Abstract
Objectives
To determine the prevalence of inappropriate prescribing in elderly patients with polypharmacy admitted to a long-term care hospital (LTCH) and to evaluate the impact of an interdisciplinary pharmacotherapy quality programme on improvement of prescribing appropriateness.
Methods
An interventional, longitudinal, prospective study was conducted in a Spanish LTCH (October 2013 to July 2014) including 162 elderly (≥70 years) patients with polypharmacy (≥5 medications). Pharmacists conducted the pharmacotherapy follow-up of patients with medication reconciliation, pharmacotherapeutic optimisation and educational interviews from admission to discharge. Reconciliation errors, potentially inappropriate medications (PIMs), potentially prescribing omissions (PPOs) and significant drug interactions rates were calculated. The impact of the programme was evaluated considering the difference between the inappropriateness score per patient (total number of reconciliation errors, PIMs, PPOs and significant drug interactions) before and after implementing pharmacotherapy recommendations.
Results
At admission, 163 reconciliation errors (median(range), 1(1-6)) in 92 (56.8%) patients (65.6% drug omissions), 335 PIMs (2(1-6)) in 147 (90.7%) patients (39.3% use ≥2 anticholinergic drugs), 43 PPOs (1(1-3)) in 32 (19.8%) patients (48.5% statin omission) and 594 significant drug interactions (4(1-19)) in 130 (80.2%) patients were detected. After implementing pharmacotherapy recommendations, statistically significant reductions in admission reconciliation errors (8.3% to 0.1%), PIMs (17.0% to 12.2%), PPOs (2.2% to 0.7%) and significant drug interactions (30.2% to 26.8%) rates were found. The programme achieved a 31% improvement in prescribing appropriateness, with a statistically significant reduction in the inappropriateness score (6(IQR:4–9) to 4(IQR:2–7)).
Conclusion
Reconciliation errors, PIMs and drug interactions are highly prevalent in elderly patients with polypharmacy admitted to an LTCH. This interdisciplinary pharmacotherapy quality programme seems to be a useful approach in the improvement of prescribing appropriateness in a high-risk older population.
Keywords: clinical pharmacy, hospital pharmacy education, therapeutics, geriatric medicine
Key messages.
What is already known on this subject?
Older patients with polypharmacy are associated with an increased risk of inappropriate prescriptions and drug-related problems (DRPs). There is a need to ensure appropriate pharmacotherapy for these patients, especially at care transitions.
Prescribing potentially inappropriate medications (PIMs) to elderly patients is quite prevalent across all care settings such as primary care, acute-care hospitals and nursing homes. In these settings, there is increasing evidence that pharmacists’ involvement in interdisciplinary teams has a positive influence on the quality of medication use and patient safety by rationalising the pharmacotherapy and reducing medication errors, PIMs and DRPs.
What this study adds?
This study determines the prevalence of inappropriate prescribing in elderly patients with polypharmacy admitted to a long-term care hospital (LTCH).
Further evidence on the need to implement a comprehensive interdisciplinary approach such as this pharmacotherapy quality programme in LTCHs. In addition, the usefulness of this programme to improve prescribing appropriateness in high-risk older populations with multimorbidity.
Introduction
Population ageing causes a higher prevalence of multiple chronic disorders, leading to polypharmacy, irrational prescribing and medication misuse.1 Therefore, any potential improvement in prescription and medication use in this growing group could have a substantial positive impact on patient safety and health resource consumption.2
Various strategies have been developed to assess appropriateness in prescribing with elderly patients, such as educational interventions, medication reconciliation reviews, geriatricians’ services, interdisciplinary teams, computerised support systems, regulatory policies and multi-faceted approaches.3 These approaches can be divided into explicit (criterion-based) and implicit (judgement-based) methods. Among the explicit methods are the STOPP-START criteria4 which detect potentially inappropriate medications (PIMs) and potentially prescription omissions (PPOs). This validated tool has demonstrated improvements in appropriate polypharmacy based on reductions in inappropriate prescribing.5 Among the implicit methods are the Medication Appropriateness Index6 or the IASER method7 (a Cipolle and Strand methodology-based tool8 focused on drug-related problems’ detection). Hence, a comprehensive approach, including more than one method for improving prescription quality, is likely to be required.3
PIM prescribing in elderly patients is quite prevalent across all care settings from community to long-term care.9–14 Some studies have been conducted in the nursing home setting11 12 15 and, recently, a study determined the prevalence of PIMs in a long-term care hospital (LTCH).16 In acute care settings, PIMs can be even more problematic due to multiple physicians and specialists who may be prescribing for a single patient as well as difficulty with medication reconciliation at transitions and limitations imposed by hospital formularies. This situation may be aggravated in patients admitted to an LTCH who are discharged from acute hospitals.
The medication reconciliation process provides opportunities to reconsider the appropriateness of patient’s medications over time as the patient’s condition may change or as other prescribers become involved. Differences between the medication lists, or medication discrepancies, are a common cause for confusion about the intended medication regimen and can lead to adverse drug events or harm due to the suboptimal use of medications or reconciliations. The latter are highly prevalent (from 3.4% to 98.2% of patients) across a broad range of settings, such as acute hospital, primary care and nursing homes.17–21 However, there is a lack of published data on their prevalence in LTCHs. Therefore, medication reconciliation is an important approach to improve the quality of the medication use and this practice should incorporate mitigation strategies to manage drug interactions such as discontinuation of drugs that do not achieve the desired end points or that are no longer needed.22 Thus, rational deprescribing of unnecessary or harmful medications is recommended to reduce the likelihood of clinically significant adverse events.23
As a part of interdisciplinary teams, pharmacists’ collaboration with other healthcare professionals is crucial to increase the effectiveness of pharmacist interventions in care transitions, and to make valuable contributions towards decision making.24 Pharmacists have shown improvements in prescribing appropriateness and patient safety by rationalising the pharmacotherapy and reducing medication errors, PIMs and drug-related problems.25–27 Nevertheless, as far as we know, no studies have been published evaluating this impact in an LTCH.
