Abstract
Background and Aims:
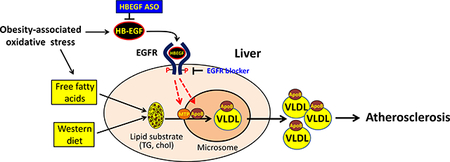
Heparin-binding EGF-like growth factor (HB-EGF) is a representative EGF family member that interacts with EGFR under diverse stress environment. Previously, we reported that the HB-EGF-targeting using antisense oligonucleotide (ASO) effectively suppressed an aortic aneurysm in the vessel wall and circulatory lipid levels. In this study, we further examined the effects of the HB-EGF ASO administration on the development of hyperlipidemia-associated atherosclerosis using an atherogenic mouse model.
Methods and Results:
The male and female LDLR deficient mice under Western diet containing 21% fat and 0.2% cholesterol content were cotreated with control and HB-EGF ASOs for 12 weeks. We observed that the HB-EGF ASO administration effectively downregulated circulatory VLDL- and LDL-associated lipid levels in circulation; concordantly, the HB-EGF targeting effectively suppressed the development of atherosclerosis in the aorta. An EGFR blocker BIBX1382 administration suppressed the hepatic TG secretion rate, suggesting a positive role of the HB-EGF signaling for the hepatic VLDL production. We newly observed that there was a significant improvement of the insulin sensitivity by the HB-EGF ASO administration in a mouse model under the Western diet as demonstrated by the improvement of the glucose and insulin tolerances.
Conclusion:
The HB-EGF ASO administration effectively downregulated circulatory lipid levels by suppressing hepatic VLDL production rate, which leads to effective protection against atherosclerosis in the vascular wall.
Keywords: HB-EGF, VLDL, hyperlipidemia, atherosclerosis, antisense oligonucleotide, insulin resistance
Graphical Abstract
INTRODUCTION
Dyslipidemia and insulin resistance associated with obesity are risk factors for the development of atherosclerosis and cardiovascular diseases [1, 2]. Heparin-binding EGF like growth factor (HB-EGF) is an EGF family member, which interacts with EGFR and ERBB4 [3]. The HB-EGF is expressed in multiple tissues including adipose and skeletal muscle tissues [4]. HB-EGF expression is upregulated in multiple cell type and tissues under oxidative stress conditions [5, 6]. The oxidation product of phospholipids induced upregulation of HB-EGF expression in the vascular endothelial cells and mediated inflammatory gene expression [7, 8]. HB-EGF expression was also upregulated in the adipose tissues under obesity [9, 10]. The expression of the HB-EGF in the liver is relatively low; however, knockdown or overexpression of the HB-EGF expression in the liver increased the sensitivity of the liver damages by CCl4, thioacetamide, and bile duct ligation [11–14].
Recently, we reported that the targeting of the HB-EGF using antisense oligonucleotide (ASO) administration significantly suppressed the development of an aortic aneurysm in a mouse model of angiotensin II (AngII) infusion model [15]. In the study, we detected that the HB-EGF ASO administration induced an effective downregulation of lipid levels in circulation. As a potential mechanism for the lipid-lowering, the HB-EGF ASO administration induced suppression of VLDL-associated triglyceride secretion from the liver [15]. In this study, we further examined the effects of the HB-EGF ASO administration on the development of aortic atherosclerosis using the atherogenic LDLR deficient mice under the Western diet.
EXPERIMENTAL PROCEDURES
Refer to the Online Supplemental Material-Extended Procedure for the details of each procedure.
Design of the antisense oligonucleotides (ASO) - Control ASO (Ionis 549144: 5′-GGCCAATACGCCGTCA −3′; with the underlines indicating cEt modified bases) and HB-EGF ASO (Ionis 597622: 5 ′-TACATTATAGTCTTGG −3′) were designed, synthesized, and purified by the Ionis Pharmaceuticals [16]. The HB-EGF ASO sequence uniquely matches with the sixth exon of the mouse HB-EGF gene. The ASOs were resuspended in sterile saline and administered into the mouse model via intraperitoneally in a volume of 200ul. We adopted 40mg/kg/week of body weight doses for the initial administration, and the dose was reduced to half for sustained suppression of the HB-EGF considering the half-life of the ASOs [17]. Generally, the range of 20–40mg/kg/week doses induced reproducible suppression of the HB-EGF gene expression in the liver tissue [17, 18].
Mouse studies - Healthy male and female LDLR deficient mice purchased from the Jackson Laboratory (Stock No: 002207) at the age of 8–10 weeks were used for the atherosclerosis study. For the induction of hyperlipidemia, the mice were fed with Western diet (Teklad, No TD-88137 containing 21% fat and 0.2% cholesterol content) ad libitum. For the glucose and insulin tolerance tests, the male C57BL/6J mice purchased from The Jackson Laboratory (Stock No: 000664). At the age of 8 weeks were treated with Western diet for an additional four weeks for the tests of glucose and insulin tolerance tests. All the mice were maintained in an American Association for Accreditation of Laboratory Animal Care (AAALAC) approved animal facility at The University of Kentucky. All animal experiments were performed under the animal protocols approved by the University of Kentucky Institutional Animal Care and Use Committee (IACUC).
Measurement of the intensity of aortic atherosclerosis - Mice was sedated by intraperitoneal injection of ketamine and xylazine (100 and 10 mg/kg body weight, respectively). Blood was collected via heart puncture at termination step using EDTA-coated syringes. Atherosclerotic lesion and aortic intimal area were quantified for the total and three separate segments aortic lumens (arch, thoracic, and abdominal) as described previously [19, 20].
Quantification of liver tissue lipid content - The details of the procedure for the lipid extraction from the liver tissues and the quantification of triglyceride (TG) and total cholesterol concentrations were described in a previous report [15, 21] and Supplemental Online-Extended Procedure with details.
Plasma lipid and liver enzyme quantification - Plasma total triglyceride (TG) and cholesterol concentrations were quantified using enzymatic assay using reagents purchased from the Wako Diagnostics (Catalog No. 994–02891/992–02892 and 999–02601 for TG and cholesterol, respectively). Plasma ALT and AST levels were quantified by the Ionis Pharmaceuticals as previously reported [18].
Immunocytochemistry with tissue sections - Refer to the Online Supplemental Materials-Extended Procedure for the details of the Oil O Red and CD68 staining procedures with the frozen and paraffin-embedded aorta, liver, and white adipose tissue samples.
