Abstract
Background
The interference in the immune response induced by biological disease-modifying antirheumatic drugs (bDMARDs) increases the risk of reactivation of infections. Treatment of patients with chronic hepatitis C virus (HCV) infection and psoriasis is complex. The efficacy and safety of the new direct-acting antiviral agents (DAA) when combined with bDMARDs remain unknown.
Case report
We present a case of a 44-year-old Caucasian man affected with psoriasis and HCV infection. Throughout the course of the psoriatic disease, this patient received several lines of treatment, including secukinumab, a new type of bDMARD. At the time of commencing secukinumab, new DAA agents (ledipasvir/sofosbuvir) were also initiated. At week 12 post-treatment, hepatitis C viral load was undetectable and the patient remained in remission of psoriasis.
Conclusion
This case report suggests that secukinumab is a therapeutic option in patients with psoriasis, particularly in those cases with HCV infection where treatment with DAA agents is warranted.
Keywords: Psoriasis, Hepatology, PHARMACOTHERAPY, CLINICAL PHARMACY, VIROLOGY
Background
Psoriasis is an immunologically driven chronic inflammatory condition which is mainly cutaneous and in which genetic predisposition plays an important role.1 The choice of therapy depends on the severity of the cutaneous symptoms; this is generally measured using the Psoriasis Area and Severity Index (PASI) score. The first line of treatment consists of topical drugs: emollients, vitamin D analogues or corticosteroids either alone or in combination with ultraviolet light.2 3 Systemic therapy with retinoids or immunosuppressive drugslike methotrexate or cyclosporine is indicated in patients not responding to topical medication.4
In the last decade, the introduction of biological disease-modifying antirheumatic drugs (bDMARDs) has completely revolutionised systemic management of severe psoriasis due to significant decreases in the number of hospital stays, reduction in use of other systemic therapies and increased patient satisfaction.5 Infliximab, etanercept and adalimumab (anti-tumour necrosis factor-α; anti-TNF-α), which block the activity of TNF-α (anti-TNF-α), were the first bDMARDs used. Later came ustekinumab, a human IgG1 monoclonal antibody, which binds with specificity to the p40 protein subunit, used by both the interleukin (IL)-12 and IL-23 cytokines. Secukinumab has been the latest addition to the group and is a first-in-class fully human monoclonal antibody which selectively binds and neutralises the proinflammatory cytokine IL-17A; this is a more specific mechanism of action for fighting psoriasis, as it upregulates keratinocyte chemokines.6
The interference in the immune response induced by bDMARDs, particularly with anti-TNF-α agents, increases the risk of reactivation of different infections.7 Therefore, it is important to adequately screen for several chronic infectious diseases before bDMARDs therapy is initiated.7 Chronic hepatitis C virus (HCV) infection is particularly problematic as different studies have shown a higher prevalence of this infection in patients with psoriasis, an association which is unrelated to previous exposure to interferon, already known to induce or exacerbate psoriasis.8 9
Treatment of patients with psoriasis and chronic HCV infection is complex. The benefits of bDMARDs controlling the psoriasis activity need to be balanced against the risk of inducing an increased replication of HCV.10 In a series of retrospective studies, the use of anti-TNF-α11 12 13 or ustekinumab14 was not associated with an increase in the viral load in the majority of patients with HCV.
On the other hand, drug treatments for hepatitis C infections have advanced greatly in the last few years with a move away from interferon-based treatments to a new generation of direct-acting antiviral agents (DAA). Different DAA drugs such as sofosbuvir, simeprevir and daclatasvir as well as combinations, for example, ombitasvir–paritaprevir–ritonavir–dasabuvir and sofosbuvir–ledipasvir were then available.15 16 The efficacy and safety of these new interferon (IFN)-free antiviral treatments when combined with bDMARDs remain unknown.10
Case presentation
We report a patient with HCV infection receiving secukinumab, the newest bDMARD for the treatment of psoriasis. The impact of secukinumab on liver function and the potential interactions with DAA are discussed.
The patient was a 44-year-old Caucasian man with plaque-type psoriasis; he was attending our dermatology outpatient unit. He was an ex-parenteral drug user with a medical history of HCV infection, genotypes 3a and 4, diagnosed in 1997. He referred no significant alcohol use.
Psoriasis was diagnosed in 2003 and, given the severity of the cutaneous involvement (PASI score of 9), the patient was prescribed topical corticosteroids and emollients combined with oral cyclosporine at a dose of 150 mg/day. He continued to be stable for 5 years, complying with the treatment and attending regular check-ups.
