Abstract
Aims
Tyrosine kinase inhibitors (TKIs) have revolutionized the treatment of chronic myelogenous leukaemia (CML). However, cardiotoxicity of these agents remains a serious concern. The underlying mechanism of these adverse cardiac effects is largely unknown. Delineation of the underlying mechanisms of TKIs associated cardiac dysfunction could guide potential prevention strategies, rescue approaches, and future drug design. This study aimed to determine the cardiotoxic potential of approved CML TKIs, define the associated signalling mechanism and identify potential alternatives.
Methods and results
In this study, we employed a zebrafish transgenic BNP reporter line that expresses luciferase under control of the nppb promoter (nppb:F-Luciferase) to assess the cardiotoxicity of all approved CML TKIs. Our in vivo screen identified ponatinib as the most cardiotoxic agent among the approved CML TKIs. Then using a combination of zebrafish and isolated neonatal rat cardiomyocytes, we delineated the signalling mechanism of ponatinib-induced cardiotoxicity by demonstrating that ponatinib inhibits cardiac prosurvival signalling pathways AKT and extra-cellular-signal-regulated kinase (ERK), and induces cardiomyocyte apoptosis. As a proof of concept, we augmented AKT and ERK signalling by administration of Neuregulin-1β (NRG-1β), and this prevented ponatinib-induced cardiomyocyte apoptosis. We also demonstrate that ponatinib-induced cardiotoxicity is not mediated by inhibition of fibroblast growth factor signalling, a well-known target of ponatinib. Finally, our comparative profiling for the cardiotoxic potential of CML approved TKIs, identified asciminib (ABL001) as a potentially much less cardiotoxic treatment option for CML patients with the T315I mutation.
Conclusion
Herein, we used a combination of in vivo and in vitro methods to systematically screen CML TKIs for cardiotoxicity, identify novel molecular mechanisms for TKI cardiotoxicity, and identify less cardiotoxic alternatives.
Keywords: Tyrosine kinase inhibitors, Zebrafish, Chronic myelogenous leukaemia, Cardiotoxicity, AKT, ERK
This article is part of the Spotlight Issue on Cardio-oncology.
1. Introduction
Over the past few decades, the survival of cancer patients has significantly improved through the collective efforts of laboratory and clinical oncology research.1,2 However, manifestation of cardiotoxic effects for many anticancer therapeutics has compromised their clinical effectiveness resulting in discontinued use or in some cases even withdrawal from the market. Hence, there is a desperate need for strategies to identify cardiotoxicity much earlier in the drug development process. The major goals of the emerging discipline of cardio-oncology are to better understand the pathophysiology of cancer therapy-associated cardiotoxicity, and to provide an early prediction, management, and treatment strategy for cardiac complications in cancer patients and survivors.3 Tremendous strides have been made to understand the mechanism of chemotherapy-induced cardiotoxicity. However, the mechanism and frequency of molecular targeted therapy-induced cardiotoxicity is largely unknown and likely underestimated.
Chronic myelogenous leukaemia (CML) is caused by a reciprocal translocation between chromosomes 9 and 22 t(9;22q34;q11) or Philadelphia (Ph) chromosome resulting in the breakpoint cluster region-Abelson (BCR-ABL) fusion gene.4–7 The constitutive activity of the oncogenic BCR-ABL tyrosine kinase is the primary event that drives CML pathogenesis and thus serves as an ideal target for therapy.8–11 Imatinib inhibits the formation of BCR-ABL oncoprotein, and has become the first-line therapy for CML patients.12–14 Unfortunately, frequent kinase domain mutations in BCR-ABL are a critical cause of imatinib resistance.8,14–20 Shortly after the realization of imatinib-insensitivity, second and third generation inhibitors were developed.21–24 Although dasatinib (Sprycel, BMS-354825), nilotinib (Tasigna, AMN107), and bosutinib (Bosulif) are potent against imatinib-resistant CML, they have been largely ineffective for patients with the T315I (gatekeeper) mutation.25–27
In 2012, ponatinib (Iclusig), a novel potent tyrosine kinase inhibitor (TKI), was granted ‘Accelerated Approval’ for the treatment of ‘Philadelphia chromosome–positive’ (Ph+) CML patients resistant or intolerant to prior TKI therapy.28–31 Importantly, ponatinib could also inhibit the activity of the BCR-ABL T315I mutation for which no alternative therapy existed. Unfortunately, serious adverse events were reported in patients treated with ponatinib, which tempered its clinical use.32 In phase II trial, 18/449 patients died during the study.29 The common adverse events were thrombocytopenia (37% of patients), abdominal pain (22%), rash (34%), vascular occlusion (23%), dry skin (32%), and neutropenia (19%).29 Patients treated with ponatinib have been reported to have increased rates of fatal myocardial infarction (Grade 3 or 4).29,33–35 In addition, ponatinib administration induces cardiovascular complications including cardiomyopathy, congestive heart failure, and vascular occlusion.29,34–36 Arterial and venous thrombosis occurred in 23% of patients within 2 weeks of commencement of ponatinib dosing.29,33 Importantly, these events occurred in patients with or without cardiovascular risk factors at any age. Thus, the adverse vascular effects of ponatinib are well recognized.37–39 Indeed, clinical trial data reported heart failure and cardiac dysfunction in 9% of ponatinib-treated patients.33,34 However, the molecular mechanism of ponatinib induced cardiac dysfunction is largely unknown which is a subject of the current study.
To gain a better understanding of the cardiotoxic potential of CML TKIs we employed both in vivo and in vitro assays to screen for cardiotoxicity of all approved CML TKIs. We report that ponatinib is the most cardiotoxic agent among the approved CML TKIs. Mechanistically, ponatinib inhibits cardiac prosurvival signalling pathways, leading to cardiomyocyte apoptosis and ventricular dysfunction. The cardiotoxic effects of ponatinib could be blunted by the administration of Neuregulin-1β, which augments cardiomyocyte prosurvival signalling. Importantly, we also identified asciminib as a less cardiotoxic alternative to ponatinib in patients harbouring the T315I CML mutation.
2. Methods
An expanded Materials and Methods section is available in the Supplementary material online.
2.1 Animal studies
Zebrafish studies were carried out at the Vanderbilt University Medical Center (VUMC). The Institutional Animal Care and Use Program (ACUP) Committee of VUMC approved all animal procedures and treatments. The VUMC ACUP is registered with the United States Department of Agriculture (USDA Registration #63-R-0129) and operates under a Public Health Service Animal Welfare Assurance Statement (PHS Assurance #A3227-01). The VUMC ACUP has been accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International (AAALAC-International) since 1967 (AAALAC file #000020) and most recently received ‘Continued Full Accreditation’ on 21 June 2017.
2.2 Zebrafish lines and dosing rationales
The transgenic zebrafish lines with cardiac expression of GFP (TG:cmlc2:GFP),40 BNP luciferase reporter line (nppb:F-Luc),41 and AB-wild type were used as described in the results section and figure legends. The dosing rationales for zebrafish were based on published literature.42,43 Zebrafish ventricular fractional shortening was measured as described in Supplementary material online, Methods.44 For drug exposures, final concentrations were achieved by preparing concentrated stock solutions of Imatinib, Dasatinib, Bosutinib, Nilotinib, and Ponatinib in DMSO and adding stock solutions to egg water to achieve final working concentrations with a DMSO concentration of <1%.
2.3 Euthanasia of zebrafish embryos
All zebrafish experiments were performed on embryos less than 5 dpf and euthanasia was performed by exposure to bleach or rapid freezing followed by maceration.45
2.4 Euthanasia of rat and neonates
Rats were euthanized by overdose of Isoflurane and there after cervical dislocation was performed to ensure the death. Neonates were euthanized by rapid decapitation.45
2.5 Statistical analysis
Statistical analysis was performed using Graph Pad Prism V8.0 (GraphPad Software Inc.). Analysis of differences between two test groups was performed using the unpaired two-tailed Student’s t-test. Statistical differences in the case of more than two groups were performed using one-way analysis of variance (ANOVA) with the Tukey post hoc test where applicable, unless otherwise indicated. Differences between data groups with two variables were evaluated for significance using two-way ANOVA followed by Tukey post hoc test. P-values were considered statistically significant if P < 0.05. All experiments were repeated at least three times, and the data are presented as mean ± standard error of the mean (SEM) unless noted otherwise.