This study aims to determine the prevalence of inappropriate prescribing in elderly patients with polypharmacy admitted to an LTCH, and to evaluate the impact of an interdisciplinary pharmacotherapy quality improvement and patient safety programme on improvement of prescribing appropriateness.
Methods
Study population and setting
From October 2013 to July 2014, an interventional, longitudinal, prospective study was performed at Pare Jofre Hospital, a 125-bed Spanish LTCH. Elderly patients (70 years of age or older) with polypharmacy (five medications or more) discharged from acute care hospitals who were admitted to the Comprehensive Medical Unit (CMU) requiring post-acute convalescent, medium-term rehabilitation care or palliative care were eligible for the programme. Patients were recruited over a 6-month period (October 2013 to April 2014).
A consensual seven-step protocol was applied to carry out the interdisciplinary pharmacotherapy quality improvement and patient safety programme in the CMU (see online supplementary appendix A). Physicians, nurses, a full-time clinical pharmacist with advanced training (eg, 4-year hospital pharmacy residency and practical knowledge in pharmaceutical care) and other healthcare providers were involved in the programme. A detailed description of the programme and its clinical impact by identifying, preventing and resolving drug-related problems, and consequently drug-related morbidity, was previously reported.28
ejhpharm-2017-001411supp001.pdf (203.1KB, pdf)
Outcome measures
Patient demographic data included age, sex, care objective (post-acute convalescent, medium-term rehabilitation care or palliative care) and hospitalisations in the last year. The following clinical characteristics were collected: reason for admission; pathologies and patient comorbidity consistent with Charlson Comorbidity Index (CCI) score and age-adjusted CCI (higher scores indicate a greater level of comorbidity); Pfeiffer questionnaire to evaluate cognitive status (scores of 0–10, score ≥3 indicates cognitive impairment); Barthel Index to assess functional status (0–100 score, greater dependence is associated with lower scores); hospital length of stay; and in-hospital mortality. The following treatment-related information was also obtained:
Prescribed medications at admission (number and type). Drugs with antimuscarinic/anticholinergic properties according to ARS scale.29
Medication reconciliation: number and type of medication discrepancies and reconciliation errors; the potential to cause patient harm was assessed by considering the severity of reconciliation errors (rated from grade 1 – would not cause harm or would cause reversible harm that would require monitoring – to grade 5 – would cause lethal harm, by agreement between pharmacist and physician according to IASER method7) and by determining if the medication involved in the reconciliation error was included or not in the Institute for Safe Medication Practices high-alert medication list: and drug classes of reconciliation errors were coded using the Anatomical Therapeutic Chemical classification system. A discrepancy was defined as ‘any difference between the three previously compared medication lists: discharge medication list from acute care hospital; current admission order; and chronic ambulatory medication list’. Whether discrepancies were to adjust the medication to a new clinical status included in clinical records, they were considered documented discrepancies. The remaining discrepancies that required clarification by a physician were undocumented discrepancies. After clarifying discrepancies, they were finally classified as intentional or unintentional discrepancies. A reconciliation error was defined as ‘an undocumented unintentional discrepancy that implied a change in prescribed medication list’.
STOPP/START criteria: number and type of PIMs and PPOs. Initially, the 2008 STOPP/START criteria (version 1) were applied, but they were recoded retrospectively to updated 2014 STOPP/START criteria (version 2). One adaptation of the original START criteria was made (six out of 34 criteria selected), by consensus of the interdisciplinary team, to make the criteria fit the elderly patient’s clinical status admitted to the LTCH.
Drug interaction: number, levels of severity (contraindicated, major, moderate, minor and unknown), scientific documentation status (excellent, good, fair, poor or unlikely) and potential risk. Only significant drug interactions were selected, as follows: contraindication; major drug interaction (may be life-threatening or require medical intervention to minimise or prevent adverse drug events); and moderate drug interaction (may result in exacerbation of the patient’s condition or require an alteration in therapy).
The decision to intervene depended on clinical judgement to find the proper balance between the need medications and the potential risk of adverse events that each additional medication could introduce. Types of pharmacotherapy recommendations were classified as individualisation of dosage regimen, cessation, initiation or change of drug, initiation of therapeutic drug monitoring or clinical monitoring, and change to more effective, safety or cost-effectiveness drug. Level of acceptance was defined as accepted, partially accepted or rejected.7 A reconciliation error, PIM, PPO or drug interaction was prevented or resolved if a pharmacotherapy recommendation was fully or partially implemented and a desired pharmacotherapeutic outcome was achieved, thus avoiding inappropriate drug use according to clinical guidelines in older patients. It was checked by a clinical pharmacist through individualised patient follow-up, by speaking to the physician or by examining the patient’s clinical records.
Initial and final reconciliation error, PIM, PPO and drug interaction rates (before and after implementing pharmacotherapy recommendations) were calculated, as follows: (i) reconciliation error rate=Total number of admission reconciliation errors/Total number of admission LTCH medicationsx100; (ii) PIM rate=Total number of PIMs/Total number of admission LTCH medicationsx100; (iii) PPO rate=Total number of PPOs/Total number of admission LTCH medicationsx100; and (iv) significant drug interaction rate=Total number of significant drug interactions/Total number of admission LTCH medicationsx100.
