherosclerotic plaques often produce events by a physical disruption, either rupture of the plaque or an erosion of the intimal endothelial lining that provokes thrombosis (Figure 1).43,44 Although these two types of ultimate complications of the pair of diseases appear disparate, they indeed also share many common mechanisms and inflammation as a driver. Atherosclerotic lesion growth can limit blood flow through affected arteries, analogous to local expansion of tumours that can cause complications by obstructing the bile duct, bowel, or by intracranial mass effect.
To metastasize cancer cells must traverse the basement membrane45 (Figure 1). Fibrillar collagens such as Type IV collagen comprise a major constituent of basement membranes. Type IV collagenases such matrix metalloproteinase (MMP)-2 and -9, overproduced by stromal cells within malignant tumours, can dissolve the ECM of the basement membrane permitting the invasion of tumours into both local tissues and the lymphatics and vasculature. Plasminogen activators, associated with transformation of cells, can activate the pro form of the MMP zymogens to their active forms licencing basement membrane degradation. Pro-inflammatory cytokines also strongly regulate both the expression of genes of non-fibrillar collagens such as MMP-2 and MMP-9, but also govern the production of their active forms.46 Such cytokines also govern the expression of chemokines—proteins that can direct the migration of tumour cells that breach the basement membrane due to its degradation by Type IV collagenases MMP-2 and -9.5
Proteolysis also participates in disruptions of atherosclerotic plaques (Figure 1). The dissolution of collagen that lends strength to the plaque’s protective fibrous cap due to production of interstitial collagenases such as MMPs-1, -8, and -13 can contribute to fibrous cap thinning and predispose toward plaque rupture and hence thrombosis.43 In the case of plaque thrombosis due to superficial erosion, mechanisms very similar to those which play a role in basement membrane dissolution in cancer may pertain.44,47 In particular, augmented activity of MMP-2 in response to inflammatory activation including cytokines such as IL-1 and TNF, or engagement of Toll-like receptors such as TLR2, can augment local production of the non-fibrillar Type IV collagenases and hasten endothelial detachment and desquamation, leading to intimal erosion and thrombosis. Thus, the ultimate complications of both cancer and atherosclerosis extensively involve proteolysis of ECM components, chemoattraction, and inflammation.
4. Conclusion
The illustration above of the commonality of biological mechanisms in oncogenesis and in atherogenesis and the shared features of progression and complication of their respective lesions provide an ever more compelling rationale for alignment and co-operation in patient care. We will encounter increasingly individuals who will benefit from co-management by cardiovascular, haematology, and oncology colleagues. We advocate broadening the perspective of cardio-oncology, beyond the unwanted actions of cancer therapeutics, to embrace scientifically and clinically the convergent biological mechanisms illustrated here.
Beyond structural or organizational changes in the care of patients and a heightened awareness of coincident pathogenic mechanisms, this broadened view of the interface between cardiovascular disease, haematology, and oncology has therapeutic implications. Inflammatory cell infiltrate burden associates with a poor prognosis in patients with certain tumours.48 The use of inflammatory biomarkers has gained a degree of clinical applicability in the practice of cardiovascular disease, and may also contribute to clinical decision making in patients with cancer. The use of aspirin and of non-steroidal anti-inflammatory drugs (NSAIDs) engenders controversy particularly in cardiology. Low-dose aspirin in primary prevention of cardiovascular events has come under considerable scrutiny in recent clinical trials, particularly given the efficacy of other contemporary standard-of-care treatments including statins.49 NSAIDs may increase thrombotic risk, gastrointestinal bleeding, and aggravate renal disfunction. Yet, NSAIDs may prove beneficial in the prevention of certain gastrointestinal tumours. The use of biomarkers to balance the risks and benefits of the use of these agents presents an opportunity for much needed future investigation.
The increasing use of biological anti-inflammatory agents constitutes a double-edged sword. While inhibiting IL-1β can reduce cardiovascular events and incident cancer and fatalities due to lung cancer, this strategy associates with a slight increase in infections. Anti-TNF agents may augment heart failure, predispose towards lymphoma, and have other adverse actions. As in the case of aspirin and NSAIDs, the appropriate selection of patients for biological therapies including checkpoint inhibitors and anti-cytokine interventions will require careful co-ordination between practitioners of cardiovascular medicine, haematology, and oncology. Bearing in mind that what might help one disease might also exacerbate the other, we advocate embracing an era of consolidation rather than fragmentation in patient care. The birth of the new subspecialty of cardio-oncology, and the broadening of scope advocated here, represent major steps in this direction.
Conflict of interest: P.L.'s laboratory has received research support from Novartis. S.K. is an inventor of several patents in the field of immunooncology and is receiving research support from TCR2 Inc, Boston, MA, all of which are unrelated to the content discussed in the present manuscript.