In January 2008, the patient was found to meet criteria to initiate antiviral HCV therapy. Laboratory analysis revealed the following results: alanine aminotransferase (ALT) 34 UI/L, aspartate aminotransferase (AST) 49 UI/L, gamma-glutamyl transpeptidase (GGT) 36 UI/L and HCV PCR 15 500 000 IU/mL (7.19log UI/mL). A fibroscan estimated the degree of liver fibrosis to be F0–F1. The physician responsible for his care discussed the treatment options with the patient and proposed commencing pegylated-interferon/ribavirin (peg-IFN/rib), which would be added to the psoriasis treatment. In consultation with the outpatient pharmacist, the patient was given recommendations on importance of adherence to treatment, medication administration, possible interactions and side effects. After careful consideration, the patient and the multidisciplinary team involved in his care decided that the risk of peg-INF worsening his dermatological condition outweighed the possible benefits from treatment. As a result, the patient declined antiviral therapy.
In November 2008, the initial psoriasis treatment based on the administration of topical corticosteroids, emollients and systemic cyclosporine proved to be insufficient for adequate control of the disease. Therapy needed to be stepped up to a biological agent. The corresponding consultant and pharmacist reviewed which would be the biological agent of choice for this case taking into consideration the type of psoriasis, information about previous treatments, contraindications, precautions and current guidelines, and a treatment request form for subcutaneous etanercept was completed and approved by a panel. Before starting therapy with etanercept, prophylactic isoniazid 300 mg/day was given for a month (which then continued for a further 9 months) as the patient was diagnosed as having a latent tuberculosis infection. Etanercept was initiated at 50 mg every fortnight, given concomitantly with a weaning dose of prednisone. The patient attended the outpatient pharmacy department for treatment initiation and during consultation with the dispensing pharmacist he received education on administration, possible side effects, storage conditions and counselling on how to proceed in the event of missed doses.
Following the first two doses of etanercept, the patient rang the pharmacy department for advice as he was experiencing generalised pruritus and hand and feet oedemas. The patient was referred to urgent care, where he was treated with ibuprofen and dexclorfeniramina. Treatment was, therefore, switched to subcutaneous adalimumab 40 mg every 2 weeks, after a new discussion of the case between the pharmacy and dermatology departments. The patient attended the pharmacy outpatient department for adalimumab initiation and then on a monthly basis for treatment monitoring (including PASI and acute phase reactants). The patient responded well, reaching PASI scores of 0–2 after 1 year of treatment, and continued to show stable psoriatic disease for a further 3-year period. No remarkable complications arose while the patient was receiving adalimumab.
After this uneventful 4-year period of treatment with adalimumab, the disease flared up again with a PASI score of 5–6. Doses were increased to 40 mg weekly and the disease remained under control for an additional 3-year period. In September 2015, after 7 years of treatment with adalimumab, the patient presented a severe exacerbation with a PASI score of 32. Consequently, adalimumab was interrupted and treatment was switched to secukinumab. Based on the half-life of adalimumab, a 4-week washout period was suggested by the specialist pharmacist, and after this period, secukinumab was initiated at a loading dose of 300 mg weekly for 4 weeks followed by a maintenance dose of 300 mg every 4 weeks from there on.
During the washout period between adalimumab and secukinumab, the patient attended a follow-up visit with the liver disease specialist. Liver fibrosis, measured by fibroscan, had progressed to F4 degree and laboratory analysis yielded the following values: ALT 56 UI/L, AST 46 UI/L, GGT 35 UI/L and HCV PCR 15 500 000 IU/mL (7.19log UI/mL). At this point in time, new drug treatments for hepatitis C infections had been made available. These newer, oral, direct-acting drugs are more effective, have fewer side-effects, are easier to take and are less debilitating for patients. A revision of the latest European and Spanish guidelines15 16 17 was conducted by the infectious diseases specialist pharmacist and this revision was then discussed with the physician and presented at the multidisciplinary committee where it was decided that the optimum regimen for this particular viral genotype would be a combination of ledipasvir/sofosbuvir and ribavirin. Therapy with ledipasvir 90 mg/sofosbuvir 400 mg and ribavirin capsules 200 mg (2–0–3) daily for 12 weeks was initiated 18 days before starting secukinumab. Figure 1 shows the course of PASI score and liver function in relation to drug intake.
Figure 1.