3. Results
3.1 Preclinical screening for cardiotoxic potential of all approved CML TKIs
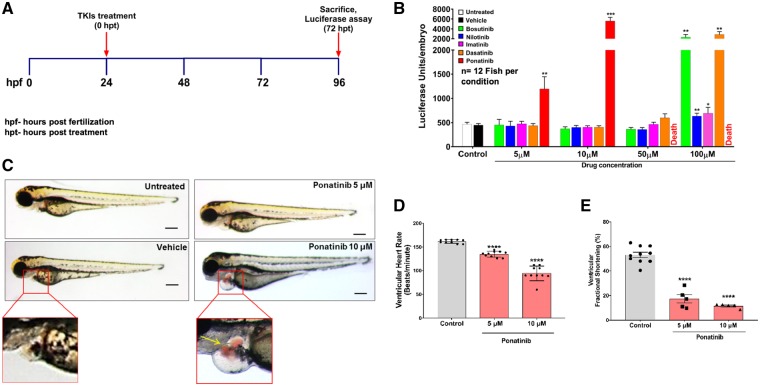
To examine the cardiotoxicity of TKIs used in CML therapy, we used a zebrafish BNP reporter transgenic line (nppb:F-Luc) that expresses firefly luciferase under the control of the nppb promoter.41 The dosing rationales were obtained from previously published literature.40,42,43,46,47 We treated the zebrafish embryos for 72 h with various concentrations of imatinib, dasatinib, nilotinib, bosutinib, ponatinib, and DMSO as vehicle control (Figure 1A). Ponatinib treatment (5 µM and 10 µM) led to a significant (10-fold) increase in luciferase levels when compared with other TKIs and control (Figure 1B). Furthermore, among all CML TKIs, ponatinib was the only drug, which caused 100% lethality at 50 µM and 100 µM concentrations. In addition, formation of pericardial oedema was very pronounced in zebrafish embryos exposed to ponatinib when compared with similar concentrations of other CML TKIs (Figure 1C, Supplementary material online, Figure S1). Formation of pericardial oedema is a common finding in zebrafish embryos with abnormal cardiac function. Importantly, at this concentration of ponatinib, we did not observe any extra-cardiac phenotypic changes, strongly suggesting that ponatinib toxicity is cardiac-specific.
Figure 1.
Cardiotoxicity screening of approved CML TKIs in zebrafish. (A) Schematic of in vivo screening of tyrosine kinase inhibitors. (B) Luciferase-based reporter assay for rapid quantitative assessment of cardiac natriuretic peptide BNP expression in zebrafish embryos upon TKIs treatment. The nppb:F-Luc transgenic zebrafish embryos were treated with different concentration of TKIs. Ponatinib showed elevation in luciferase level when compared with control and other TKIs at 5 μM and 10 μM concentrations, while 50 μM and 100 μM ponatinib treatments leads to 100% lethality. Data are presented as mean ± SEM. Statistical analysis was conducted using two-way ANOVA post hoc Tukey test for comparison between DMSO vs. TKIs (*P < 0.05, **P < 0.01, ***P < 0.001); n=12 fish per condition. (C) Ponatinib cause pericardial oedema in zebrafish embryos. At 1 dpf, wild-type (AB) zebrafish embryos were treated with increasing concentrations of ponatinib and were assessed for phenotypic changes. After 72 h of treatment with ponatinib, zebrafish had extensive pericardial oedema at 5 μm and 10 μM when compared with vehicle-treated embryos. The red box displays the pericardial sac area. In ponatinib treated (10 µM) fish, ventricular contractions got perturbed, allowing blood to accumulate in both chambers, and pool just posterior to the atrium (yellow arrow). Scale bar-100 µm. (D) Ponatinib treatment reduces heart rate. Fish were treated for 72 h with ponatinib at 5 µM and 10 µM concentrations and ventricular contraction rate was determined (b.p.m.). Ponatinib treatment led to significant decline in the heart rate when compared with control. Data are presented as mean ± SEM. Statistical analysis was conducted using one-way ANOVA post hoc Tukey test for comparison between DMSO (control) vs. ponatinib (****P < 0.0001); n = 8–10 fish per condition. (E) Ponatinib treatment reduces fractional shortening. Ventricular function was assessed on zebrafish embryos exposed from 1 dpf to 4 dpf with vehicle or 5 μM, 10 μM concentrations of ponatinib. Ventricular end-diastolic dimension (EDD) and end-systolic dimension (ESD) in both long and short axes were quantified. Fractional shortening (FS) was then calculated as a measure of contractile function; n = 5–10 fish per condition. Data are presented as mean ± SEM. Statistical analysis was conducted using one-way ANOVA post hoc Tukey test for comparison between DMSO (control) vs. ponatinib (****P < 0.0001).
3.2 Ponatinib treatment leads to abnormal cardiac function in vivo
To determine the effect of ponatinib on heart rate, we employed transgenic cmlc2:GFP zebrafish embryos. Ponatinib treatment led to a significant decline in ventricular heart rate at both 5 µM and 10 µM concentrations (Figure 1D) without any atrioventricular block. We next assessed the ponatinib effect on ventricular systolic function (ventricular fractional shortening). Ventricular systolic function was severely impaired in embryos exposed to ponatinib (Figure 1E). Taken together, these findings show that ponatinib exposure leads to abnormal cardiac function.
3.3 Ponatinib treatment induces cardiomyocyte apoptosis
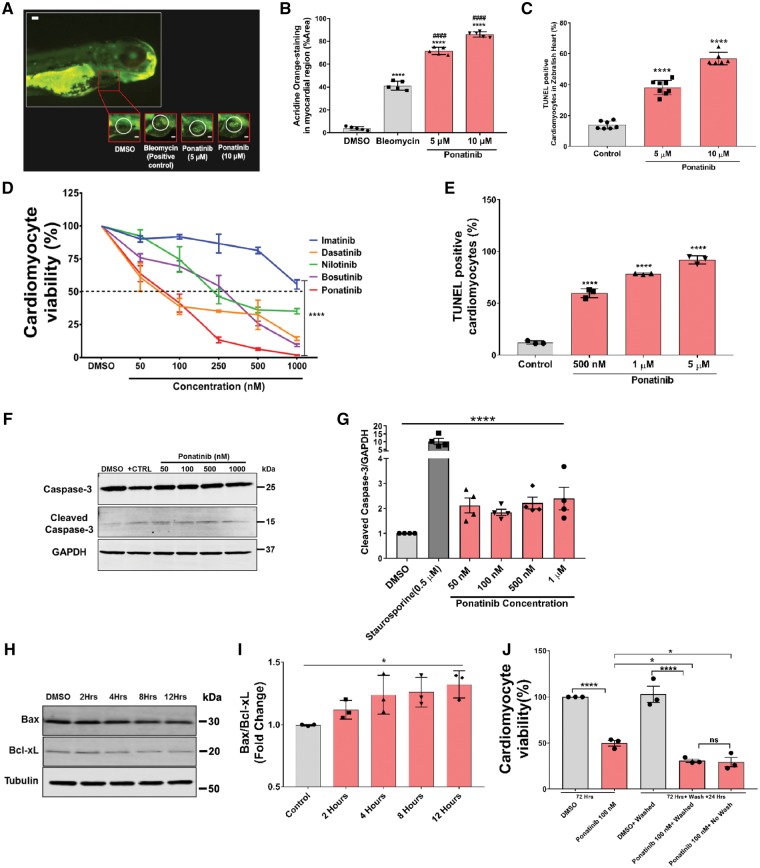
Based on our findings of decreased heart rate and impaired cardiac function in zebrafish embryos exposed to ponatinib, we next measured if there is evidence of cardiomyocytes apoptosis. To determine the extent of apoptosis, we treated zebrafish embryos (AB-wild type) with 5 μM and 10 μM ponatinib at 1 dpf and then stained whole fish at 4 dpf with the acridine orange (AO). Ponatinib exposure led up to 70–80% AO-stained myocardial area showed apoptotic cardiomyocytes in heart, and this was significantly higher than the vehicle (4%) or the positive control bleomycin (40%) (Figure 2A and B). TUNEL assay of ponatinib-exposed zebrafish also identified increased cardiomyocyte apoptosis (Figure 2C). These results suggest that ponatinib mediated cardiomyocyte apoptosis contributes to cardiotoxicity in zebrafish.