Hepatic triglyceride (TG) secretion assay- The details of the procedure were described in a previous report [15]. We adopted intraperitoneal injection of poloxamer-407 (1g/kg of body weight, Sigma-Aldrich, Cat No. 16758) to suppress the enzyme that hydrolyzes TG in the bloodstream and measured the time-dependent increases of circulatory TG levels by collecting 50μl of blood at 0, 1, 2, and 5-hour points via the retro-orbital route. Blood collection and injections were conducted on mice sedated under anesthesia using isoflurane (inhalation with 1–4% in O2). The apoB concentrations, as surrogates of VLDL, and triglyceride levels in the plasma samples were measured by the Western blotting and enzymatic assay kits from the Wako Diagnostics, respectively.
Heparin-releasable circulatory triglyceride (TG) hydrolytic activity assay - To measure the relative rate of the circulatory TG hydrolysis in circulation, we measured the TG hydrolytic activity of the plasma samples collected before and after 20 min-point of heparin injection (1.5 unit/g of body weight in saline) as described previously [15].
Glucose and insulin tolerance tests - After 5- and 3-hour fasting, one-time intraperitoneal injection of glucose (Sigma-Aldrich) and insulin (Novolin) at the dose of 1 g/kg and 0.15 Unit/kg of body weight were applied, respectively. Tail-vein blood glucose levels at 0, 15, 30, 60, 90, and 120 min points were quantified using Ascensia Contour Blood Glucose Meter following the procedure recommended by the manufacturer (Bayer HealthCare, LLC). The glucose and insulin tolerance assay values were presented as a mean ± standard error (SE).
HB-EGF quantification in the plasma – The ELISA kit for the mouse HB-EGF (LSBio, Cat No LS-F26245–1) was used for the quantification of HB-EGF levels in the plasma samples following the procedure recommended by the manufacturer. Recombinant mouse HB-EGF accompanied with the kit was used for the standard curve plotting.
Western blotting and qRT-PCR – Refer to the Online Supplemental Material-Extended Procedure for the details of the Western blotting and qRT-PCR procedures. Antibody information is described in the Online Supplemental Material-Extended Procedure. Primer DNA sequence information for the qRT-PCR is described in the Suppl. Table I.
Fast Protein Liquid Chromatography (FPLC) fractionation analysis for the lipoprotein-associated cholesterol - Blood was collected from the mice in EDTA-coated tubes by cardiac puncture at termination and plasma was isolated by centrifugation by spinning 3,000×g for 10min at 4°C. The cholesterol distribution among lipoprotein classes was determined by separation of plasma lipoproteins by gel filtration chromatography based upon the method described previously [15, 21].
Cell culture and treatment – Human hepatocarcinoma-derived HepG2 cell line purchased from the ATCC (Cat No. HB-8065) and cultured in DMEM complemented with 10% fetal bovine serum (FBS) (Hyclone, No SH30070) containing 4.5g/L glucose with supplementation with penicillin/streptomycin (100 Units/ml and 100 μg/ml, respectively). Cells at about 80% confluence were serum-starved one day before treatment. Cells were treated with different doses of recombinant HB-EGF (LSBio, Cat No LS-G49) for 16 hours in a DMEM media supplemented with 0.1% BSA (fatty acid-free, Sigma-Aldrich, Cat No A2059) and 0.6 mM oleic acid (Cayman Chemical, Cat No 112–80-1). We quantified the apoB protein contents in the cell supernatant as a surrogate for the VLDL particles by sequential procedures of immunoprecipitation and Western blotting using two different apoB antibodies (Millipore-Sigma Cat No AB742 and Meridian Bioscience Cat No K34005G-1), respectively.
Statistical analysis – If not described separately, the results were presented as the mean ± standard deviation (SD). Student’s t- or Two-way ANOVA tests were applied for the comparison between or among groups. If applicable, multiple comparison adjustment corrections were applied using the Bonferroni method.
RESULTS
HB-EGF ASO administration effectively downregulated circulatory lipid levels in a hyperlipidemic mouse model.
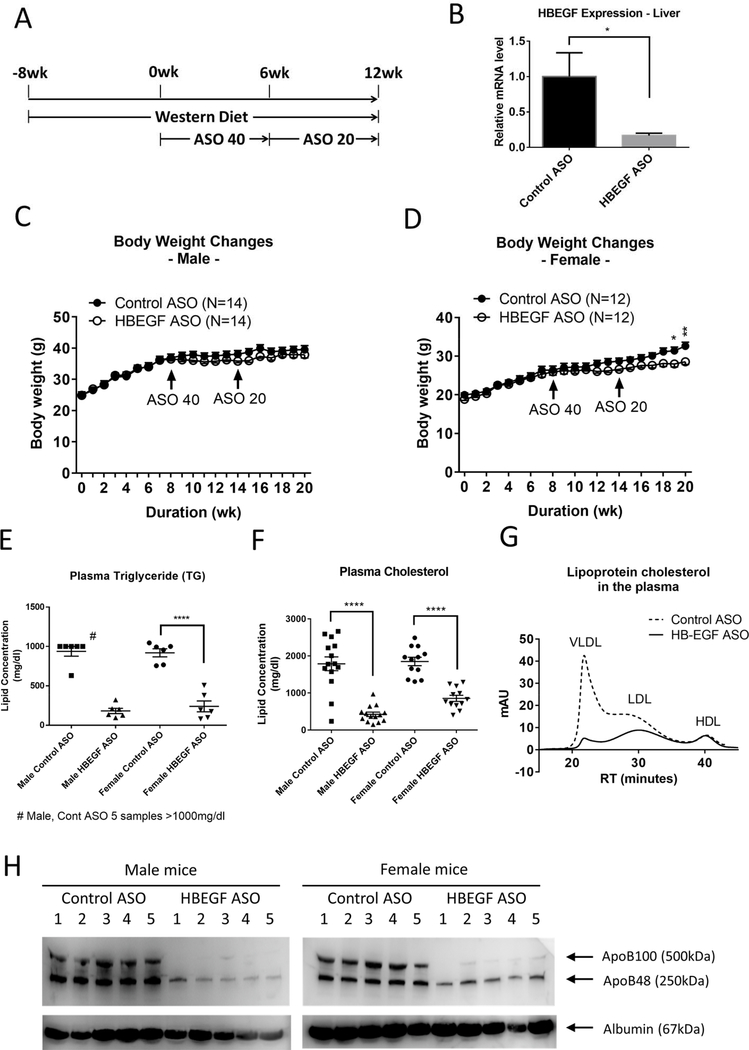
The male and female LDLR deficient mice under Western diet were cotreated with control and HB-EGF ASOs for 12 weeks for the test of the effects of the HB-EGF targeting on the development of hyperlipidemia and hyperlipidemia-associated atherosclerosis formation (Figure 1A for the treatment scheme). The HB-EGF ASO administration induced a significant suppression of the HB-EGF gene expression in the liver (Figure 1B). Body weight change in male mice was not significant, but there was a tendency of reduction during the HB-EGF ASO administration (Figure 1C). The reduction of the body weight by the HB-EGF ASO in female mice was significant (Figure 1D). In a separate control experiment, we confirmed that the HB-EGF ASO administration did not induce significant changes in the food and water intakes in the male and female mice (Suppl. Figure 1A–B). However, there was a significant reduction in the size of epididymal white adipose tissue (eWAT) in the female mice by the HB-EGF ASO administration but not in male mice (Suppl. Figure 1C–H). The HB-EGF ASO administration did not change the size of the interscapular brown adipose tissue (BAT) in both male and female mice (Suppl. Figure 1C–H). The cell size or morphology of the adipocytes in the eWAT were not different in both genders of mice (Suppl. Figure 2).