The impact of the programme on the prescribing appropriateness was assessed by the change in the inappropriateness score after implementing pharmacotherapy recommendations. This score was calculated by totalling the number of reconciliation errors, PIMs, PPOs and significant drug interactions met per patient in prescribed medications at admission, and calculating a total score for each patient. A higher inappropriateness score signified more reconciliation errors, PIMs, PPOs and/or drug interactions being met, thus, lower prescribing appropriateness.
Statistical analysis
Descriptive statistics were utilised to summarise the population and the Shapiro–Wilks normality test was used. The Wilcoxon signed rank was used to examine the difference in reconciliation errors, PIMs, PPOs and drug interactions, and inappropriateness score after implementing pharmacotherapy recommendations. The association between inappropriateness score and number of prescribed medications was assessed using Spearman’s ρ correlation coefficient. χ2 testing was used to compare the number of patients with ≥1 inappropriate item after implementing pharmacotherapy recommendations. Statistical significance at P<0.05 was assumed. Data analyses were performed using SPSS version 17.0.
Results
Two hundred and eighty-five candidates were admitted to the CMU during the recruitment period. Following exclusion of the patients who were younger than 70 years (n=99), took less than five medications (n=7), were actively dying (n=15) or declined to participate in the programme (n=2), 162 (56.8%) elderly patients with polypharmacy were included. The most frequent reasons for admission were cancer (n=44, 27.2%), cerebral vascular disease (n=35, 21.6%) and pneumonia (n=22, 13.6%). The main characteristics of the patients included in the study are described in table 1.
Table 1.
Main characteristics of the 162 patients included in the programme
| Mean ± SD age in years | 81.1 ± 6.2 |
| Female, n (%) | 86 (53.1) |
| Care objectives, n (%) | |
| Post-acute convalescent care | 90 (55.6) |
| Palliative care | 50 (30.9) |
| Median of hospitalisations in the last year (range) | 2 (1–6) |
| Pathologies | |
| Median (range) per patient | 3 (2–6) |
| Hypertension, n (%) | 124 (76.5) |
| Moderate-severe renal disease, n (%) | 87 (53.7) |
| Dyslipidaemia, n (%) | 84 (51.9) |
| Dementia, n (%) | 78 (48.1) |
| Congestive heart failure, n (%) | 71 (43.8) |
| Comorbidity, n (%) | |
| High | |
| CCI ≥ 3 points | 126 (77.8) |
| Very high | |
| CCI ≥ 5 points | 83 (51.2) |
| Age-adjusted CCI ≥ 5 points | 156 (96.3) |
| Cognitive impairment, n (%) | |
| Pfeiffer score ≥3 errors | 119 (73.5) |
| Total dependence, n (%) | |
| Barthel Index ≤20 points | 137 (84.6) |
| Median hospital LOS in days (range) | 42.5 (3–160) |
| In-hospital mortality, n (%) | 83 (51.2) |
| Prescribed medication at admission | |
| Total number, n | 1970* |
| Mean±SD per patient | 12.2±3.7 |
| ≥10 medications, n (%) | 118 (72.8) |
| ≥15 medications, n (%) | 43 (26.5) |
| ≥20 medications, n (%) | 5 (3.1) |
CCI Charlson Comorbidity Index, LOS length of stay.
*Total number of admission long-term care hospital medications used to calculate reconciliation error, PIM, PPO and drug interaction rates.
Medication reconciliation
Overall, 1315 medication discrepancies were detected in all patients with a median of 8 (2–12) discrepancies per patient at admission to the LTCH. Of these, 967 (73.5%) were documented discrepancies and 185 (14.1%) were undocumented intentional discrepancies. The remaining 163 (12.4%) were reconciliation errors, and represented an 8.3% initial reconciliation error rate. There were no unresolved discrepancies. In total, 92 (56.8%) of the patients suffered at least one reconciliation error, with a median of 1 (1-6) reconciliation error per patient. The most common reconciliation error was drug omission (65.6%), followed by different dosage, frequency or route of administration (20.2%), wrong drug (7.4%), drug commission (6.1%) and incomplete prescription (0.6%).
Regarding the potential clinical impact of reconciliation errors if undetected on admission, 93 (57.1%) were categorised as potential severity grade 2 (reversible harm that would require change of therapy) and 55 (33.7%) as grade 3 (reversible harm that would require additional therapy or increased length of stay). No potentially lethal harm was detected in our study. High-risk medications accounted for 16 (9.8%) reconciliation errors, including anticoagulants (n=8), opioids (n=4), digoxin (n=1), oral hypoglycaemics (n=1), parenteral nutrition preparations (n=1) and subcutaneous insulin (n=1).
The most frequently prescribed drug classes involved in detected reconciliation errors at admission were medications acting on the nervous system (ATC group N; 24.5%), cardiovascular system (ATC group C; 22.1%) and alimentary tract and metabolism (ATC group A; 15.3%). table 2 summarises the five drug classes most commonly implicated in reconciliation errors.
Table 2.