Figure 2.
Ponatinib induces apoptosis in zebrafish hearts and isolated neonatal rat CMs. (A) At 1 dpf, fish were treated with ponatinib, positive control bleomycin or vehicle, as indicated. Representative images depict acridine orange (AO)-stained and AO-negative hearts in the myocardial region in fish at 4 dpf. The heart is outlined with ovals. Scale bar-125 µm. (B) The AO-staining in the pericardial area were quantified (using image J) and displayed in bar diagram. Data are presented as means ± SEM. Statistical analysis was conducted using one-way ANOVA post hoc Tukey test for comparison between DMSO (control) vs. ponatinib (****P < 0.0001) and ponatinib vs. bleomycin (####P < 0.0001); n=5 fish per condition. (C) Cardiomyocyte apoptosis in zebrafish heart was quantified by TdT-mediated dUTP Nick-End Labelling (TUNEL) method. TUNEL positive nuclei showed a significant percentage of apoptotic cells in ponatinib treated zebrafish hearts. Data are presented as means ± SEM. Statistical analysis was conducted using one-way ANOVA post hoc Tukey test for comparison between DMSO (control) vs. ponatinib (****P < 0.0001); n = 6–10 fish per condition. (D) Concentration-dependent effect of TKIs on cardiomyocyte survival. Neonatal rat ventricular cardiomyocytes were incubated for 72 h with TKIs from 50 nM to 1000 nM drug or vehicle (0.01% DMSO). Cell viability was determined (via cell-titer glo) as a percentage of vehicle control. Three biological replicates conducted per TKIs. Data are presented as mean ± SEM. ***P < 0.001 (two way-ANOVA). (E) NRVMs were treated with increasing dose of ponatinib for 24 h followed by TUNEL assay. TUNEL positive nuclei in displayed apoptotic cardiomyocytes in ponatinib treated samples, Data are presented as means ± SEM. Statistical analysis was conducted using one-way ANOVA post hoc Tukey test for comparison between DMSO (control) vs. ponatinib (****P < 0.0001). (F) Western blot was used to detect caspase-3 activity in ponatinib treated NRVMs. Cells were treated with increasing concentration of ponatinib for 24 h followed by lysate collection and western blot. Positive control was achieved by the treatment of 0.5 μM staurosporine. (G) Band intensities in bar diagrams clearly demonstrate the increased level of cleaved caspase-3 expression in ponatinib treated cardiomyocytes in a concentration dependent manner. ****P < 0.0001 considered significant using one way-ANOVA. (H) NRVMs were treated with 50 nM ponatinib and lysates were collected at various time points, as indicated. Western blot analysis was performed to determine the expression of Bax and Bcl-xL. (I) Bax/Bcl-xL ratio was calculated after densitometric analysis of blots. Ponatinib treatment led to increased Bax/Bcl-XL ratio as early as 2 h after ponatinib treatment, *P < 0.05 considered significant using one way-ANOVA. (J) Ponatinib-induced cardiotoxicity is irreversible. NRVMs were treated with 100 nM ponatinib 72 h. After 72 h drug containing media was removed. Cells were washed once with PBS and fresh media was added. Cells were further incubated for 24 h followed by cell viability assay. DMSO was used as vehicle control. Data are presented as means ± SEM. Statistical analysis was conducted using two-way ANOVA post hoc Tukey test for comparison between groups (*P < 0.05, **P < 0.01, ****P < 0.0001).
We then examined if mammalian cardiomyocytes responded in a similar fashion to zebrafish cardiomyocytes. To elucidate direct effects of TKIs on cardiomyocytes, neonatal rat ventricular myocytes (NRVMs) were treated with various concentrations (50–1000 nM) of imatinib, dasatinib, nilotinib, bosutinib, ponatinib, or DMSO as a vehicle control up to 72 h and cell viability was determined. As expected, cardiomyocyte viability was significantly decreased in response to various concentrations of TKIs (Figure 2D). Similar to the zebrafish in vivo experiments, ponatinib was the most toxic CML TKI. Ponatinib treatment dose-dependently induced cardiomyocytes apoptotic cell death as determined by TUNEL assay (Figure 2D and E). Consistently, ponatinib treatment induced the activation of pro-apoptotic caspase pathway as evident by significantly increased caspase-3 cleavage (Figure 2F and G). In addition to these results, we also used a comparatively low dose of ponatinib and earlier time point to assess the ponatinib mediated activation of apoptotic pathways. For this, NRVMs were treated with a clinically relevant dose of ponatinib, (50 nM, the blood concentration in ponatinib-treated patients)28,29 for up to 12 h and expressions of Bax and Bcl-xL were determined. As shown in Figure 2H and I, a clinically relevant dose of ponatinib was sufficient to induce the activation of cardiomyocytes apoptotic signalling as evident by dysregulated Bax/Bcl-xL ratio. The balance of Bax/Bcl-xL is critical to mitochondrial membrane integrity and cell fate.48–50 These findings suggest that ponatinib induces the cardiomyocyte apoptosis by altering the Bax/Bcl-xL ratio.
Next, we investigated whether removal of ponatinib after treatment improves the cardiomyocyte viability. NRVMs were treated with ponatinib (100 nM) for 72 h, thereafter, ponatinib was removed and cells were washed with PBS. Cells were further grown for another 24 h and cell viability assay was performed. We found that ponatinib treatment led to a significant decrease in cell viability even after its removal (Figure 2J). Thus, once the cardiomyocytes are injured by ponatinib exposure and apoptotic pathways are activated, removal of ponatinib was not sufficient to drive the reversal of the adverse effects.
3.4 Molecular mechanism of ponatinib-induced cardiotoxicity
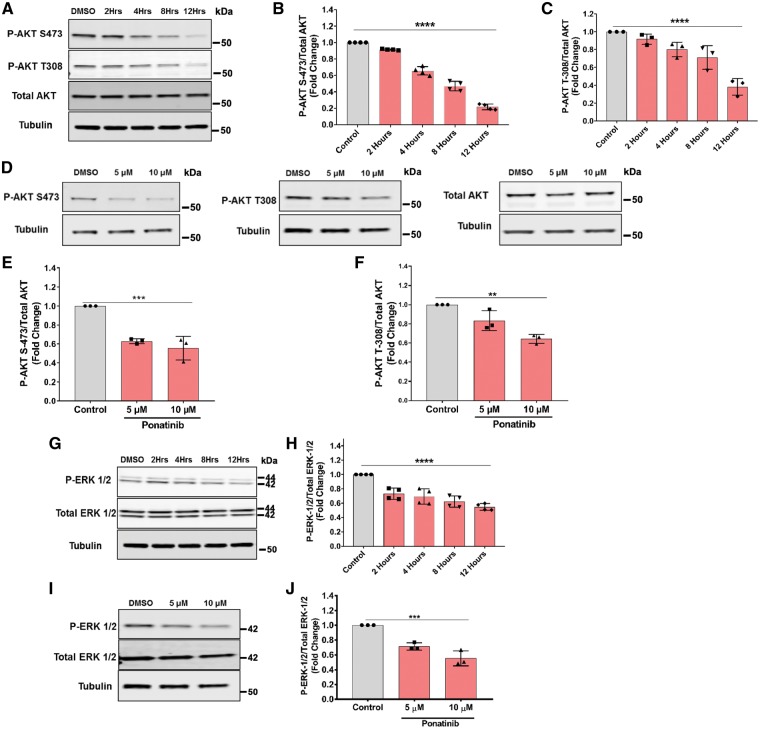
We next examined signalling pathways regulating ponatinib cardiotoxicity. Previous studies suggest that ponatinib is a multi-targeted kinase inhibitor that suppresses more than 60 kinases, including PDGFR, c-KIT, SFK, VEGFR, and FGFR activities. This makes it difficult to pinpoint the specific target(s) mediating toxicity. Therefore, in this study, we focused on PI3K/AKT and extra-cellular-signal-regulated kinase (ERK) signalling pathways, which are key growth-signalling pathways critical to cardiomyocyte survival and cardiac homeostasis.51 As shown in Figure 3A–C, treatment of NRVMs with a clinically relevant dose of ponatinib (50 nM) for various time points inhibited phosphorylation of AKT at both sites, Thr308 and Ser473. These findings were also consistent in ponatinib-exposed zebrafish (Figure 3D–F).