Figure 1. HB-EGF ASO administration effectively downregulated circulatory lipid levels.
(A) Animal treatment scheme. Male and female LDLR deficient mice under Western-diet were intraperitoneally injected weekly with control and HB-EGF ASOs for additional 12 weeks (40 and 20 mg/kg/week ASO doses in a 6-week interval) (N=12–14). (B) The HB-EGF mRNA level in the liver was determined by qRT-PCR for randomly selected three mice. (C-D) Body weight changes by weekly for the male (C) and female (D) mice. Initiation time points of ASO administration at doses of 40 and 20 mg/kg/week are marked with arrows. (E-F) Triglyceride (TG) and total cholesterol concentrations in the plasma samples collected at the termination step. For the TG quantification assay, six samples nearest median values of cholesterol concentration were selected for the quantification. (G) FPLC fractionation of the lipoprotein-associated cholesterol in the plasma samples. Four plasma samples from the male groups in a median range of cholesterol levels were pooled together for the FPLC fractionation analysis. (H) ApoB levels in the five plasma samples selected from the median range of cholesterol levels were compared. Albumin level was determined as a loading control of plasma samples. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
First, we detected that the HB-EGF ASO administration induced an effective downregulation of circulatory lipid levels including triglyceride (TG) and cholesterol in both male and female mice (Figure 1E–F; Suppl. Figure 3A). The FPLC fractionation of the lipoprotein-associated cholesterol in the plasma samples indicated an effective downregulation of the VLDL and LDL particles by the HB-EGF ASO administration (Figure 1G and Suppl. Figure 3B). The level of apoB protein, which is a permanently associated with VLDL and LDL particles, was also downregulated by the HB-EGF ASO administration (Figure 1H). However, the level of HDL was not changed by the HB-EGF ASO administration (Figure 1G and Suppl. Figure 3C).
We also measured the changes of the circulatory HB-EGF levels using ELISA procedure. Interestingly, the HB-EGF ASO administration induced a significant increase of the HB-EGF levels in circulation (Suppl. Figure 3D). We compared the HB-EGF transcript levels in multiple internal organs and adipose tissues to find a potential source of the HB-EGF in circulation. Internal organs including liver and lung, and eWAT but not BAT showed suppression of the HB-EGF expression (Suppl. Figure 3E).
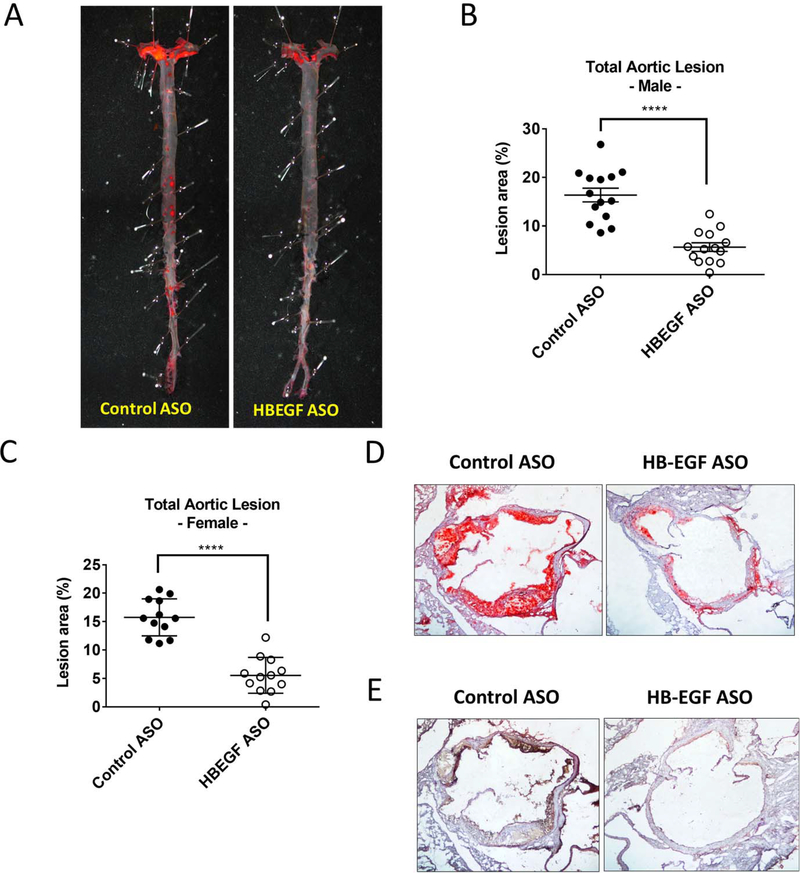
The HB-EGF ASO administration effectively suppressed atherosclerosis development in the aorta.