The five drug classes most commonly involved in reconciliation errors detected on admission
| Therapeutic groups and subgroups (ATC classification) | n (%) |
| Nervous system (N) | 40 (24.5) |
| Psycholeptics (N05) | 13 |
| Analgesics (N02) | 10 |
| Psychoanaleptics (N06) | 10 |
| Antiepileptics (N03) | 6 |
| Anti-Parkinson drugs (N04) | 1 |
| Cardiovascular system (C) | 36 (22.1) |
| Lipid modifying agents (C10) | 9 |
| Diuretics (C03) | 8 |
| Beta-blocking agents (C07) | 6 |
| Agents acting on the renin-angiotensin system (C09) | 5 |
| Cardiac therapy (C01) | 4 |
| Calcium channel blockers (C08) | 4 |
| Alimentary tract and metabolism (A) | 25 (15.3) |
| Drugs for acid-related disorders (A02) | 12 |
| Drugs for constipation (A06) | 5 |
| Drugs for functional gastrointestinal disorders (A03) | 2 |
| Drugs used in diabetes (A10) | 2 |
| Mineral supplements (A12) | 2 |
| Antiemetics and antinauseants (A04) | 1 |
| Digestives, incl. enzymes (A09) | 1 |
| Blood and blood forming organs (B) | 25 (15.3) |
| Antithrombotic agents (B01) | 18 |
| Antianaemic preparations (B03) | 5 |
| Blood substitutes and perfusion solutions (B05) | 2 |
| Sensory organs (S) | 16 (9.8) |
| Ophthalmologicals (S01) | 16 |
| Total | 142/163 (87.1) |
ATC, anatomical therapeutic chemical.
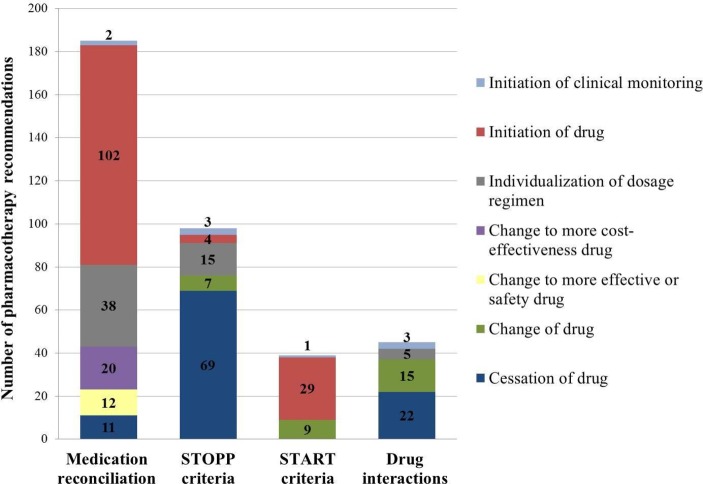
Of 185 pharmacotherapy recommendations (figure 1), 179 (96.8%) were accepted and implemented by the physicians leading to a decrease of two reconciliation errors in two (1.2%) patients, representing a 0.1% final reconciliation error rate. A Wilcoxon signed rank test reported a statistically significant reduction in the reconciliation errors (z=−12.649, P<0.001).
Figure 1.
Proposed pharmacotherapy recommendations during hospital admission.
STOPP/START criteria
Considering the STOPP criteria, 335 PIMs were identified in 147 (90.7%) patients at admission. A median of 2 (1–6) PIMs per patient was detected, representing a 17.0% initial PIM rate. Among participants with PIMs, 95 (64.6%) were prescribed two or more PIMs and 61 (41.5%) were prescribed three or more PIMs. The most common PIM was the concomitant use of two or more drugs with antimuscarinic/anticholinergic properties (39.3%) with a median ARS scale of 5 (1–10), followed by a drug prescribed without an evidence-based clinical indication (17.6%) and benzodiazepines for ≥4 weeks (9.6%).
The most frequently prescribed drugs without an evidence-based clinical indication were medications acting on the nervous system (ATC group N; 18.6%), blood and blood forming organs (ATC group B; 16.9%), and respiratory system (ATC group R; 15.3%). Classification of detected PIMs is reported in table 3. The 91.8% (90/98) of pharmacotherapy recommendations (figure 1) were accepted and implemented by the physicians, leading to a decrease of 240 PIMs in 140 (86.4%) patients, representing a 12.2% final PIM rate. A Wilcoxon signed rank test reported a statistically significant reduction in the PIMs (z=− 7.173, P<0.001).
Table 3.