Figure 3.
Ponatinib inhibits phosphorylation of AKT and ERK. (A–C) NRVMs treated with the ponatinib at the 50 nM concentration for various time points, as indicate and lysates were analysed by western blotting to determine the levels of phosphorylated and total AKT. (D–F) Zebrafish embryos were treated with ponatinib from 1 dpf to 4 dpf at the indicated concentrations. Lysate were collected and levels of phosphorylated and total AKT were detected. (G, H) NRVMs exposed to vehicle or ponatinib were analysed for phosphorylated ERK and total ERK. (I, J) Zebrafish embryos exposed to vehicle or ponatinib from 1 dpf to 4 dpf were analysed for phosphorylated ERK and total ERK. Band intensities in bar diagrams clearly demonstrate the significant decline in phosphorylation of AKT and ERK proteins. **P < 0.01, ***P < 0.001, ****P < 0.0001 were considered significant using one way-ANOVA.
Serine-threonine kinase ERK signalling is another essential cardiomyocyte pathway, which promotes myocyte survival, proliferation, and differentiation.52 Ponatinib treatment led to a marked decrease in ERK1/2 phosphorylation in cultured cardiomyocytes (Figure 3G and H), and zebrafish (Figure 3I and J). Inhibition of the ERK pathway is known to cause cardiomyocyte death52–55 and thus also may be contributing to ponatinib-mediated cardiotoxicity. Importantly, other CML-TKIs did not significantly inhibited the prosurvival AKT and ERK signalling pathways at a very comparable doses, suggesting a unique effect of ponatinib on cardiomyocyte prosurvival signalling and cardiotoxicity (Supplementary material online, Figure S2).
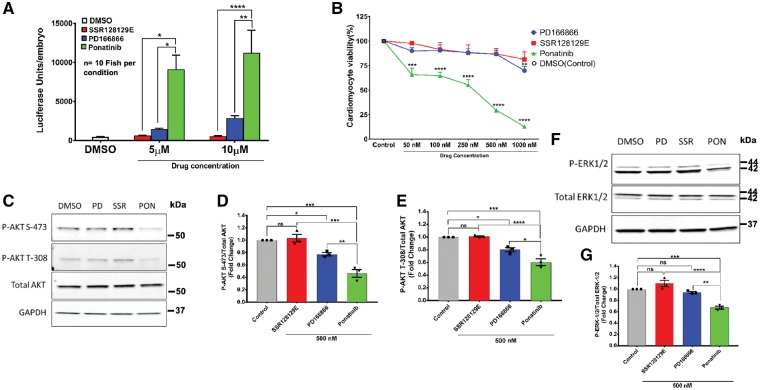
Ponatinib is known to target fibroblast growth factor (FGF) signalling pathway, which is critical to cardiac pathophysiology.56–59 To define the potential contribution of anti-FGFR activity in ponatinib-mediated cardiotoxicity, we used two distinct and selective inhibitors of the FGFR1 kinase. These inhibitors (PD166866 and SSR128129E) did not cause the same level of BNP reporter induction as ponatinib (Figure 4A). Likewise, the FGFR inhibitors did not recapitulate the same cytotoxic effect when compared with ponatinib in NRVMs (Figure 4B). In addition, the effect of FGFR inhibitors on cardiomyocyte AKT and ERK signalling was significantly less compared with ponatinib (Figure 4C–G). Taken together, these data confirm that selective FGFR inhibitors could not reiterate the toxic effect of ponatinib on cardiomyocytes, suggesting that FGFR inhibition contributes minimally to the ponatinib-induced cardiotoxicity.
Figure 4.
FGFR1 inhibition does not specifically contribute to ponatinib associated cardiotoxicity. (A) The nppb:F-Luc transgenic zebrafish embryos were treated with FGFR1 inhibitors (SSR128129E and PD166866) and ponatinib for 72 h and then luciferase assay was done. Ponatinib showed significant elevation of luciferase compared with FGFR1 inhibitors. Data are presented as means ± SEM. Statistical analysis was conducted using two-way ANOVA post hoc Tukey test for comparison between groups (*P < 0.05, **P < 0.01, ****P < 0.0001); n = 10 fish per condition. (B) NRVMs were treated with FGFR1 inhibitors (SSR128129E and PD166866) and ponatinib for 72 h with increasing range of concentrations. After that, cell viability was determined by Cell-titer Glo-assay. Data are presented as means ± SEM. Statistical analysis was conducted using two-way ANOVA post hoc Tukey test for comparison between control vs. ponatinib or FGFR inhibitors at each concentration (**P < 0.01, ***P < 0.001, ****P < 0.0001). (C–G) Effects of FGFR1 inhibition with SSR128129E and PD166866 on cardiac AKT and ERK pathway was determined by western blot. Cells were treated with inhibitors and ponatinib at 500 nM concentrations for 24 h followed by lysate preparation and western blot. Representative western blots and densitometric evaluations are shown. Data are presented as means ± SEM. Statistical analysis was conducted using one-way ANOVA post hoc Tukey test for comparison between groups (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001), ns, not significant.
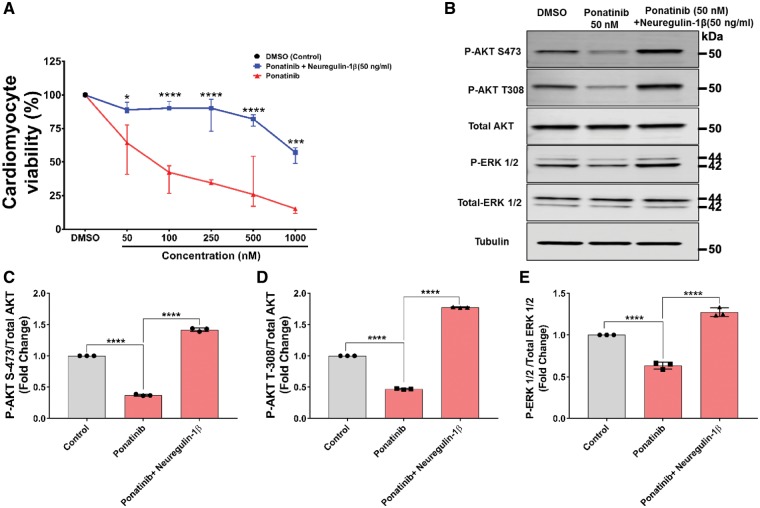
3.5 Neuregulin-1β attenuates the ponatinib-induced cardiotoxicity through activation of AKT and ERK signalling pathways
The above data suggests that dysregulation of ERK and AKT signalling might be the key mechanism for ponatinib-induced cardiac toxicity. Based on these findings, we hypothesized that activation of these signalling pathways may protect the heart from ponatinib-induced cardiotoxicity. Neuregulin-1β (NRG-1β) synergistically activates ERK and PI3K-AKT pathways and has been shown to be cardioprotective in various experimental settings.60–62 Furthermore, NRG-1β has been used in clinical trials to evaluate the efficacy and safety for chronic systolic heart failure (ClinicalTrials.gov Identifier: NCT01214096)63 and currently being used in trial to confirm the efficacy of NRG-1β in reducing the death rate of heart failure patients (ClinicalTrials.gov Identifier: NCT03388593). To examine the potential of NRG-1β to mitigate the ponatinib-induced cardiotoxicity, NRVMs were pretreated with 50 ng/mL NRG-1β for 30 min, thereafter, increasing concentrations of ponatinib (50 nM to 1 µM) were added, and cardiomyocyte viability was assessed. Indeed, NRG-1β pretreatment significantly reduced ponatinib mediated cardiomyocyte death (Figure 5A). Consistently, NRG-1β treatment also prevented the ponatinib-mediated reduction of AKT and ERK phosphorylation (Figure 5B–E). Our findings are consistent with previous reports supporting the cardioprotective nature of NRG-1β via ERK and AKT signalling.64–66 Taken together, these data further support the role of reduced AKT and ERK signalling as the mechanism for ponatinib induced cardiotoxicity and suggest a potential strategy to prevent cardiotoxicity by this agent.
Figure 5.