The Western diet feeding induced a substantial atherosclerotic lesion formation in the LDLR deficient mice (about 15% of the total aortic lumen area) as shown by the Oil Red O staining of the aortic lumen (Figure 2A–C). The HB-EGF ASO administration induced an effective suppression of the atherosclerosis formation in both male and female mice (Figure 2B–C). The suppression of lesion development was shown in the entire aortic lumen area including arch, thoracic, and abdominal aorta (Suppl. Figure 4A–E). Correspondingly, there was a significant reduction of neutral lipid and inflammatory cell accumulation in the aortic root as determined by the Oil Red O and CD68 staining, respectively (Figure 2D–E). The atherosclerotic lesion size was significantly correlated with the total cholesterol levels in circulation but not with triglyceride levels, suggesting a central role of LDL particle level for the atherosclerotic development (Suppl. Figure 4F).
Figure 2. HB-EGF ASO administration effectively suppressed atherosclerosis development in the aorta.
(A) Representative images of Oil Red O (ORO) staining for the lumen side of aortas isolated from the control and HB-EGF ASO treatment group of male mice. (B-C) En face measurement of intimal atherosclerotic lesions as measured at the termination step as a percent of the total aortic area for the male (B) and female (C) mice. (D-E) Representative images of in situ intimal lesion in the aortic root sections stained using Oil Red O (ORO) and CD68 antibody, respectively. **** p<0.0001.
HB-EGF ASO administration affected hepatic lipid composition and function.
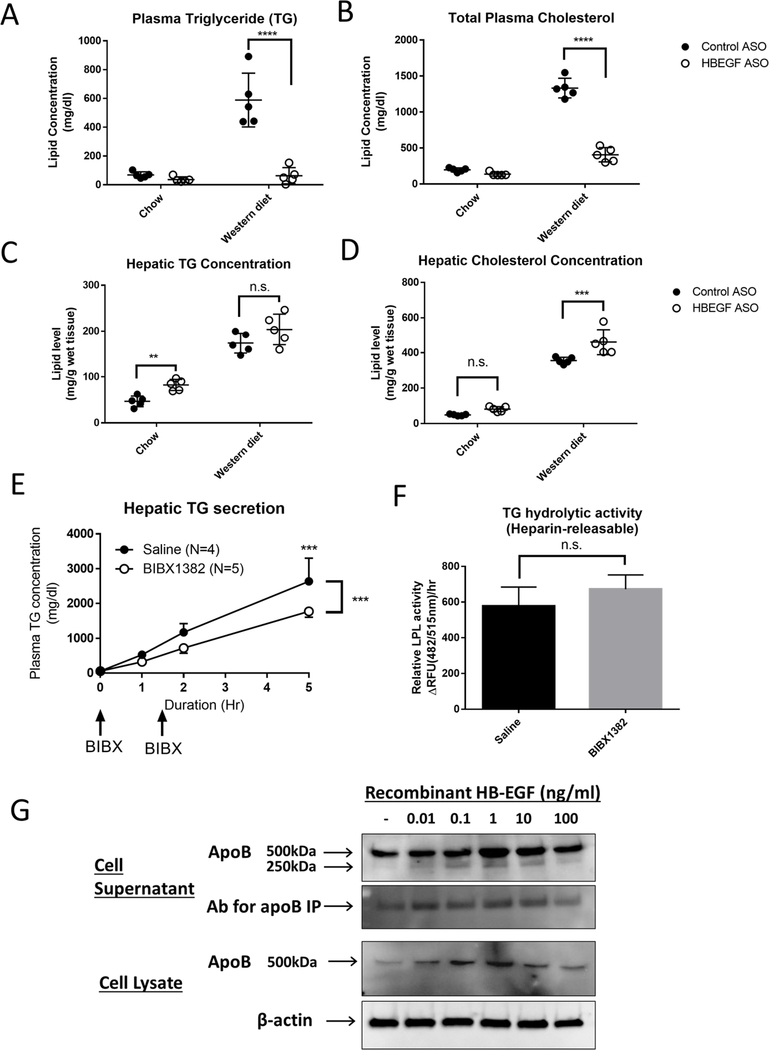
Next, we examined the changes of the hepatic lipid composition and function by the HB-EGF ASO administration in the LDLR deficient mice under chow and Western diet in a separate set of mouse experiment. We confirmed that the HB-EGF ASO administration induced effective suppression of circulatory TG and cholesterol levels (Figure 3A–B). The Western diet feeding induced a robust increase in the TG and cholesterol concentrations in the liver tissue (Figure 3C–D). The combination of the HB-EGF ASO administration showed a further increase or tendency of increase in the TG and cholesterol concentrations in the mice under both chow and the Western diets. We also detected a significant increase of the liver size by the HB-EGF ASO administration in the hyperlipidemic mice model (Suppl. Figure 5A). We measured liver enzyme ALT and AST levels in circulation as indices of liver function (Suppl. Figure 5B–C). The HB-EGF ASO administration under chow diet condition did not induce elevation of the enzyme levels in circulation; however, the combination of the HB-EGF ASO with the Western diet induced a significant elevation of the liver enzyme levels in circulation.
Figure 3. HB-EGF ASO administration affected the hepatic lipid content and VLDL production.
(A-D) The LDLR deficient mice (female, 8–10 weeks of age) under chow and Western diet were cotreated with control and HB-EGF ASOs at a dose for 40mg/kg/week for 16 weeks (N=5 per group). (A-B) The plasma and hepatic concentrations of the TG and total cholesterol levels were quantified at the termination step. (C-D) The hepatic concentrations of triglyceride and cholesterol were quantified. (E) Healthy C57BL/6 male mice fasted for 4 hours were intraperitoneally injected with poloxamer-407 (1g/kg) at the 0-time point (N=5). At 0 and 1.5-hour points, the mice were injected with saline and BIBX1382 (40mg/kg) via tail-vein. The time-dependent elevation of the TG concentration in circulation was measured by retro-orbital bleeding at 0, 1, 2 and 5-hour points. (F) Pre and heparin-releasable plasma TG hydrolytic activities were measured in C57BL/6 male mice after tail vein injections of saline or BIBX1382 (40mg/kg) at 4 and 2 hours before the heparin injection. Plasma samples collected at 0 and 20 min of heparin injection were used for the calculation of the heparin-releasable TG hydrolytic activity in circulation (G) Recombinant HB-EGF positively regulates apoB production in the liver cells. Confluent HepG2 cells were treated with HB-EGF for 16 hours in DMEM supplemented with 0.1% BSA and 0.6 mM oleic acid. Immunoprecipitation and Western blotting using apoB specific antibodies measured the apoB content in the cell supernatant and cell lysates. ** p<0.01, *** p<0.001, **** p<0.0001, and n.s. means no significance.