Potentially inappropriate medications identified on admission during the programme
| PIMs type | n (%) |
| Drug indication criteria | 84 (25.1) |
| Any drug prescribed without an evidence-based clinical indication | 59 |
| Any drug prescribed beyond the recommended duration, where treatment duration is well defined | 18 |
| Any duplicate drug class prescription | 7 |
| Cardiovascular system criteria | 6 (1.8) |
| Amiodarone as first-line antiarrhythmic therapy in supraventricular tachyarrhythmia | 4 |
| Aldosterone antagonists with concurrent potassium-conserving drugs without monitoring of serum potassium | 2 |
| Coagulation system criteria | 9 (2.7) |
| Long-term aspirin at doses greater than 160 mg per day | 7 |
| Aspirin with a past history of peptic ulcer disease without concomitant proton pump inhibitor | 2 |
| Nervous system criteria | 59 (17.6) |
| Benzodiazepines for ≥ 4 weeks | 32 |
| Neuroleptic antipsychotic in patients with behavioural and psychological symptoms of dementia unless symptoms are severe and other treatments have failed | 20 |
| Neuroleptics as hypnotics, unless sleep disorder is due to psychosis or dementia | 2 |
| Prolonged use of first-generation antihistamines | 5 |
| Renal system criteria | 2 (0.6) |
| Digoxin at a long-term dose greater than 125 µg/day if eGFR < 30 mL/min / 1.73 m2 | 2 |
| Gastrointestinal system criteria | 3 (0.9) |
| Drugs likely to cause constipation in patients with chronic constipation where non-constipating alternatives are appropriate | 3 |
| Respiratory system criteria | 2 (0.6) |
| Anti-muscarinic bronchodilators with a history of narrow angle glaucoma or bladder outflow obstruction | 1 |
| Benzodiazepines with acute or chronic respiratory failure | 1 |
| Drugs that predictably increase the risk of falls in older people | 25 (7.5) |
| Benzodiazepines | 11 |
| Hypnotic Z-drugs (eg, zolpidem) | 14 |
| Analgesic drugs | 14 (4.2) |
| Use of regular (as distinct from PRN) opioids without concomitant laxative | 10 |
| Long-acting opioids without short-acting opioids for break-through pain | 4 |
| Antimuscarinic/anticholinergic drug burden | 131 (39.3) |
| Concomitant use of two or more drugs with antimuscarinic/anticholinergic properties | 131 |
| Total | 335 (100) |
PIM potentially inappropriate medication, eGFR estimated glomerular filtration rate
Considering the six included START criteria, 43 PPOs were identified in 32 (19.8%) patients at admission, representing a 2.2% initial PPO rate. A median of 1 (1–3) PPO per patient was detected. Statin in coronary, cerebral or peripheral vascular disease was the most frequent PPO (n=16, 37.2%). The second most frequent PPO was ACE inhibitor in diabetes with evidence of renal disease (n=10, 23.3%), followed by bisphosphonate, vitamin D and calcium in osteoporosis (n=8, 18.6%), antiplatelet therapy in coronary, cerebral or peripheral vascular disease (n=6, 14.0%) and bisphosphonate, vitamin D and calcium in patients taking long-term systemic corticosteroid therapy (n=3, 7.0%).
The 79.5% (31/39) of pharmacotherapy recommendations (figure 1) were accepted and implemented by the physicians leading to a decrease of 14 PPOs in nine (5.6%) patients, representing a 0.7% final PPO rate. A Wilcoxon signed rank test reported a statistically significant reduction in the PPOs (z=−4.564, P<0.001).
Drug interactions
Five hundred and ninety-four potential significant drug interactions were identified in 130 (80.2%) patients at admission. Of all, 19 (3.2%) were contraindications, 289 (48.7%) were major drug interactions and 286 (48.1%) were moderate drug interactions. A median of 4 (1–19) drug interactions per patient was detected, representing a 30.2% initial potential drug interaction rate. Fifty-one (39.2%) patients were exposed to five or more potential significant drug interactions. One hundred and ten (84.6%) patients were exposed to at least one potential contraindication or major drug interaction, and 13 (10.0%) patients were exposed to at least one potential contraindication.
The most common potential contraindications were metoclopramide-haloperidol (n=4, 21.1%), metoclopramide-trazodone (n=4, 21.1%) and metoclopramide-quetiapine (n=3, 15.8%). These drug interactions may result in an increased risk of extrapyramidal reactions. The 10 potential major drug interactions most frequently encountered at admission, along with their documentation status and potential risk, are reported in table 4.
Table 4.
The 10 potential major drug interactions most commonly detected at admission
| Major drug interactions | Documentation status | Potential risk | n (%) |
| Aspirin-enoxaparin | Good | Increased risk of bleeding | 21 (7.3) |
| Clopidogrel-omeprazole | Excellent | Increased risk for thrombosis | 11 (3.8) |
| Clopidogrel-enoxaparin | Fair | Increased risk of bleeding | 10 (3.5) |
| Fentanyl-morphine | Fair | Increased risk of CNS depression | 10 (3.5) |
| Aspirin-clopidogrel | Fair | Increased risk of bleeding | 8 (2.8) |
| Fentanyl-lorazepam | Fair | Increased risk of CNS depression | 6 (2.1) |
| Omeprazole-citalopram | Fair | Increased risk of QT interval prolongation | 6 (2.1) |
| Citalopram-enoxaparin | Good | Increased risk of bleeding | 5 (1.7) |
| Lorazepam-zolpidem | Fair | Increased risk of CNS depression | 5 (1.7) |
| Quetiapine-trazodone | Fair | Increased risk of QT interval prolongation | 5 (1.7) |
| Total | 87/289 (30.1) |
CNS, central nervous system.
The 91.1% (41/45) of pharmacotherapy recommendations (figure 1) were accepted and implemented by the physicians, leading to a decrease of 528 drug interactions in 125 (77.2%) patients, representing a 26.8% final potential drug interaction rate. A Wilcoxon signed rank test reported a statistically significant reduction in the drug interactions (z=−5.158, P<0.001).
Inappropriateness score
As a result of the programme, the implementation of pharmacotherapy recommendations revealed a 30.9% (1135 vs 784) decrease in the inappropriateness prescribing. The initial inappropriateness score showed a median of 6 (0–23) (IQR: 4–9) inappropriate items per patient: in contrast, the final inappropriateness score revealed a median of 4 (0–21) (IQR: 2–7) inappropriate items per patient. A statistically significant reduction was reported by Wilcoxon signed rank test (z=−9.639, P<0.001).
A weak but statistically significant correlation was found between the number of prescribed medications and the initial inappropriateness score per patient at admission (correlation coefficient (rs)=0.394, P<0.001).