Neuregulin-1β attenuates the ponatinib induced cardiac growth signalling perturbation in NRVMs. (A) NRVMs pretreated with 50 ng/mL Neuregulin-1β for 30 min followed by ponatinib treatments (50 nM–1µM) for 72 h. Cell viability assay was performed after 72 h. DMSO was used as vehicle control. Only ponatinib treated NRVMs were used for viability comparison. Data are presented as means ± SEM. Statistical analysis was conducted using two-way ANOVA post hoc Tukey test for comparison between ponatinib vs. ponatinib + Neuregulin-1β at each concentration (*P < 0.05, ***P < 0.001, ****P < 0.0001). (B–E) NRVMs were pretreated with 50 ng/mL for 30 min followed by 50 nM ponatinib treatment upto 12 h. After that, lysates were prepared using cell lysis buffer. Lysates were then subjected to western blot and probed with respective antibodies mentioned in the diagram. Tubulin serves as loading control. Bar diagram represents a densitometric analysis of western blots. Western blots are representative of three independent experiments. Data are presented as means ± SEM. Statistical analysis was conducted using two-way ANOVA post hoc Tukey test for comparison between groups (****P < 0.0001).
3.6 Searching for a potential alternative of ponatinib; comparative cardiotoxicity of an allosteric inhibitor asciminib (ABL001)
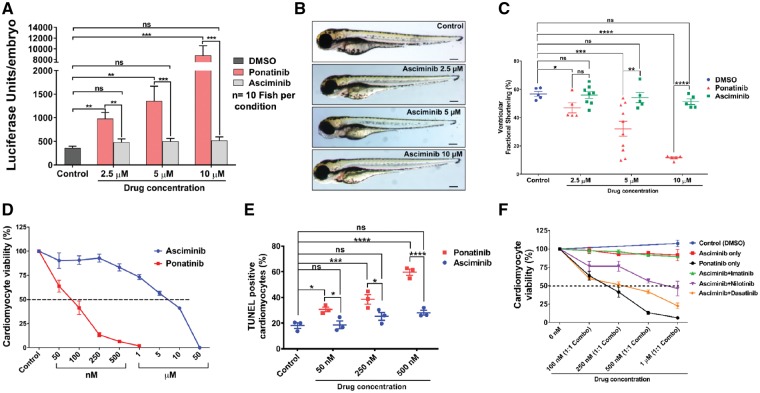
A recently developed TKI asciminib (ABL001) has shown a potent activity against T315I mutations. Asciminib inhibits BCR-ABL kinase by allosteric inhibition of myristoyl pocket.67,68 Of note, ponatinib inhibits tyrosine kinase activity of BCR-ABL by binding to its ATP site (catalytic inhibition). This has led us to investigate the comparative cardiotoxicity of ponatinib vs. asciminib. Interestingly, increasing dose of asciminib showed minimal induction of the BNP reporter compared with ponatinib (Figure 6A). Likewise, there was an absence of pericardial oedema after asciminib treatment (Figure 6B) and ventricular systolic function was not affected (Figure 6C). To further investigate the comparative cardiotoxic potential of asciminib vs. ponatinib, NRVMs were exposed to asciminib and ponatinib. Indeed, our results reveal that asciminib has 50 times higher IC50 (∼5 μM) than ponatinib IC50 (∼100 nM) (Figure 6D). Furthermore, the TUNEL assay confirms that asciminib mediated induction of apoptosis was markedly less compared with ponatinib (Figure 6E).
Figure 6.
Allosteric inhibitor asciminib (ABL001) as an option for ponatinib. (A) The nppb:F-Luc transgenic zebrafish embryos were treated with asciminib and ponatinib for 72 h and then luciferase assay was performed. Asciminib treatment compared with ponatinib did not induce the BNP reporter. Data are presented as means ± SEM. Statistical analysis was conducted using two-way ANOVA post hoc Tukey test for comparison between groups (P < 0.01, ***P < 0.001); n = 10 fish per condition. ns, not significant. (B) Asciminib when compared with ponatinib was unable to induce the formation of pericardial oedema in zebrafish (AB wildtype). n = 5–10 fish per condition. Scale bar 100 µm. (C) Reduction in fractional shortening post-treatment of asciminib in comparison to ponatinib. Data are presented as means ± SEM. Statistical analysis was conducted using two-way ANOVA post hoc Tukey test for comparison between groups (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). n = 5–10 fish per condition. ns, not significant. (D) Asciminib treatment when compared with ponatinib depicts 50 times higher IC50 value (∼5μM) in cell viability assay. (E) Asciminib does not induce apoptosis in NRVMs when compare with ponatinib by TUNEL assay. Data are presented as means ± SEM. Statistical analysis was conducted using two-way ANOVA post hoc Tukey test for comparison between groups (*P < 0.05, ***P < 0.001, ****P < 0.0001). (F) Cardiomyocyte viability comparison of the combination of TKIs possesses less cardiotoxicity than ponatinib. Cells were treated with equimolar combination (1:1 ratio) of two TKIs upto 72 h followed by cell viability assay.
Based on ongoing clinical trial (ClinicalTrials.gov Identifier: NCT02081378) to assess the combination therapy of asciminib with various approved CML TKIs, we also evaluated the cardiotoxicity of the combination of asciminib with approved TKIs. The comparative analysis of cardiomyocyte viability shows that equimolar combinations of Asciminib + Nilotinib, Asciminib + Imatinib, and Asciminib + Dasatinib are comparatively less cardiotoxic than ponatinib alone (Figure 6F). Collectively, these results demonstrate that asciminib appears to be less cardiotoxic than ponatinib and could be a therapeutic alternative to treat patients suffering from CML secondary to the T315I ‘gatekeeper’ mutation.
4. Discussion
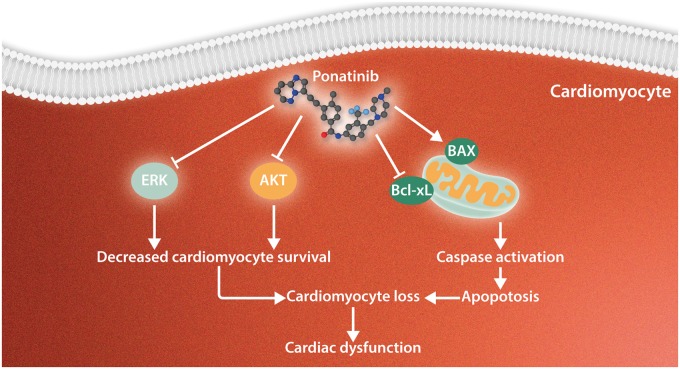
Our targeted in vivo and in vitro screen of all approved CML TKIs identified ponatinib as the most cardiotoxic drug. Ponatinib causes a dose-dependent reduction in cardiac function secondary to cardiomyocyte apoptosis. Mechanistically, we demonstrated that ponatinib exerts its cardiotoxic effect by blunting the essential cardiomyocyte prosurvival signalling pathways, ERK and AKT (Figure 7). Furthermore, pharmacologically augmenting these prosurvival pathways prevented cardiomyocyte apoptosis. Importantly, we utilized this screening system to identify a T315I selective TKI that is much less cardiotoxic than ponatinib.
Figure 7.
Proposed model of ponatinib-induced cardiotoxicity. Our findings suggest that ponatinib exerts its cardiotoxic effects by blunting the essential prosurvival pathways (AKT and ERK) to cardiomyocytes and through the activation of classical apoptotic pathways, including the up-regulation of Bax/Bcl-xL ratio and caspase cleavage. Thus, ponatinib induces cardiomyocyte death leading to cardiac dysfunction.
Our findings are consistent with previous reports with cell-based models suggesting the severe cardiotoxic potential of ponatinib.46,47 Sharma et al.46 employed a hiPSC-CM model for a high-throughput cardiotoxicity screen, among 21 small-molecule TKIs; ponatinib induced the most cell death with a median lethal dose (LD50) of 4.3 µM. Another report47 with hiPSC-CMs also demonstrated ponatinib induced cytotoxicity. Importantly, due to the well-known limitations of the cell-based models, these studies were unable to provide any extra-cardiac or organ-specific effects. Indeed, our findings suggest a cardiac-specific adverse effect of ponatinib, as we did not observe any extra-cardiac abnormalities in the zebrafish embryos. Furthermore, consistent with the previous reports, all the employed drugs caused some level of cardiomyocyte death, but ponatinib was by far the most toxic.