To test the dose effect of the HB-EGF ASO on the liver function, we compared the effects of different doses of ASO administration on the hepatic lipid composition and liver enzyme levels in circulation. The range of 5 to 20mg/kg/week HB-EGF ASO administration still induced effective lipid-lowering in circulation (for example, 79%, 71%, and 61% reduction of the circulatory TG levels by 20, 10, and 5 mg/kg/week doses, respectively) (Suppl. Figure 5D–E). The hepatic lipid accumulation induced by the HB-EGF ASO administration was moderate at the range of the doses compared with control ASO treatment group (Suppl. Figure 5F–G). The 20mg/kg/week dose of HB-EGF ASO administration still induced an increase of ALT and AST elevation in circulation, but the 5 and 10 mg/kg/week doses induced minimal changes of the enzyme levels (Suppl. Figure 5H–I). We compared the gene expressions involved in the hepatic VLDL production, lipid synthesis, fibrogenesis, inflammation, bile acid synthesis, and lipid β-oxidation in the liver (Suppl. Figure 6). There were no significant differences except MCP1 (Suppl. Figure 6D) in the list of gene expressions by the HB-EGF ASO administration compared with control ASO treatment groups.
Previously, we reported that the HB-EGF ASO administration significantly inhibited VLDL-TG secretion from the liver [15]; in contrast, a bolus injection of recombinant HB-EGF induced a transient increase of VLDL-TG secretion from the liver. In this study, we additionally tested the effects of the treatment of EGFR blocker on the hepatic TG secretion. As shown in Figure 3E, administration of water-soluble EGFR blocker BIBX1382 [22] significantly suppressed the hepatic TG secretion in vivo. In a separate control experiment, we confirmed that the BIBX1382 administration did not affect the TG clearance in circulation (Figure 3F).
To directly test the role of HB-EGF in the production of the VLDL particles in the liver cells, we tested the effects of recombinant HB-EGF in the apoB production in the HepG2 cell line, which has been widely used for the mechanistic study of hepatic VLDL production [23]. A range of 0.1–10ng/ml of recombinant HB-EGF significantly increased apoB protein content in the cells and the secretion from the cells (Figure 3G). Different from the protein level, the apoB mRNA level was not significantly changed by the recombinant HB-EGF in the cells (Suppl. Figure 7A). The expression of the microsomal triglyceride transfer protein (MTP), which is another essential component for the VLDL assembly, was upregulated by 0.1ng/ml of recombinant HB-EGF. The expression of CPT1A, which is involved in lipid β-oxidation, was also upregulated by the recombinant HB-EGF with dose-dependent fashion (Suppl. Figure 7B). The gene expressions involved in the lipid synthesis (SREBP-1a, −1c, and −2 target genes) in the HepG2 cells were not significantly affected by the recombinant HB-EGF although there was a trend of bell-showed elevation of the SREBP-1c target genes (ACACA, SCD1, and FASN) in the cells (Suppl. Figure 7B).
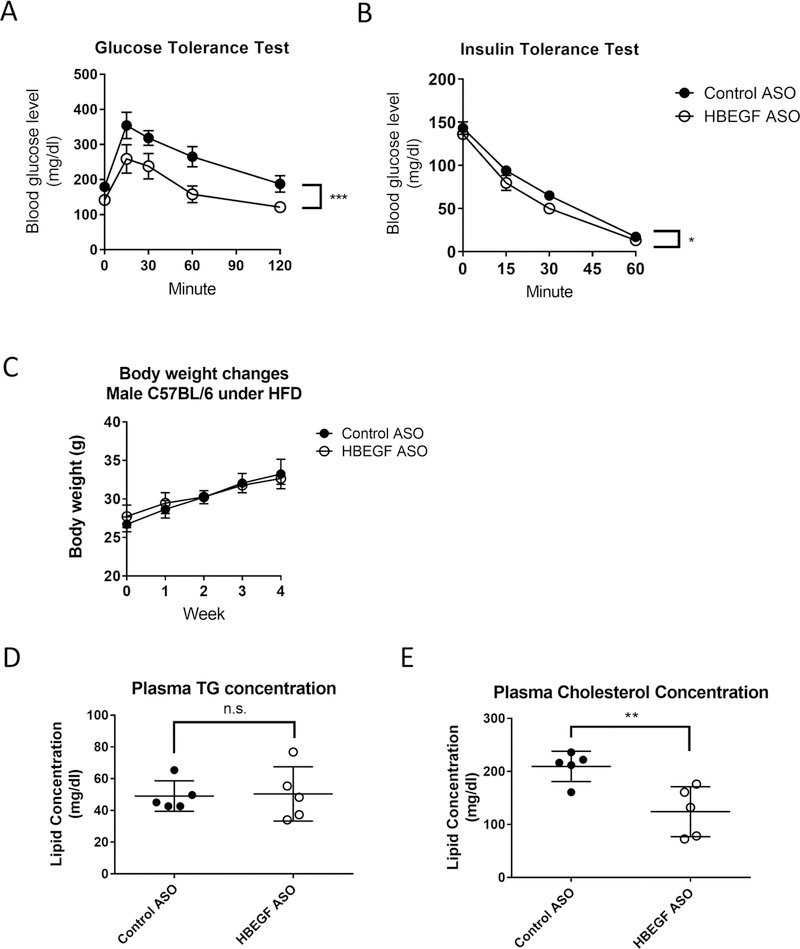
HB-EGF ASO administration significantly improved insulin sensitivity in the mouse under the Western diet.
Dyslipidemia is closely associated with insulin resistance in obese individuals [2, 24]. To test the effects of the HB-EGF ASO administration in the systemic insulin sensitivity, we performed glucose and insulin tolerance tests using the male C57BL/6J mice after four weeks of Western diet feeding. As shown in Figure 4A–in B, the HB-EGF ASO administration significantly improved the glucose and insulin tolerances in the mice. There were no significant body weight changes by the HB-EGF ASO administration (Figure 4C). The basal level of TG and cholesterol in the model was relatively low compared with the lipid levels in the LDLR deficient mice under the Western diet; thus, the lipid-lowering effects induced by the HB-EGF ASO administration was moderate in the model mice (Figure 4D–E).