Nevertheless, the total number of prescribed medications (2110) was similar after implementing pharmacotherapy recommendations, with a mean of 12.4 (3.5) medications per patient. In total, 122 (75.3%) patients had a lower inappropriateness score and 40 (24.7%) patients had no change in their inappropriateness score. There was a slight but statistically significant reduction in the number of patients with≥1 inappropriate item, from 158 (97.5%) to 149 (92.0%) patients (P<0.001).
Discussion
This study implemented a pharmacotherapy quality improvement and patient safety programme with a prospective design and a multifactorial interdisciplinary intervention, including medication reconciliation, structured comprehensive assessment of pharmacotherapy, face-to-face discussion with the patient’s physician and individualised pharmacotherapy follow-up during hospital stay. The intervention was conducted in a real-life context. Along with the high clinical impact previously shown,28 this study also goes one step further than previous observational studies on prescribing appropriateness, by providing all implemented pharmacotherapy recommendations. Our findings suggest that this programme seems to be helpful in improving the prescribing appropriateness among these high-risk elderly patients recently discharged from acute hospitals, and consequently, improve procedures on LTCHs.
In our setting, defining the precise appropriateness of medication considering multimorbidity, functional dependence, cognitive impairment, potential drug interactions and high in-hospital mortality can be a complicated goal. Ensing et al,24 showed that pharmacists have additional value in multifaceted and interdisciplinary programmes by performing a clinical medication review in addition to patient-involved medication reconciliation, as implemented in our programme.
Although medication discrepancies were detected in all the patients at admission, only 12.4% of these discrepancies were proved to be reconciliation errors, in line with other studies focused on elderly patients with polypharmacy admitted in acute hospitals.18 21 Eighty-eight per cent of the medication discrepancies were clarified at admission, either by reviewing the patient’s medical records or by directly questioning the physician, patient and/or caregiver. As previously reported in other studies of aged populations,18 21 more than half of patients had at least one reconciliation error and a median of one reconciliation error per patient was detected.
Despite being conducted in different research settings, most studies also reported drug omission as the most frequent type of medication reconciliation error identified, which accounted for 40% to 100%.17–21 Lack of information on outpatient medication lists, incomplete anamnesis or complexity of medication regimens might contribute to drug omissions at admission, suggesting the need for pharmacotherapy quality programmes during care transitions, as some other authors have recognised.30 In contrast with other published studies,17–21 the majority of detected reconciliation errors were judged to be of clinical importance and a higher percentage of them involved high-alert medications. These differences could be justified, mainly, by different clinical patient status and evaluation methodologies used to rate the potential clinical significance of reconciliation errors, by agreement between the pharmacist and physician in our study.
There is emerging evidence supporting an interdisciplinary systematic approach to deprescribing or ceasing unnecessary medications.23 31 Structured medication review with STOPP criteria is considered a useful strategy to achieve it, where patients' remaining life expectancy and care goals are also taken into account.31 In our study, 91 per cent of our patients had at least one PIM at admission. This prevalence is considerably higher than identified in other care settings9–14 16 for older patients, ranging from 21.4%13 to 79.0%.11 However, interpretation of this range should be made with caution due to the heterogeneous sample population and study design, differences in drug availability, prescribing practices and pharmacist routine review of prescriptions across the different studies. Our higher prevalence could also be explained by the fact that our patients were transitioning from an acute-care setting to another as a nursing home or their own home and a high percentage of them presented major polypharmacy.
In our study sample, two or more drugs with antimuscarinic or anticholinergic properties and a drug without an evidence-based clinical indication were the most common PIMs. These differed from the most frequent PIMs found in other research settings9–11 13 14 such as proton pump inhibitors for peptic ulcer disease at full therapeutic dosage for >8 weeks10 11 13 or use of long-acting benzodiazepines.9 11 These medication classes have also been frequently described in the literature as being overprescribed and unnecessary.32
The START criteria identified at least one PPO in 20 per cent of patients at admission. It should be noted that the prevalence of PPOs varies greatly between studies (from 22.7%13 to 74.0%),11 depending on the research setting and the range of the START criteria that were taken into account (six out of 34 criteria in our study). In keeping with previous reports,9 10 13 14 the majority of PPOs involved statins in coronary, cerebral or peripheral vascular disease and ACE inhibitors in diabetes with evidence of renal disease. The evidence for clear-cut benefit from these drugs in secondary prevention of major morbidity and mortality is well established. Nevertheless, the real applicability of START criteria in our study population is uncertain and their routine incorporation in the pharmaceutical validation may be not be necessary in LTCHs.16
Despite different classifications for drug interactions, which make comparisons between studies difficult, the prevalence of potentially significant drug interactions in our study was a great deal higher than other elderly-based studies.22 This may be due to the nature of our study population, including very elderly patients with high multimorbidity and polypharmacy. Similar to our results, Doan et al33 showed that the prevalence of potential cytochrome P450-mediated drug interactions was 80% in elderly patients with polypharmacy. Although a high number of potential significant drug interactions were detected by the Micromedex database, in the LTCH most interactions were considered of minor clinical importance by the interdisciplinary team or risk-benefit analyses were justified by the clinical characteristics of these patients. Thus, interventions were lower than detected drug interactions.
Overall, most frequent pharmacotherapy recommendations were starting or stopping a drug. It is remarkable that more than 90% of pharmacotherapy recommendations were accepted and implemented by healthcare professionals, except where pharmacotherapy recommendations involved START criteria, where implementation was over 80%. It could be mainly justified by face-to-face communication with the interdisciplinary team when recommendations were suggested and pharmacists' experience in conducting comprehensive medication assessment with a systematic methodology. Consequently, substantial reductions in admission reconciliation errors, PIMs, PPOs and significant drug interactions rates were found.