In early preclinical studies with ponatinib, very low rates of cardiotoxicity were reported.28,29 However, in phase 2 clinical trial, fatal or serious heart failure or left ventricular dysfunction occurred in 6% of ponatinib-treated patients and 9% of patients experienced different grades of heart failure or left ventricular dysfunction.33–36 The most frequently reported heart failure events were congestive heart failure and decreased ejection fraction. The fact that typical pre-clinical toxicology studies failed to detect significant problems, highlights the inadequacy of current approaches. Herein, we have successfully utilized zebrafish to screen the cardiotoxic potential of all approved CML TKIs and believe that a very similar approach could be employed for the screening of multiple other settings. Importantly, previous studies have demonstrated a high degree of correlation between bradycardia in zebrafish and QT prolongation in humans.69 With that said, it’s important to note that while there are several advantages to the embryonic zebrafish model, in order to extrapolate the findings to humans, undoubtedly warrants further validation in mammalian in vivo models.
As Ponatinib is the only approved TKI that is effective against CML T315I mutations, it’s crucial to delineate the molecular mechanism of ponatinib-induced cardiotoxicity. Mechanistic studies with isolated cardiomyocytes and zebrafish reveal that ponatinib treatment impaired AKT and ERK signalling pathways, which are crucial for cardiomyocyte survival. It’s well established that AKT serves as a nodal point of convergence of cardiomyocyte survival signalling and several lines of evidence have shown the necessity of AKT signalling for cardiomyocyte cell survival.70–73 Furthermore, transgenic mice with cardiomyocyte-specific overexpression of AKT improved heart function.74 In light of these reports and considering the degree of ponatinib-mediated AKT inhibition (∼70% in 12 h), the observed cardiotoxicity is not entirely surprising. Ponatinib also blunted ERK signalling, albeit to a lesser degree. ERK is also essential for cardiomyocyte survival and cardiac homeostasis.53–55 Mice with heart-specific deletion of ERK1/2 showed spontaneous cardiac dysfunction and chamber dilation, leading to severe heart failure and death.55 Conversely, transgenic mice with cardiac-specific activation of ERK1/2 signalling (via MEK1 expression) showed a physiologic hypertrophy response associated with augmented cardiac function and partial resistance to apoptosis.53 Based on our findings and previous reports, we hypothesize that pharmacologically augmenting AKT and ERK signalling would protect against ponatinib-induced cardiotoxicity. As a proof of concept, we used Neuregulin-1β to synergistically augment AKT and ERK signalling. Indeed, Neuregulin-1β treatment restored phosphorylation of AKT and ERK and cardiomyocyte viability after ponatinib treatment. In theory, if ponatinib-induced AKT and ERK inhibition does not contribute to the drug efficacy, it is conceivable to pharmacologically supplement these cascades to protect the heart from the adverse effects of the drug. On the other hand, it is completely possible that supplementing these pathways may hamper the drug efficacy or even aggravate the malignancy due to their well-known prosurvival nature. Studies with CML mouse models are warranted to further investigate these critical issues.75 However, it is reasonable to conclude that strategies for cardiac-specific augmentation of AKT and ERK signalling will potentially protect the heart from the ponatinib-induced cardiotoxicity without interfering with the anti-cancer effect of the compound.
More recently, a new allosteric TKI asciminib was developed which also works against T315I mutations at nanomolar concentrations. The target spectra of asciminib and ponatinib have been analysed previously and show extensive overlap on tyrosine kinase mutations.67,68 In a pursuit to identify a safer alternative of ponatinib for CML patients with gatekeeper T315I mutation, we investigated the comparative cardiotoxicity of ponatinib vs. asciminib. The results from our study suggest that asciminib has much less cardiomyocyte toxicity than ponatinib and could be a safer therapeutic option for CML patients carrying the T315I mutation. Ongoing clinical trials are testing the efficacy of combinational therapy of asciminib with various approved first and second generation CML TKIs (ClinicalTrials.gov Identifier: NCT02081378). Our findings suggest that such a combination therapy would be a safer alternative than ponatinib alone.
In summary, we have shown that ponatinib has significantly increased in vivo and in vitro cardiotoxicity compared with other FDA-approved CML TKIs. Importantly, we identified the AKT and ERK pathways as key targets of ponatinib, and demonstrate that inhibition of these pathways likely mediates, at least in part, the cardiotoxicity associated with this agent. Furthermore, this study suggests asciminib may be a safer treatment option compared with ponatinib for CML patients carrying the T315I ‘gatekeeper’ mutation. Finally, our findings strongly suggest the need for more comprehensive pre-clinical cardiotoxicity screening for new oncology agents using a combination of in vitro and in vivo models.
Supplementary Material
Acknowledgements
The authors like to thank Dr Javid Moslehi and Richard Gumina for their valuable expert advice.
Confict of interest: none declared.
Funding
This work was supported by the NHLBI (R01HL133290, R01HL119234 to H.L.), American Heart Association (13SDG16930103 to H.L.), and K08HL116803 and R01HL136824 (to J.R.B).
Footnotes
Time for primary review: 36 days
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 3.International Cardioncology Society North America. Mission. 2014. http://icosna.org/mission/.
- 4. Shtivelman E, Lifshitz B, Gale RP, Canaani E.. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature 1985;315:550–554. [DOI] [PubMed] [Google Scholar]
- 5. Ben-Neriah Y, Daley GQ, Mes-Masson AM, Witte ON, Baltimore D.. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science 1986;233:212–214. [DOI] [PubMed] [Google Scholar]
- 6. Daley GQ, Van Etten RA, Baltimore D.. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science 1990;247:824–830. [DOI] [PubMed] [Google Scholar]
- 7. Melo JV. The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype. Blood 1996;88:2375–2384. [PubMed] [Google Scholar]
- 8. Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, Sawyers CL.. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001;293:876–880. [DOI] [PubMed] [Google Scholar]
- 9. O'Hare T, Eide CA, Deininger MW.. Bcr-Abl kinase domain mutations, drug resistance, and the road to a cure for chronic myeloid leukemia. Blood 2007;110:2242–2249. [DOI] [PubMed] [Google Scholar]