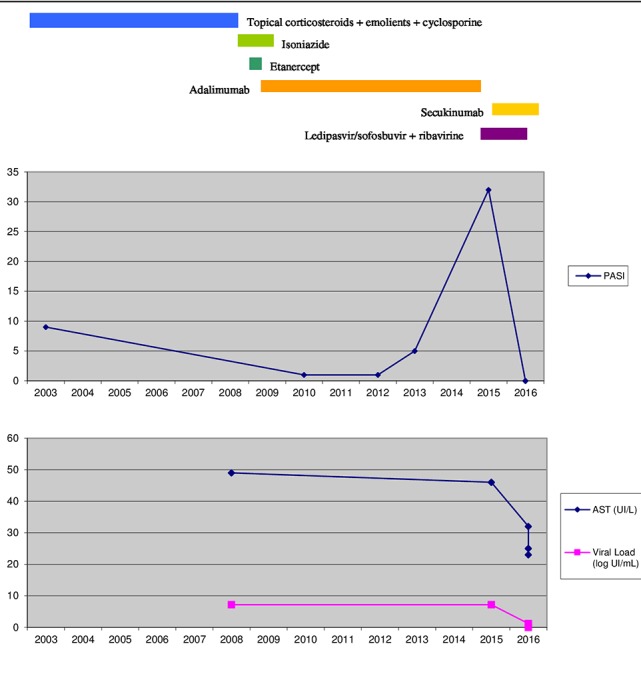
Schematic representation of the course of the liver function and PASI score in relation to drug intake. AST, aspartate aminotransferase; PASI, Psoriasis Area and Severity Index.
Investigations
The effect of biological therapy on reactivation of HCV is at present unknown and is very much in debate.10 This is an important issue due to the higher frequency of HCV infection in patients with psoriasis who may have indication for bDMARDs.
There is published evidence on the safe use of anti-TNF-α and ustekinumab in patients infected with HCV who are undergoing treatment with peg-INF and ribavirin.11 12 14 18 However, there is no data published to date in relation to the use of new DAA and other biological agents not targeting TNF-α, such as secukinumab. To our knowledge, this is the first case published in the corresponding literature.
Prior to the beginning of the treatment for this patient, a medicines information enquiry was directed to the manufacturer (Gilead Labs) by the pharmacy department requesting information on the concomitant use of the association of ledipasvir–sofosbuvir plus ribavirin and secukinumab in this population of patients and particularly regarding the risk of increasing replication of HCV. A report was obtained confirming that pharmacological or pharmacokinetic interactions were not expected, taking into account the drug’s mechanism of action and the metabolic pathways used by these molecules.
Treatment
Choice of therapy for psoriasis after failure to adalimumab was decided taking into account two factors: previous lines of treatment and maximum PASI values reached during the different previous therapies. It has been reported that anti-TNF efficacy decreases with time and the number of anti-TNF treatments received. This is due to antibody production against these molecules. Consequently, it is recommended to switch to a different treatment option with a different therapeutic target in those patients that have received two previous lines of treatment with anti-TNF molecules, which was our patient’s case.19 A switch in the therapeutic target was suggested and ustekinumab or secukinumab was the two options available. At that time (March 2016), our hospital protocol for treatment of moderate to severe psoriasis was updated. In this version of the protocol, a treatment decision algorithm was established, and secukinumab was positioned as the biological drug of choice for the treatment of psoriasis vulgaris with PASI ≥20, based on the results of two studies: FEATURE20 and JUNCTURE.21
Therefore, secukinumab was chosen and before initiation, the patient was seen in consultation by the dermatology/rheumatology specialist pharmacist who reviewed the patient’s medical records to verify that there was no medical history of infectious disease, explained treatment to the patient and clarified how future monitoring appointments would be scheduled. During treatment with secukinumab, the patient attended the pharmacy consultation room monthly for monitoring of treatment efficacy and safety.