Figure 4. HB-EGF ASO administration improved systemic insulin sensitivity in vivo.
The male C57BL/6 mice under the Western diet were treated with control or HB-EGF ASOs for four weeks at the dose of 40- and 20mg/kg/week by a 2-week interval (N=5 per group). At four week point After 5 and 3-hour fasting, the glucose and insulin tolerance tests were performed. (C) Body weight change profile during Western diet and ASO administration. (D-E) The circulatory levels of triglyceride and total cholesterol were quantified at four-week point by bleeding 50ul of the blood samples via the retro-orbital route. * p<0.05, ** p<0.01, and *** p<0.001.
Using HepG2 cells, we examined the direct effects of the recombinant HB-EGF pre- and cotreatment in the insulin signaling. The acute insulin treatment effectively induced the activation of the Akt signaling in the cells as shown by the induction of phosphorylation of Ser473-and Ser308-Akt (Suppl. Figure 8A). The pre- and cotreatment of the recombinant HB-EGF did not affect the insulin action for the activation of Akt (Suppl. Figure 8B). Thus, the mechanism of the improvement of the systemic insulin sensitivity in vivo by the HB-EGF ASO administration appears to be mediated by an indirect mechanism or via a non-hepatic tissue function.
DISCUSSION
In this study, the targeting of HB-EGF using ASO administration induced effective downregulation of circulatory TG and cholesterol levels and parallel protection against the development of atherosclerosis in a hyperlipidemic mouse model. Suppression of the hepatic VLDL production appears to be a central mechanism for the lipid-lowering in circulation and increased lipid content in the liver tissue by the HB-EGF ASO administration. Interestingly, we observed that the HB-EGF ASO administration induced a tendency of the reduction of body weight and white adipose tissue size, and an improvement of the systemic insulin sensitivity. The reduction of the body weight and white adipose tissue size was more evident in female than male mice.
The HepG2 cell data showing the production of the apoB by the recombinant HB-EGF suggested that the HB-EGF could be an autonomous stimulator for the VLDL production in the liver as shown in the HepG2 cell results (Figure 4G). Additionally, we observed inhibition of the hepatic TG secretion by the administration of EGFR blocker BIBX1382. In HepG2 cells, the apoB protein content in the cells was increased without a change of apoB transcript, suggesting that an enhanced translation of apoB mRNA or increased stability of the apoB protein is associated with HB-EGF signaling. Previous reports showed that the increased apoB translation and enhanced stability of the ApoB are the mechanisms for the increased VLDL production in the liver [25, 26]. We performed a series of control experiments to test the role of the other candidate mechanisms for the lipid-lowering by HB-EGF ASO administration. Previously, we reported that the administration of HB-EGF ASO or the bolus injection of recombinant HB-EGF did not affect the rate of TG clearance in circulation [15]. EGFR blocker BIBX1382 injection did not affect the TG clearance rate as shown in Figure 3F. We also reported that the HB-EGF ASO administration did not affect the fecal neutral sterol excretion rate, which is an index for the estimation of the net amount of cholesterol excretion via hepatobiliary pathway [15, 21]. There were no significant changes of gene expressions involved in the bile acid synthesis and lipid β-oxidation in the liver tissues by the HB-EGF ASO administration or in the HepG2 cells by the recombinant HB-EGF treatment (Suppl. Figures 6E–F).
When the hyperlipidemic mouse models were treated with the range of 20 to 40 mg/kg/week of HB-EGF ASO, there were significant increases or tendency of increase of lipid contents in the liver under both chow and Western diet feeding conditions (Figures 3C–D). At the same time, the HB-EGF ASO administration induced a significant increase in liver enzyme levels in circulation (Suppl. Figures 5B–C). A similar phenotype of hepatic lipid accumulation was also reported by the administration of apoB or MTP ASOs in the same strain of mouse model [18]. The liver enzyme elevation in circulation by HB-EGF ASO administration appears to be closely linked with the lipid accumulation in the liver because the HB-EGF ASO administration under chow diet condition did not induce the elevation. The increase of hepatic lipid content or liver enzyme elevation in circulation were also dependent on the dose of HB-EGF ASO applied. A lower range of HB-EGF ASO administration (for example, 5 to 10mg/kg/week), which still induced lipid-lowering, but showed less lipid accumulation in the liver and elevation of liver enzymes in circulation (Suppl. Figures 5F–I). A recent report demonstrated that the apoB ASO administration induced TG disposal autophagy pathway as the duration of the ASO administration increased [27]. The MTP ASO did not induce this protective pathway because the MTP function is required for the function of the TG disposal by the autophagic pathway [27]. Thus, it would be interesting to check whether the HB-EGF ASO administration can induce a similar autophagic protective pathway or not.
Normal insulin signaling increased lipid synthesis and deposition in the liver cells but suppressed the hepatic VLDL production as typical anabolic signaling at the postprandial period [24, 28]. The occurrence of insulin resistance in an obese person, paradoxically, still allows the lipid synthesis and accumulation in the liver but selectively induces resistance on the suppression of VLDL production in the liver; thus, the insulin resistance frequently leads to a combination of the hepatic lipid accumulation (fatty liver) and the elevation of the TG levels in circulation (hypertriglyceridemia) [24, 28, 29]. We newly observed that the HB-EGF ASO administration improved systemic insulin sensitivity in a mouse model under the Western diet (Figures 4A–B). The underlying mechanism of the improvement of the insulin sensitivity is still unclear; but, it appears to be mediated by an indirect mechanism through the improvement of lipid homeostasis or enhanced insulin sensitivity in non-hepatic tissues because the pre- or cotreatment recombinant HB-EGF did not affect the acute insulin signaling for Akt activation in a HepG2 cell system (Suppl. Figure 8B).
Surprisingly, the HB-EGF ASO administration significantly increased circulatory HB-EGF levels (Suppl. Figure 3D). It appears to be induced by a compensatory mechanism although the source of the elevated HB-EGF in circulation is unclear yet. Fukatsu et al. reported that the skeletal muscle tissue-specific HB-EGF overexpression significantly increased the systemic insulin sensitivity and glucose uptake by the skeletal muscle tissue [30]. Because the lipid-lowering by the HB-EGF ASO administration in the C57BL/6 mice was moderate (Figures 4D–E), the underlying mechanism of the improvement of the insulin sensitivity appears to be different from the mechanism of the lipid-lowering effects. Potentially, the elevated HB-EGF level in circulation may interact with the skeletal muscle to increase of the glucose uptake in the tissue. Because of the importance of the prevention of insulin resistance in obese people, further mechanistic studies on the improvement of the insulin sensitivity by the HB-EGF ASO administration might be important.