Funding
P.L. has received funding from the National Heart, Lung, and Blood Institute (R01HL080472), the American Heart Association (18CSA34080399), and the RRM Charitable Fund. S.K. is supported by the international doctoral program “i-Target: Immunotargeting of cancer” funded by the Elite Network of Bavaria, the Melanoma Research Alliance (grant number 409510 to S.K.), the Marie-Sklodowska-Curie “Training Network for the Immunotherapy of Cancer (IMMUTRAIN)” funded by the H2020 program of the European Union (to S.E. and S.K.), and by the European Research Council Starting Grant (grant number 756017 to S.K.)”.
References
- 1. Virchow R. Cellular Pathology. London: John Churchill; 1858. [Google Scholar]
- 2. Prochnicki T, Latz E.. Inflammasomes on the crossroads of innate immune recognition and metabolic control. Cell Metab 2017;26:71–93. [DOI] [PubMed] [Google Scholar]
- 3. Lecker SH, Goldberg AL, Mitch WE.. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol 2006;17:1807–1819. [DOI] [PubMed] [Google Scholar]
- 4. Netea MG, Balkwill F, Chonchol M, Cominelli F, Donath MY, Giamarellos-Bourboulis EJ, Golenbock D, Gresnigt MS, Heneka MT, Hoffman HM, Hotchkiss R, Joosten LAB, Kastner DL, Korte M, Latz E, Libby P, Mandrup-Poulsen T, Mantovani A, Mills KHG, Nowak KL, O'Neill LA, Pickkers P, van der Poll T, Ridker PM, Schalkwijk J, Schwartz DA, Siegmund B, Steer CJ, Tilg H, van der Meer JWM, van de Veerdonk FL, Dinarello CA.. A guiding map for inflammation. Nat Immunol 2017;18:826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galdiero MR, Marone G, Mantovani A.. Cancer inflammation and cytokines. Cold Spring Harb Perspect Biol 2018;10. doi:10.1101/cshperspect.a028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grivennikov SI, Greten FR, Karin M.. Immunity, inflammation, and cancer. Cell 2010;140:883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Candido J, Hagemann T.. Cancer-related inflammation. J Clin Immunol 2013;33 Suppl 1:S79–S84. [DOI] [PubMed] [Google Scholar]
- 8. Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol 2012;30:677–706. [DOI] [PubMed] [Google Scholar]
- 9. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ.. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 10. Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ.. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017;390:1833–1842. [DOI] [PubMed] [Google Scholar]
- 11. Libby P. Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J Am Coll Cardiol 2017;70:2278–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL.. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel AP, Natarajan P. Completing the genetic spectrum influencing coronary artery disease: from germ line to somatic variation. Cardiovasc Res 2019;115:830–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Balkwill FR, Mantovani A.. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol 2012;22:33–40. [DOI] [PubMed] [Google Scholar]
- 15. Sceneay J, McAllister SS.. The skinny on obesity and cancer. Nat Cell Biol 2017;19:887–888. [DOI] [PubMed] [Google Scholar]
- 16. Chaffer CL, Weinberg RA.. A perspective on cancer cell metastasis. Science 2011;331:1559–1564. [DOI] [PubMed] [Google Scholar]
- 17. Ridker PM. Inflammation, C-reactive protein, and cardiovascular disease: moving past the marker versus mediator debate. Circ Res 2014;114:594–595. [DOI] [PubMed] [Google Scholar]
- 18. Wesseling M, Sakkers TR, de Jager SCA, Pasterkamp G, Goumans MJ.. The morphological and molecular mechanisms of epithelial/endothelial-to-mesenchymal transition and its involvement in atherosclerosis. Vascul Pharmacol 2018;106:1–8. [DOI] [PubMed] [Google Scholar]
- 19. Costantino S, Libby P, Kishore R, Tardif JC, El-Osta A, Paneni F.. Epigenetics and precision medicine in cardiovascular patients: from basic concepts to the clinical arena. Eur Heart J 2018;39:4150–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ross R, Glomset JA.. The pathogenesis of atherosclerosis I. N Engl J Med 1976;295:369–377. [DOI] [PubMed] [Google Scholar]
- 21. Ross R, Glomset JA.. The pathogenesis of atherosclerosis II. N Engl J Med 1976;295:420–425. [DOI] [PubMed] [Google Scholar]
- 22. Libby P, Warner SJC, Salomon RN, Birinyi LK.. Production of platelet-derived growth factor-like mitogen by smooth-muscle cells from human atheromata. N Engl J Med 1988;318:1493–1498. [DOI] [PubMed] [Google Scholar]