As for the treatment for HCV, it was approved according to the guidelines outlined by AEEH (Spanish Liver Society): ‘II consensus document on hepatitis C treatment’.15 This document is in line with the National Strategic Plan for treatment of HCV which was approved by the Spanish National Health System in March 2015 and the recommendations of the European Association for the Study of the Liver.16 After review of these guidelines, it was decided that first-line therapy for a patient showing genotype 3 and 4 and F4 degree fibrosis was ledipasvir/sofosbuvir one tablet OD for 12 weeks. At present, these guidelines have been updated and this treatment option is no longer first line. Newer guidelines have now been published: ‘Guidelines AEEH/SEIMC for hepatitis C treatment’, edited in 2016 in collaboration with SEIMC (Spanish Society of Clinical Microbiology and Infectious Diseases).17 In consultation with the infectious disease (ID) specialist pharmacist, chronic medication taken by the patient was checked for interactions before initiation of new treatment, posology and possible side effects were explained and further follow-up visits were scheduled for monitoring, in order to maximise efficacy and safety of treatment. A multidisciplinary approach is key for success in treatment, and at this point, the ID specialist pharmacist and the dermatology/rheumatology specialist pharmacist were working together and in collaboration with the multidisciplinary team to provide the best pharmaceutical care to their patient
Outcome and follow-up
At week 4 of DAA treatment (January 2016), hepatitis C viral load was less than 15 IU/mL (1.16log UI/mL) and analytical liver parameters were: ALT 30 UI/L, AST 23 UI/L and GGT 21 UI/L. The patient complained of some minor degree of dizziness and pain. At this stage, the psoriasis had completely subsided with a PASI score of 0.
At week 12 (March 2016), hepatitis C viral load was still under 15 UI/mL (1.16log UI/mL) and clinical parameters read: ALT/AST=34/25, GGT=21. At week 12 post-treatment (June 2016), viral load was undetectable and analytical parameters were stable at ALT 30 UI/L, AST 23 UI/L and GGT 21 UI/L, meaning sustained viral response was achieved. Regarding psoriasis, the patient remains with a PASI score of 0 to date (September 2016).
Discussion
A review of available literature was undertaken with the terms ‘hepatitis C AND psoriasis’. As a result, isolated case reports and two systematic reviews (Pompili et al 12 and Brunasso et al 11) were listed. Pompili et al 12 researched the use of anti-TNF-α in patients with HCV concluding that TNF-α has a minimal influence in HCV replication. These findings were the result of the analysis of small retrospective cohort studies and case reports. Of the 216 patients included, three abandoned treatment because of a worsening of their liver disease; however, these negative outcomes could not be attributed to concomitant biological treatment. In all other 213 patients, the viral load was stable or had diminished.
Brunasso et al 11 also conducted a systematic review on the use of anti-TNF-α in patients diagnosed with HCV infection. Their conclusions were very similar to those reached by Pompili et al. Patients treated concomitantly with anti-TNF-α and INF-ribavirin showed improvement of their liver disease.
On another note, there are two studies showing controversial information on the use of ustekinumab in this population of HCV infected patients. Chiu et al 18 published a series of four cases where patients had a diagnosis of HCV infection and they had initiated treatment with ustekinumab, but they were not on active treatment with antivirals. Viral load increased in three out of those four cases. On the contrary, the three cases followed by Navarro et al 14 showed no worsening of the liver disease.
It is well established that the presence of HCV is not a contraindication to therapy with TNF-α inhibitors, except in cirrhotic patients, in whom the benefit:risk ratio should be individually evaluated before treatment initiation.12 Moreover, the Spanish Society of Rheumatology outlines recommendations for monitoring treatment in this population of patients in the ‘Guideline for risk management in prescribing biological therapies’, where it is stated that anti-HCV antibody titres should be measured at initiation of treatment and, in case of positive result, continue to be monitored closely throughout.7 However, the presentation of this case to our day clinic raised a new question: would the biological agent affect the antiviral effect of DAA? The favourable outcomes of HCV infection and psoriasis seen in our patient suggest that secukinumab is a safe treatment in patients receiving DAA such as ledipasvir–sofosbuvir.
In conclusion, the presence of HCV infection under treatment is not a contraindication for initiating a biological agent to treat psoriasis. Both evaluation of each individual case and close monitoring, carried out by a multidisciplinary team, are strongly recommended.
Learning points.
The efficacy and safety of the new direct-acting antiviral agents, when combined with biological agents, remain largely unknown.
This case shows that the presence of hepatitis C virus infection under treatment is not a contraindication for initiating a biological agent.
Evaluation of each individual case is necessary.
Close monitoring by a multidisciplinary team during treatment is strongly recommended.
Footnotes
Competing interests: None declared.