There was a significant reduction of the body weight and white adipose tissue size in the female mice by the HB-EGF ASO administration (Figure 1D and Suppl. Figures 1F–G). In male mice, the change was not significant, but there was a tendency of the body weight reduction by the HB-EGF ASO administration. Previous reports suggested that the HB-EGF is a central regulator for the stem cell proliferation and differentiation as recently reviewed by the Vinante et al. [31]. Zhou et al. reported that the HB-EGF is involved in the transdifferentiation of the fibroblasts to adipose tissue-like cells [32]. Lee et al. reported that the recombinant HB-EGF is an inhibitor of the initial commitment of differentiation of pluripotent mesenchymal stem cells to the adipogenesis [33]. Because of the multi-functions of the HB-EGF depending on the cell type and tissue of its expression, for further clarification, the adipose tissue-specific HB-EGF gene-deficient model system might be required.
Multiple reports indicated that the vascular HB-EGF expression was upregulated in the hyperlipidemic condition and the HB-EGF expression was positively associated with the progress of atherosclerosis [34, 35]. The HB-EGF expression was also closely associated with the arterial intimal media thickness in a flow-induced arterial remodeling mouse model [36]. The HB-EGF ASO administration also downregulated the HB-EGF gene expression in the mouse aorta [15]. In addition to the lipid-lowering in circulation, the downregulation of local HB-EGF expression by the HB-EGF ASO administration may also contribute to the protection against the development of atherosclerosis in the vessel wall. Because the change of circulatory lipid levels may cause the changes of the local vessel HB-EGF gene expression [7], more study on the relative contributions of the local HB-EGF gene downregulation and lipid-lowering to the protection against atherosclerosis need to be addressed further. Use of vascular endothelial- or smooth muscle cell-specific HB-EGF gene deletion models might be useful tools for the determination.
Though we demonstrated that the HB-EGF ASO is effective in suppressing the circulatory lipid levels and protecting the atherosclerosis formation in the vessel wall, there are still many questions to be addressed to understand the mechanism of the phenotype changes associated with the HB-EF ASO. First, we need to understand the details of the cellular and signaling mechanism of the regulation of hepatic VLDL production by HB-EGF signaling. Additionally, the study on the role of the HB-EGF in the adipose and skeletal muscle tissues in regulating the lipid metabolism, adipogenesis, and insulin sensitivity would be important for understanding the HB-EGF ASO-induced phenotype changes like the reduction of the adipose tissue size and improvement of the insulin sensitivity. Further in-depth evaluation of any deleterious effects of the HB-EGF ASO on the hepatic lipid accumulation and function should be required to determine the usefulness of the ASO in treating dyslipidemia and insulin resistance.
Supplementary Material
Highlights:
HB-EGF targeting using ASO administration suppressed hepatic VLDL secretion leading to a remarkable downregulation of circulatory triglyceride and cholesterol levels.
HB-EGF ASO administration also induced an effective suppression of atherosclerosis.
Newly we observed that the HB-EGF ASO administration significantly improved systemic insulin sensitivity in vivo.
Acknowledgments:
This study was financially supported by National Institutes of Health grants of HL105577(SL), GM103527 (PI: Lisa Cassis), HL030568 (AJL), and HL064731 (JAB), and American Heart Association 17GRNT33700302 (SL). The content is solely the responsibility of the authors and does not necessarily represent official views of the fund providers.
Abbreviations:
- ApoB
apolipoprotein B
- ASO
antisense oligonucleotide
- EGFR
epidermal growth factor receptor
- HB-EGF
heparin-binding EGF-like growth factor
- LDL
low-density lipoprotein
- LDLR
LDL receptor
- MTP
microsomal triglyceride transfer protein
- TG
triglyceride
- VLDL
very low-density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ginsberg HN, Maccallum PR. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: II. Therapeutic management of atherogenic dyslipidemia. Journal of clinical hypertension. 2009;11:520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ginsberg HN, MacCallum PR. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. Journal of the cardiometabolic syndrome. 2009;4:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Experimental cell research. 2003;284:2–13. [DOI] [PubMed] [Google Scholar]
- [4].Abraham JA, Damm D, Bajardi A, Miller J, Klagsbrun M, Ezekowitz RA. Heparin-binding EGF-like growth factor: characterization of rat and mouse cDNA clones, protein domain conservation across species, and transcript expression in tissues. Biochemical and biophysical research communications. 1993;190:125–33. [DOI] [PubMed] [Google Scholar]
- [5].Dreux AC, Lamb DJ, Modjtahedi H, Ferns GA. The epidermal growth factor receptors and their family of ligands: their putative role in atherogenesis. Atherosclerosis. 2006;186:38–53. [DOI] [PubMed] [Google Scholar]
- [6].Shepard HM, Brdlik CM, Schreiber H. Signal integration: a framework for understanding the efficacy of therapeutics targeting the human EGFR family. The Journal of clinical investigation. 2008;118:3574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee S, Springstead JR, Parks BW, Romanoski CE, Palvolgyi R, Ho T, et al. Metalloproteinase processing of HB-EGF is a proximal event in the response of human aortic endothelial cells to oxidized phospholipids. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Romanoski CE, Lee S, Kim MJ, Ingram-Drake L, Plaisier CL, Yordanova R, et al. Systems Genetics Analysis of Gene-by-Environment Interactions in Human Cells. Am J Hum Genet. 2010;86:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Matsumoto S, Kishida K, Shimomura I, Maeda N, Nagaretani H, Matsuda M, et al. Increased plasma HB-EGF associated with obesity and coronary artery disease. Biochemical and biophysical research communications. 2002;292:781–6. [DOI] [PubMed] [Google Scholar]