- 10. Zhang J, Adrian FJ, Jahnke W, Cowan-Jacob SW, Li AG, Iacob RE, Sim T, Powers J, Dierks C, Sun F, Guo GR, Ding Q, Okram B, Choi Y, Wojciechowski A, Deng X, Liu G, Fendrich G, Strauss A, Vajpai N, Grzesiek S, Tuntland T, Liu Y, Bursulaya B, Azam M, Manley PW, Engen JR, Daley GQ, Warmuth M, Gray NS.. Targeting Bcr-Abl by combining allosteric with ATP-binding-site inhibitors. Nature 2010;463:501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hantschel O, Grebien F, Superti-Furga G.. The growing arsenal of ATP-competitive and allosteric inhibitors of BCR-ABL. Cancer Res 2012;72:4890–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A, Powell BL, Gabrilove JL, Rousselot P, Reiffers J, Cornelissen JJ, Hughes T, Agis H, Fischer T, Verhoef G, Shepherd J, Saglio G, Gratwohl A, Nielsen JL, Radich JP, Simonsson B, Taylor K, Baccarani M, So C, Letvak L, Larson RA, Investigators I.. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 2006;355:2408–2417. [DOI] [PubMed] [Google Scholar]
- 13. Alvarez RH, Kantarjian H, Cortes JE.. The biology of chronic myelogenous leukemia: implications for imatinib therapy. Semin Hematol 2007;44:S4–14. [DOI] [PubMed] [Google Scholar]
- 14. Hochhaus A, O'Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, Goldman JM, Muller MC, Radich JP, Rudoltz M, Mone M, Gathmann I, Hughes TP, Larson RA, Investigators I.. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia 2009;23:1054–1061. [DOI] [PubMed] [Google Scholar]
- 15. Neville K, Parise RA, Thompson P, Aleksic A, Egorin MJ, Balis FM, McGuffey L, McCully C, Berg SL, Blaney SM.. Plasma and cerebrospinal fluid pharmacokinetics of imatinib after administration to nonhuman primates. Clin Cancer Res 2004;10:2525–2529. [DOI] [PubMed] [Google Scholar]
- 16. Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, Rosenzweig A, Salomon RN, Van Etten RA, Alroy J, Durand JB, Force T.. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med 2006;12:908–916. [DOI] [PubMed] [Google Scholar]
- 17. Wolf A, Couttet P, Dong M, Grenet O, Heron M, Junker U, Laengle U, Ledieu D, Marrer E, Nussher A, Persohn E, Pognan F, Riviere GJ, Roth DR, Trendelenburg C, Tsao J, Roman D.. Imatinib does not induce cardiotoxicity at clinically relevant concentrations in preclinical studies. Leuk Res 2010;34:1180–1188. [DOI] [PubMed] [Google Scholar]
- 18. Castagnetti F, Gugliotta G, Breccia M, Stagno F, Iurlo A, Albano F, Abruzzese E, Martino B, Levato L, Intermesoli T, Pregno P, Rossi G, Gherlinzoni F, Leoni P, Cavazzini F, Venturi C, Soverini S, Testoni N, Alimena G, Cavo M, Martinelli G, Pane F, Saglio G, Rosti G, Baccarani M, Party GCW.. Long-term outcome of chronic myeloid leukemia patients treated frontline with imatinib. Leukemia 2015;29:1823–1831. [DOI] [PubMed] [Google Scholar]
- 19. Kalmanti L, Saussele S, Lauseker M, Muller MC, Dietz CT, Heinrich L, Hanfstein B, Proetel U, Fabarius A, Krause SW, Rinaldetti S, Dengler J, Falge C, Oppliger-Leibundgut E, Burchert A, Neubauer A, Kanz L, Stegelmann F, Pfreundschuh M, Spiekermann K, Scheid C, Pfirrmann M, Hochhaus A, Hasford J, Hehlmann R.. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia 2015;29:1123–1132. [DOI] [PubMed] [Google Scholar]
- 20. Soverini S, Hochhaus A, Nicolini FE, Gruber F, Lange T, Saglio G, Pane F, Muller MC, Ernst T, Rosti G, Porkka K, Baccarani M, Cross NC, Martinelli G.. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood 2011;118:1208–1215. [DOI] [PubMed] [Google Scholar]
- 21. Kamath AV, Wang J, Lee FY, Marathe PH.. Preclinical pharmacokinetics and in vitro metabolism of dasatinib (BMS-354825): a potent oral multi-targeted kinase inhibitor against SRC and BCR-ABL. Cancer Chemother Pharmacol 2008;61:365–376. [DOI] [PubMed] [Google Scholar]
- 22. Muller MC, Cortes JE, Kim DW, Druker BJ, Erben P, Pasquini R, Branford S, Hughes TP, Radich JP, Ploughman L, Mukhopadhyay J, Hochhaus A.. Dasatinib treatment of chronic-phase chronic myeloid leukemia: analysis of responses according to preexisting BCR-ABL mutations. Blood 2009;114:4944–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lambert GK, Duhme-Klair AK, Morgan T, Ramjee MK.. The background, discovery and clinical development of BCR-ABL inhibitors. Drug Discov Today 2013;18:992–1000. [DOI] [PubMed] [Google Scholar]
- 24. Reinwald M, Schleyer E, Kiewe P, Blau IW, Burmeister T, Pursche S, Neumann M, Notter M, Thiel E, Hofmann WK, Kolb HJ, Burdach S, Bender HU.. Efficacy and pharmacologic data of second-generation tyrosine kinase inhibitor nilotinib in BCR-ABL-positive leukemia patients with central nervous system relapse after allogeneic stem cell transplantation. Biomed Res Int 2014;2014:637059.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Golas JM, Arndt K, Etienne C, Lucas J, Nardin D, Gibbons J, Frost P, Ye F, Boschelli DH, Boschelli F.. SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice. Cancer Res 2003;63:375–381. [PubMed] [Google Scholar]
- 26. Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL.. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 2004;305:399–401. [DOI] [PubMed] [Google Scholar]
- 27. Weisberg E, Manley PW, Breitenstein W, Brüggen J, Cowan-Jacob SW, Ray A, Huntly B, Fabbro D, Fendrich G, Hall-Meyers E, Kung AL, Mestan J, Daley GQ, Callahan L, Catley L, Cavazza C, Azam M, Mohammed A, Neuberg D, Wright RD, Gilliland DG, Griffin JD.. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell 2005;7:129–141. [DOI] [PubMed] [Google Scholar]
- 28. Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn I, O'Hare T, Hu S, Narasimhan NI, Rivera VM, Clackson T, Turner CD, Haluska FG, Druker BJ, Deininger MW, Talpaz M.. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med 2012;367:2075–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cortes JE, Kim D-W, Pinilla-Ibarz J, Le Coutre P, Paquette R, Chuah C, Nicolini FE, Apperley JF, Khoury HJ, Talpaz M, DiPersio J, DeAngelo DJ, Abruzzese E, Rea D, Baccarani M, Müller MC, Gambacorti-Passerini C, Wong S, Lustgarten S, Rivera VM, Clackson T, Turner CD, Haluska FG, Guilhot F, Deininger MW, Hochhaus A, Hughes T, Goldman JM, Shah NP, Kantarjian H; PACE Investigators. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med 2013;369:1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, Adrian LT, Zhou T, Huang WS, Xu Q, Metcalf CA 3rd, Tyner JW, Loriaux MM, Corbin AS, Wardwell S, Ning Y, Keats JA, Wang Y, Sundaramoorthi R, Thomas M, Zhou D, Snodgrass J, Commodore L, Sawyer TK, Dalgarno DC, Deininger MW, Druker BJ, Clackson T.. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell 2009;16:401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang WS, Metcalf CA, Sundaramoorthi R, Wang Y, Zou D, Thomas RM, Zhu X, Cai L, Wen D, Liu S, Romero J, Qi J, Chen I, Banda G, Lentini SP, Das S, Xu Q, Keats J, Wang F, Wardwell S, Ning Y, Snodgrass JT, Broudy MI, Russian K, Zhou T, Commodore L, Narasimhan NI, Mohemmad QK, Iuliucci J, Rivera VM, Dalgarno DC, Sawyer TK, Clackson T, Shakespeare WC.. Discovery of 3-[2-(imidazo[1,2-b]pyridazin-3-yl)ethynyl]-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzamide (AP24534), a potent, orally active pan-inhibitor of breakpoint cluster region-abelson (BCR-ABL) kinase including the T315I gatekeeper mutant. J Med Chem 2010;53:4701–4719. [DOI] [PubMed] [Google Scholar]
- 32. Gainor JF, Chabner BA.. Ponatinib: accelerated disapproval. Oncologist 2015;20:847–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoy SM. Ponatinib: a review of its use in adults with chronic myeloid leukaemia or Philadelphia chromosome-positive acute lymphoblastic leukaemia. Drugs 2014;74:793–806. [DOI] [PubMed] [Google Scholar]
- 34.U.S. Food and Drug Administration. ICLUSIG® (ponatinib) tablets for oral use. U.S. FDA. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203469s030lbl.pdf.