Ethics approval: Hospital Esperit Sant Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Parisi R, Symmons DPM, Griffiths CEM, et al. Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013;133:377–85. [DOI] [PubMed] [Google Scholar]
- 2. Menter A, Korman NJ, Elmets CA, et al. American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol 2009;60:643–59. 10.1016/j.jaad.2008.12.032 [DOI] [PubMed] [Google Scholar]
- 3. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol 2010;62:114–35. 10.1016/j.jaad.2009.08.026 [DOI] [PubMed] [Google Scholar]
- 4. Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris--update 2015--short version--EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol 2015;29:2277–94. 10.1111/jdv.13354 [DOI] [PubMed] [Google Scholar]
- 5. Fathi R, Armstrong AW. The role of biologic therapies in dermatology. Med Clin North Am 2015;99:1183–94. 10.1016/j.mcna.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 6. Wasilewska A, Winiarska M, Olszewska M, et al. Interleukin-17 inhibitors. A new era in treatment of psoriasis and other skin diseases. Postepy Dermatol Alergol 2016;33:247–52. 10.5114/ada.2016.61599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gómez Reino J, Loza E, Andreu JL, et al. Sociedad Española de Reumatología. [Consensus statement of the Spanish Society of Rheumatology on risk management of biologic therapy in rheumatic patients]. Reumatol Clin 2011;7:284–98. 10.1016/j.reumae.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 8. Tsai TF, Wang TS, Hung ST, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci 2011;63:40–6. 10.1016/j.jdermsci.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 9. Taglione E, Vatteroni ML, Martini P, et al. Hepatitis C virus infection: prevalence in psoriasis and psoriatic arthritis. J Rheumatol 1999;26:370–2. [PubMed] [Google Scholar]
- 10. Caso F, Cantarini L, Morisco F, et al. Current evidence in the field of the management with TNF-α inhibitors in psoriatic arthritis and concomitant hepatitis C virus infection. Expert Opin Biol Ther 2015;15:641–50. 10.1517/14712598.2015.1011616 [DOI] [PubMed] [Google Scholar]
- 11. Brunasso AM, Puntoni M, Gulia A, et al. Safety of anti-tumour necrosis factor agents in patients with chronic hepatitis C infection: a systematic review. Rheumatology 2011;50:1700–11. 10.1093/rheumatology/ker190 [DOI] [PubMed] [Google Scholar]
- 12. Pompili M, Biolato M, Miele L, et al. Tumor necrosis factor-α inhibitors and chronic hepatitis C: a comprehensive literature review. World J Gastroenterol 2013;19:7867–73. 10.3748/wjg.v19.i44.7867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salvi M, Macaluso L, Luci C, et al. Safety and efficacy of anti-tumor necrosis factors α in patients with psoriasis and chronic hepatitis C. World J Clin Cases 2016;4:49–55. 10.12998/wjcc.v4.i2.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Navarro R, Vilarrasa E, Herranz P, et al. Safety and effectiveness of ustekinumab and antitumour necrosis factor therapy in patients with psoriasis and chronic viral hepatitis B or C: a retrospective, multicentre study in a clinical setting. Br J Dermatol 2013;168:609–16. 10.1111/bjd.12045 [DOI] [PubMed] [Google Scholar]
- 15. SECRETARIA GENERAL DE SANIDAD Y CONSUMO. PLAN ESTRATÉGICO PARA EL ABORDAJE DE LA HEPATITIS CEN EL SISTEMA NACIONAL DE SALUD. 2016. http://www.msssi.gob.es/ciudadanos/enfLesiones/enfTransmisibles/docs/plan_estrategico_hepatitis_C.pdf
- 16. European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol 2015;63:199–236. 10.1016/j.jhep.2015.03.025 [DOI] [PubMed] [Google Scholar]
- 17. AEEH/SEIMC de manejo de la Hepatitis C. 2016. https://www.seimc.org/contenidos/documentoscientificos/guiasclinicas/seimc-clinicasclinicas-2016-Manejo_HepatitisC.pdf.
- 18. Chiu HY, Chen CH, Wu MS, et al. The safety profile of ustekinumab in the treatment of patients with psoriasis and concurrent hepatitis B or C. Br J Dermatol 2013;169:1295–303. 10.1111/bjd.12461 [DOI] [PubMed] [Google Scholar]
- 19. Gniadecki R, Kragballe K, Dam TN, et al. Comparison of drug survival rates for adalimumab, etanercept and infliximab in patients with psoriasis vulgaris. Br J Dermatol 2011;164:1091–6. 10.1111/j.1365-2133.2011.10213.x [DOI] [PubMed] [Google Scholar]
- 20. Blauvelt A, Prinz JC, Gottlieb AB, et al. FEATURE Study Group. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol 2015;172:484–93. 10.1111/bjd.13348 [DOI] [PubMed] [Google Scholar]
- 21. Paul C, Lacour JP, Tedremets L, et al. JUNCTURE study group. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol 2015;29:1082–90. 10.1111/jdv.12751 [DOI] [PubMed] [Google Scholar]