It is also noteworthy that pharmacist intervention integrated in an interdisciplinary team led to a 31% improvement in prescribing appropriateness. In fact, after implementing the pharmacotherapy recommendations, a statistically significant reduction from six to four in the inappropriateness score was observed. Similar to O’Sullivan et al,26 the intervention showed improvements in prescribing appropriateness for three-quarters of our patients.
Instead of using the Medication Appropriateness Index,6 we decided to use a non-previously validated score as a comprehensive approach that gathered explicit and implicit methods to improve prescribing quality, including medication reconciliation. This score allows for the comparison of prescribing appropriateness between populations and studies by considering the total number of PIMs, PPOs, reconciliation errors and drug interactions.
Limitations
It is important to consider the limitations of this study. The main one is the absence of a control group. Patients were their own controls before the interventions of the interdisciplinary team because it was not ethically feasible to keep a control group deprived of interventions in the LTCH. Furthermore, it is unclear whether the implemented pharmacotherapy recommendations to reduce inappropriate prescribing resulted in clinically significant improvements such as a reduction in healthcare utilisation, drug-related readmissions or patients’ quality of life. Other limitations include the non-randomised and single-centre design that requires comparing these findings with other future elderly population-based studies.
Our findings are of interest as a description of the types of inappropriateness of prescribing and associated pharmacotherapy recommendations most often implemented in elderly patients with polypharmacy of an LTCH. These results tend to support the expansion of the pharmacist’s role in this setting.
Conclusion
Reconciliation errors, STOPP criteria-related PIMs and significant drug interactions are highly prevalent in elderly patients with polypharmacy admitted to an LTCH. The interdisciplinary pharmacotherapy quality programme revealed a positive impact by leading to a 31% improvement in prescribing appropriateness in our population of study. Thus, our findings confirm the usefulness of the programme to improve prescribing appropriateness in older patients with multimorbidity admitted to an LTCH.
These results indicate the need for clinical pharmacists as active members of interdisciplinary teams to reduce admission reconciliation errors, PIMs, PPOs and significant drug interactions rates by implementing pharmacotherapy recommendations. This study also provides further evidence of the need to implement a comprehensive interdisciplinary approach such as this programme in LTCHs.
ejhpharm-2017-001411supp002.doc (33.5KB, doc)
Acknowledgments
The authors would like to thank Jose Luis Gorriz and Ana M Vicedo-Cabrera for their suggestions on manuscript preparation, and Ana M Galbis-Bernácer for her support on data registration.
Footnotes
Contributors: OR-M, MC-M and JRN-S were the authors of the manuscript. Ana M Galbis-Bernácer participated in data registration. Jose Luis Gorriz and Ana M Vicedo-Cabrera revised the draft paper.
Funding: Funding Nofunding sources were received for the conduct of this study or the preparationof this article.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Ethics approval (74/13) was received from the Clinical Research Ethics Committee of the Doctor Peset University Hospital (reference acute-care hospital). Written informed consent was obtained from all participating patients prior to enrolment in the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Fialová D, Desplenter F. Aging of the population, clinical pharmacy services, and interdisciplinary cooperation in the optimization of pharmacotherapy in older patients. Drugs Aging 2016;33:163–7. 10.1007/s40266-016-0361-6 [DOI] [PubMed] [Google Scholar]
- 2.Gallagher J, O’Sullivan D, McCarthy S, et al. Structured pharmacist review of medication in older hospitalised patients: a cost-effectiveness analysis. Drugs Aging 2016;33:285–94. 10.1007/s40266-016-0348-3 [DOI] [PubMed] [Google Scholar]
- 3.Kaur S, Mitchell G, Vitetta L, et al. Interventions that can reduce inappropriate prescribing in the elderly: a systematic review. Drugs Aging 2009;26:1013–28. 10.2165/11318890-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 4.O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015;44:213–8. 10.1093/ageing/afu145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper JA, Cadogan CA, Patterson SM, et al. Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. BMJ Open 2015;5:e009235 10.1136/bmjopen-2015-009235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol 1992;45:1045–51. 10.1016/0895-4356(92)90144-C [DOI] [PubMed] [Google Scholar]
- 7.Climente Martí M, Jiménez Torres N. Manual for pharmaceutical care. 3rd ed Valencia: AFAHPE. Dr. Peset University Hospital, 2005. [Google Scholar]
- 8.Cipolle RJ, Strand LM, Morley PC. Pharmaceutical care practice: the clinician’s guide. 3rd ed New York: The McGraw-Hill Companies, Inc, 2004. [Google Scholar]
- 9.Cruz-Esteve I, Marsal-Mora JR, Galindo-Ortego G, et al. Potentially inappropriate prescribing in older Spanish population according to STOPP/START criteria (STARTREC study). Aten Primaria 2017;49:166–76. 10.1016/j.aprim.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frankenthal D, Lerman Y, Lerman Y. The impact of hospitalization on potentially inappropriate prescribing in an acute medical geriatric division. Int J Clin Pharm 2015;37:60–7. 10.1007/s11096-014-0040-9 [DOI] [PubMed] [Google Scholar]