- 35. Prasad V, Mailankody S.. The accelerated approval of oncologic drugs: lessons from ponatinib. JAMA 2014;311:353–354. [DOI] [PubMed] [Google Scholar]
- 36. Jain P, Kantarjian H, Jabbour E, Gonzalez GN, Borthakur G, Pemmaraju N, Daver N, Gachimova E, Ferrajoli A, Kornblau S, Ravandi F, O'Brien S, Cortes J.. Ponatinib as first-line treatment for patients with chronic myeloid leukaemia in chronic phase: a phase 2 study. Lancet Haematol 2015;2:e376–e383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ai N, Chong CM, Chen W, Hu Z, Su H, Chen G, Lei Wong QW, Ge W. . Ponatinib exerts anti-angiogenic effects in the zebrafish and human umbilical vein endothelial cells via blocking VEGFR signaling pathway. Oncotarget 2018;9:31958–31970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gover-Proaktor A, Granot G, Shapira S, Raz O, Pasvolsky O, Nagler A, Lev DL, Inbal A, Lubin I, Raanani P, Leader A.. Ponatinib reduces viability, migration, and functionality of human endothelial cells. Leuk Lymphoma 2017;58:1455–1467. [DOI] [PubMed] [Google Scholar]
- 39. Valent P, Hadzijusufovic E, Schernthaner GH, Wolf D, Rea D, Le Coutre P.. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood 2015;125:901–906. [DOI] [PubMed] [Google Scholar]
- 40. Burns CG, Milan DJ, Grande EJ, Rottbauer W, MacRae CA, Fishman MC.. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat Chem Biol 2005;1:263–264. [DOI] [PubMed] [Google Scholar]
- 41. Becker JR, Robinson TY, Sachidanandan C, Kelly AE, Coy S, Peterson RT, MacRae CA.. In vivo natriuretic peptide reporter assay identifies chemical modifiers of hypertrophic cardiomyopathy signalling. Cardiovasc Res 2012;93:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheng H, Kari G, Dicker AP, Rodeck U, Koch WJ, Force T.. A novel preclinical strategy for identifying cardiotoxic kinase inhibitors and mechanisms of cardiotoxicity. Circ Res 2011;109:1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wiley DS, Redfield SE, Zon LI.. Chemical screening in zebrafish for novel biological and therapeutic discovery. Methods Cell Biol 2017;138:651–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Becker JR, Deo RC, Werdich AA, Panakova D, Coy S, MacRae CA.. Human cardiomyopathy mutations induce myocyte hyperplasia and activate hypertrophic pathways during cardiogenesis in zebrafish. Dis Model Mech 2011;4:400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Association AVM. AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. American Veterinary Medical Association; 2013. https://www.avma.org/KB/Policies/Documents/euthanasia.pdf. [Google Scholar]
- 46. Sharma A, Burridge PW, McKeithan WL, Serrano R, Shukla P, Sayed N, Churko JM, Kitani T, Wu H, Holmstrom A, Matsa E, Zhang Y, Kumar A, Fan AC, Del Alamo JC, Wu SM, Moslehi JJ, Mercola M, Wu JC.. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci Transl Med 2017;9:eaaf2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Talbert DR, Doherty KR, Trusk PB, Moran DM, Shell SA, Bacus S.. A multi-parameter in vitro screen in human stem cell-derived cardiomyocytes identifies ponatinib-induced structural and functional cardiac toxicity. Toxicol Sci 2015;143:147–155. [DOI] [PubMed] [Google Scholar]
- 48. Czabotar PE, Lessene G, Strasser A, Adams JM.. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 2014;15:49–63. [DOI] [PubMed] [Google Scholar]
- 49. Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, Walensky LD.. BAX activation is initiated at a novel interaction site. Nature 2008;455:1076–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW.. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 1997;275:983–986. [DOI] [PubMed] [Google Scholar]
- 51. Vanhaesebroeck B, Alessi DR.. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J 2000;346 Pt 3:561–576. [PMC free article] [PubMed] [Google Scholar]
- 52. Lu Z, Xu S.. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 2006;58:621–631. [DOI] [PubMed] [Google Scholar]
- 53. Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD.. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J 2000;19:6341–6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu R, van Berlo JH, York AJ, Vagnozzi RJ, Maillet M, Molkentin JD.. DUSP8 regulates cardiac ventricular remodeling by altering ERK1/2 signaling. Circ Res 2016;119:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kehat I, Davis J, Tiburcy M, Accornero F, Saba-El-Leil MK, Maillet M, York AJ, Lorenz JN, Zimmermann WH, Meloche S, Molkentin JD.. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res 2011;108:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ren M, Hong M, Liu G, Wang H, Patel V, Biddinger P, Silva J, Cowell J, Hao Z.. Novel FGFR inhibitor ponatinib suppresses the growth of non-small cell lung cancer cells overexpressing FGFR1. Oncol Rep 2013;29:2181–2190. [DOI] [PubMed] [Google Scholar]
- 57. Chase A, Bryant C, Score J, Cross NC.. Ponatinib as targeted therapy for FGFR1 fusions associated with the 8p11 myeloproliferative syndrome. Haematologica 2013;98:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gozgit JM, Wong MJ, Moran L, Wardwell S, Mohemmad QK, Narasimhan NI, Shakespeare WC, Wang F, Clackson T, Rivera VM.. Ponatinib (AP24534), a multitargeted pan-FGFR inhibitor with activity in multiple FGFR-amplified or mutated cancer models. Mol Cancer Ther 2012;11:690–699. [DOI] [PubMed] [Google Scholar]
- 59. Ren M, Qin H, Ren R, Cowell JK.. Ponatinib suppresses the development of myeloid and lymphoid malignancies associated with FGFR1 abnormalities. Leukemia 2013;27:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P.. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res 2002;90:390–397. [DOI] [PubMed] [Google Scholar]
- 61. Brar BK, Stephanou A, Liao Z, O'Leary RM, Pennica D, Yellon DM, Latchman DS.. Cardiotrophin-1 can protect cardiac myocytes from injury when added both prior to simulated ischaemia and at reoxygenation. Cardiovasc Res 2001;51:265–274. [DOI] [PubMed] [Google Scholar]
- 62. Liao Z, Brar BK, Cai Q, Stephanou A, O'Leary RM, Pennica D, Yellon DM, Latchman DS.. Cardiotrophin-1 (CT-1) can protect the adult heart from injury when added both prior to ischaemia and at reperfusion. Cardiovasc Res 2002;53:902–910. [DOI] [PubMed] [Google Scholar]
- 63. Lenihan DJ, Anderson SA, Lenneman CG, Brittain E, Muldowney JAS, Mendes L, Zhao PZ, Iaci J, Frohwein S, Zolty R, Eisen A, Sawyer DB, Caggiano AO.. A phase I, single ascending dose study of cimaglermin alfa (Neuregulin 1β3) in patients with systolic dysfunction and heart failure. JACC Basic Transl Sci 2016;1:576.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM.. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation 2002;105:1551–1554. [DOI] [PubMed] [Google Scholar]
- 65. Pentassuglia L, Timolati F, Seifriz F, Abudukadier K, Suter TM, Zuppinger C.. Inhibition of ErbB2/neuregulin signaling augments paclitaxel-induced cardiotoxicity in adult ventricular myocytes. Exp Cell Res 2007;313:1588–1601. [DOI] [PubMed] [Google Scholar]
- 66. Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB.. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol 2003;35:1473–1479. [DOI] [PubMed] [Google Scholar]
- 67. Wylie AA, Schoepfer J, Jahnke W, Cowan-Jacob SW, Loo A, Furet P, Marzinzik AL, Pelle X, Donovan J, Zhu W, Buonamici S, Hassan AQ, Lombardo F, Iyer V, Palmer M, Berellini G, Dodd S, Thohan S, Bitter H, Branford S, Ross DM, Hughes TP, Petruzzelli L, Vanasse KG, Warmuth M, Hofmann F, Keen NJ, Sellers WR.. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature 2017;543:733–737. [DOI] [PubMed] [Google Scholar]
- 68. Adrian FJ, Ding Q, Sim T, Velentza A, Sloan C, Liu Y, Zhang G, Hur W, Ding S, Manley P, Mestan J, Fabbro D, Gray NS.. Allosteric inhibitors of Bcr-abl-dependent cell proliferation. Nat Chem Biol 2006;2:95–102. [DOI] [PubMed] [Google Scholar]
- 69. Milan DJ, Peterson TA, Ruskin JN, Peterson RT, MacRae CA.. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation 2003;107:1355–1358. [DOI] [PubMed] [Google Scholar]
- 70. Sussman MA, Volkers M, Fischer K, Bailey B, Cottage CT, Din S, Gude N, Avitabile D, Alvarez R, Sundararaman B, Quijada P, Mason M, Konstandin MH, Malhowski A, Cheng Z, Khan M, McGregor M.. Myocardial AKT: the omnipresent nexus. Physiol Rev 2011;91:1023–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A.. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 2001;104:330–335. [DOI] [PubMed] [Google Scholar]
- 72. Matsui T, Rosenzweig A.. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol 2005;38:63–71. [DOI] [PubMed] [Google Scholar]
- 73. Matsui T, Nagoshi T, Rosenzweig A.. Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle 2003;2:220–223. [PubMed] [Google Scholar]
- 74. Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J Jr.. Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci USA 2002;99:12333–12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Peng C, Li S.. Chronic myeloid leukemia (CML) mouse model in translational research. Methods Mol Biol 2016;1438:225–243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.