- 23. Kaplanov I, Carmi Y, Kornetsky R, Shemesh A, Shurin GV, Shurin MR, Dinarello CA, Voronov E, Apte RN.. Blocking IL-1beta reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc Natl Acad Sci U S A 2019;116:1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Libby P, Nahrendorf M, Swirski FK.. Monocyte heterogeneity in cardiovascular disease. Semin Immunopathol 2013;35:553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caronni N, Savino B, Bonecchi R.. Myeloid cells in cancer-related inflammation. Immunobiology 2015;220:249–253. [DOI] [PubMed] [Google Scholar]
- 26. Klingenberg R, Gerdes N, Badeau RM, Gisterå A, Strodthoff D, Ketelhuth DFJ, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, Zoller S, Lohmann C, Lüscher TF, Jauhiainen M, Sparwasser T, Hansson GK.. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest 2013;123:1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mantovani A, Allavena P, Sica A, Balkwill F.. Cancer-related inflammation. Nature 2008;454:436–444. [DOI] [PubMed] [Google Scholar]
- 28. Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med 1998;338:436–445. [DOI] [PubMed] [Google Scholar]
- 29. Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HWM, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M.. Myocardial infarction accelerates atherosclerosis. Nature 2012;487:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gistera A, Hansson GK.. The immunology of atherosclerosis. Nat Rev Nephrol 2017;13:368–380. [DOI] [PubMed] [Google Scholar]
- 31. Kobold S. Innate and adaptive immunity combined for cancer treatment. Proc Natl Acad Sci USA 2019;116:1087–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Voigt C, May P, Gottschlich A, Markota A, Wenk D, Gerlach I, Voigt S, Stathopoulos GT, Arendt KAM, Heise C, Rataj F, Janssen K-P, Königshoff M, Winter H, Himsl I, Thasler WE, Schnurr M, Rothenfußer S, Endres S, Kobold S.. Cancer cells induce interleukin-22 production from memory CD4(+) T cells via interleukin-1 to promote tumor growth. Proc Natl Acad Sci U S A 2017;114:12994–12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Geng Y-J, Henderson L, Levesque E, Muszynski M, Libby P.. Fas is expressed in human atherosclerotic intima and promotes apoptosis of cytokine-primed human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 1997;17:2200–2208. [DOI] [PubMed] [Google Scholar]
- 34. Zeboudj L, Maître M, Guyonnet L, Laurans L, Joffre J, Lemarie J, Bourcier S, Nour-Eldine W, Guérin C, Friard J, Wakkach A, Fabre E, Tedgui A, Mallat Z, Tharaux PL, Ait-Oufella H. Selective EGF-receptor inhibition in CD4+ T cells induces anergy and limits atherosclerosis. J Am Coll Cardiol 2017;71:160–172. [DOI] [PubMed] [Google Scholar]
- 35. Libby P, Hansson GK.. Taming immune and inflammatory responses to treat atherosclerosis. J Am Coll Cardiol 2018;71:173–176. [DOI] [PubMed] [Google Scholar]
- 36. Folkman J. Angiogenesis and angiogenesis inhibition: an overview In Goldberg ID, Rosen EM (eds). Regulation of Angiogenesis. Switzerland: Birkhauser Verlag; 1997(79). p1–8. [DOI] [PubMed] [Google Scholar]
- 37. Vander Heiden MG, Cantley LC, Thompson CB.. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324:1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dvorak HF, Brown LF, Detmar M, Dvorak AM.. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 39. Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol Res 2015;3:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fredman G, Tabas I.. Boosting inflammation resolution in atherosclerosis: the next frontier for therapy. Am J Pathol 2017;187:1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J 2017;31:1273–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res 2015;116:1254–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 2013;369:2004–2013. [DOI] [PubMed] [Google Scholar]
- 44. Libby P, Pasterkamp G, Crea F, Jang IK.. Reassessing the mechanisms of acute coronary syndromes. Circ Res 2019;124:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stetler-Stevenson WG, Aznavoorian S, Liotta LA.. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 1993;9:541–573. [DOI] [PubMed] [Google Scholar]
- 46. Galis ZS, Muszynski M, Sukhova GK, Simon-Morrissey E, Unemori EN, Lark MW, Amento E, Libby P.. Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ Res 1994;75:181–189. [DOI] [PubMed] [Google Scholar]
- 47. Quillard T, Franck G, Mawson T, Folco E, Libby P.. Mechanisms of erosion of atherosclerotic plaques. Curr Opin Lipidol 2017;28:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Diakos CI, Charles KA, McMillan DC, Clarke SJ.. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493–e503. [DOI] [PubMed] [Google Scholar]
- 49. Ridker PM. Should aspirin be used for primary prevention in the post-statin era? N Engl J Med 2018;379:1572–1574. [DOI] [PubMed] [Google Scholar]