- 11.García-Gollarte F, Baleriola-Júlvez J, Ferrero-López I, et al. Inappropriate drug prescription at nursing home admission. J Am Med Dir Assoc 2012;13:83.e9–15. 10.1016/j.jamda.2011.02.009 [DOI] [PubMed] [Google Scholar]
- 12.Morin L, Laroche ML, Texier G, et al. Prevalence of potentially inappropriate medication use in older adults living in nursing homes: a systematic review. J Am Med Dir Assoc 2016;17:862.e1–e9. 10.1016/j.jamda.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 13.Ryan C, O’Mahony D, Kennedy J, et al. Potentially inappropriate prescribing in an Irish elderly population in primary care. Br J Clin Pharmacol 2009;68:936–47. 10.1111/j.1365-2125.2009.03531.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher P, Lang PO, Cherubini A, et al. Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur J Clin Pharmacol 2011;67:1175–88. 10.1007/s00228-011-1061-0 [DOI] [PubMed] [Google Scholar]
- 15.Ryan C, O’Mahony D, Kennedy J, et al. Potentially inappropriate prescribing in older residents in Irish nursing homes. Age Ageing 2013;42:116–20. 10.1093/ageing/afs068 [DOI] [PubMed] [Google Scholar]
- 16.Hernandez Martin J, Merino-Sanjuán V, Peris-Martí J, et al. Applicability of the STOPP/START criteria to older polypathological patients in a long-term care hospital. Eur J Hosp Pharm. doi: 10.1136/ejhpharm-2017-001262[Epub ahead of print 9 Aug 2017]. 10.1136/ejhpharm-2017-001262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehnbom EC, Stewart MJ, Manias E, et al. Impact of medication reconciliation and review on clinical outcomes. Ann Pharmacother 2014;48:1298–312. 10.1177/1060028014543485 [DOI] [PubMed] [Google Scholar]
- 18.Delgado Sánchez O, Nicolás Picó J, Martínez López I, et al. Reconciliation errors at admission and departure in old and polymedicated patients. Prospective, multicenter randomized study. Med Clin 2009;133:741–4. [DOI] [PubMed] [Google Scholar]
- 19.Climente-Martí M, García-Mañón ER, Artero-Mora A, et al. Potential risk of medication discrepancies and reconciliation errors at admission and discharge from an inpatient medical service. Ann Pharmacother 2010;44:1747–54. 10.1345/aph.1P184 [DOI] [PubMed] [Google Scholar]
- 20.Quélennec B, Beretz L, Paya D, et al. Potential clinical impact of medication discrepancies at hospital admission. Eur J Intern Med 2013;24:530–5. 10.1016/j.ejim.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez Vargas B, Delgado Silveira E, Iglesias Peinado I, et al. Prevalence and risk factors for medication reconciliation errors during hospital admission in elderly patients. Int J Clin Pharm 2016;38:1164–71. 10.1007/s11096-016-0348-8 [DOI] [PubMed] [Google Scholar]
- 22.Hines LE, Murphy JE. Potentially harmful drug-drug interactions in the elderly: a review. Am J Geriatr Pharmacother 2011;9:364–77. 10.1016/j.amjopharm.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 23.Gnjidic D, Le Couteur DG, Kouladjian L, et al. Deprescribing trials: methods to reduce polypharmacy and the impact on prescribing and clinical outcomes. Clin Geriatr Med 2012;28:237–53. 10.1016/j.cger.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 24.Ensing HT, Stuijt CC, van den Bemt BJ, et al. Identifying the optimal role for pharmacists in care transitions: a systematic review. J Manag Care Spec Pharm 2015;21:614–36. 10.18553/jmcp.2015.21.8.614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanlon JT, Lindblad CI, Gray SL. Can clinical pharmacy services have a positive impact on drug-related problems and health outcomes in community-based older adults? Am J Geriatr Pharmacother 2004;2:3–13. 10.1016/S1543-5946(04)90002-5 [DOI] [PubMed] [Google Scholar]
- 26.O’Sullivan D, O’Mahony D, O’Connor MN, et al. The impact of a structured pharmacist intervention on the appropriateness of prescribing in older hospitalized patients. Drugs Aging 2014;31:471–81. 10.1007/s40266-014-0172-6 [DOI] [PubMed] [Google Scholar]
- 27.Walsh KA, O’Riordan D, Kearney PM, et al. Improving the appropriateness of prescribing in older patients: a systematic review and meta-analysis of pharmacists' interventions in secondary care. Age Ageing 2016;45:201–9. 10.1093/ageing/afv190 [DOI] [PubMed] [Google Scholar]
- 28.Ruiz-Millo O, Climente-Martí M, Galbis-Bernácer AM, et al. Clinical impact of an interdisciplinary patient safety program for managing drug-related problems in a long-term care hospital. Int J Clin Pharm 2017;39:1201–10. 10.1007/s11096-017-0548-x [DOI] [PubMed] [Google Scholar]
- 29.Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr 2015;15:31 10.1186/s12877-015-0029-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marvin V, Kuo S, Poots AJ, et al. Applying quality improvement methods to address gaps in medicines reconciliation at transfers of care from an acute UK hospital. BMJ Open 2016;6:e010230 10.1136/bmjopen-2015-010230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med 2015;175:827–34. 10.1001/jamainternmed.2015.0324 [DOI] [PubMed] [Google Scholar]
- 32.Lucchetti G, Lucchetti AL. Inappropriate prescribing in older persons: a systematic review of medications available in different criteria. Arch Gerontol Geriatr 2017;68:55–61. 10.1016/j.archger.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 33.Doan J, Zakrzewski-Jakubiak H, Roy J, et al. Prevalence and risk of potential cytochrome P450-mediated drug-drug interactions in older hospitalized patients with polypharmacy. Ann Pharmacother 2013;47:324–32. 10.1345/aph.1R621 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2017-001411supp001.pdf (203.1KB, pdf)
ejhpharm-2017-001411supp002.doc (33.5KB, doc)