- [10].Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:29–33. [DOI] [PubMed] [Google Scholar]
- [11].Guo Y, Ding Q, Chen L, Ji C, Hao H, Wang J, et al. Overexpression of Heparin-Binding Epidermal Growth Factor-Like Growth Factor Mediates Liver Fibrosis in Transgenic Mice. Am J Med Sci. 2017;354:199–210. [DOI] [PubMed] [Google Scholar]
- [12].Takemura T, Yoshida Y, Kiso S, Kizu T, Furuta K, Ezaki H, et al. Conditional loss of heparin-binding EGF-like growth factor results in enhanced liver fibrosis after bile duct ligation in mice. Biochemical and biophysical research communications. 2013;437:185–91. [DOI] [PubMed] [Google Scholar]
- [13].Takemura T, Yoshida Y, Kiso S, Saji Y, Ezaki H, Hamano M, et al. Conditional knockout of heparin-binding epidermal growth factor-like growth factor in the liver accelerates carbon tetrachloride-induced liver injury in mice. Hepatology research : the official journal of the Japan Society of Hepatology. 2013;43:384–93. [DOI] [PubMed] [Google Scholar]
- [14].Huang G, Besner GE, Brigstock DR. Heparin-binding epidermal growth factor-like growth factor suppresses experimental liver fibrosis in mice. Laboratory investigation; a journal of technical methods and pathology. 2012;92:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim S, Yang L, Kim S, Lee RG, Graham MJ, Berliner JA, et al. Targeting hepatic heparin-binding EGF-like growth factor (HB-EGF) induces anti-hyperlipidemia leading to reduction of angiotensin II-induced aneurysm development. PloS one. 2017;12:e0182566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Seth PP, Vasquez G, Allerson CA, Berdeja A, Gaus H, Kinberger GA, et al. Synthesis and biophysical evaluation of 2’,4’-constrained 2’O-methoxyethyl and 2’,4’-constrained 2’O-ethyl nucleic acid analogues. The Journal of organic chemistry. 2010;75:1569–81. [DOI] [PubMed] [Google Scholar]
- [17].Geary RS, Norris D, Yu R, Bennett CF. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Advanced drug delivery reviews. 2015;87:46–51. [DOI] [PubMed] [Google Scholar]
- [18].Lee RG, Fu W, Graham MJ, Mullick AE, Sipe D, Gattis D, et al. Comparison of the pharmacological profiles of murine antisense oligonucleotides targeting apolipoprotein B and microsomal triglyceride transfer protein. Journal of lipid research. 2013;54:602–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arteriosclerosis, thrombosis, and vascular biology. 2002;22:456–61. [DOI] [PubMed] [Google Scholar]
- [20].Daugherty A, Whitman SC. Quantification of atherosclerosis in mice. Methods in molecular biology. 2003;209:293–309. [DOI] [PubMed] [Google Scholar]
- [21].Temel RE, Lee RG, Kelley KL, Davis MA, Shah R, Sawyer JK, et al. Intestinal cholesterol absorption is substantially reduced in mice deficient in both ABCA1 and ACAT2. Journal of lipid research. 2005;46:2423–31. [DOI] [PubMed] [Google Scholar]
- [22].Solca FF, Baum A, Langkopf E, Dahmann G, Heider KH, Himmelsbach F, et al. Inhibition of epidermal growth factor receptor activity by two pyrimidopyrimidine derivatives. The Journal of pharmacology and experimental therapeutics. 2004;311:502–9. [DOI] [PubMed] [Google Scholar]
- [23].Bostrom K, Boren J, Wettesten M, Sjoberg A, Bondjers G, Wiklund O, et al. Studies on the assembly of apo B-100-containing lipoproteins in HepG2 cells. The Journal of biological chemistry. 1988;263:4434–42. [PubMed] [Google Scholar]
- [24].Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends in endocrinology and metabolism: TEM. 2011;22:353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mobin MB, Gerstberger S, Teupser D, Campana B, Charisse K, Heim MH, et al. The RNA-binding protein vigilin regulates VLDL secretion through modulation of Apob mRNA translation. Nature communications. 2016;7:12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ginsberg HN, Fisher EA. The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. Journal of lipid research. 2009;50 Suppl:S162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Conlon DM, Thomas T, Fedotova T, Hernandez-Ono A, Di Paolo G, Chan RB, et al. Inhibition of apolipoprotein B synthesis stimulates endoplasmic reticulum autophagy that prevents steatosis. The Journal of clinical investigation. 2016;126:3852–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2104–12. [DOI] [PubMed] [Google Scholar]
- [29].Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell metabolism. 2008;7:95–6. [DOI] [PubMed] [Google Scholar]
- [30].Fukatsu Y, Noguchi T, Hosooka T, Ogura T, Kotani K, Abe T, et al. Muscle-specific overexpression of heparin-binding epidermal growth factor-like growth factor increases peripheral glucose disposal and insulin sensitivity. Endocrinology. 2009;150:2683–91. [DOI] [PubMed] [Google Scholar]
- [31].Vinante F, Rigo A. Heparin-binding epidermal growth factor-like growth factor/diphtheria toxin receptor in normal and neoplastic hematopoiesis. Toxins. 2013;5:1180–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhou Z, Darwal MA, Cheng EA, Taylor SR, Duan E, Harding PA. Cellular reprogramming into a brown adipose tissue-like phenotype by co-expression of HB-EGF and ADAM 12S. Growth factors. 2013;31:185–98. [DOI] [PubMed] [Google Scholar]
- [33].Lee JS, Suh JM, Park HG, Bak EJ, Yoo YJ, Cha JH. Heparin-binding epidermal growth factor-like growth factor inhibits adipocyte differentiation at commitment and early induction stages. Differentiation. 2008;76:478–87. [DOI] [PubMed] [Google Scholar]
- [34].Nakata A, Miyagawa J, Yamashita S, Nishida M, Tamura R, Yamamori K, et al. Localization of heparin-binding epidermal growth factor-like growth factor in human coronary arteries. Possible roles of HB-EGF in the formation of coronary atherosclerosis. Circulation. 1996;94:2778–86. [DOI] [PubMed] [Google Scholar]
- [35].Reape TJ, Wilson VJ, Kanczler JM, Ward JP, Burnand KG, Thomas CR. Detection and cellular localization of heparin-binding epidermal growth factor-like growth factor mRNA and protein in human atherosclerotic tissue. Journal of molecular and cellular cardiology. 1997;29:1639–48. [DOI] [PubMed] [Google Scholar]
- [36].Zhang H, Sunnarborg SW, McNaughton KK, Johns TG, Lee DC, Faber JE. Heparin-binding epidermal growth factor-like growth factor signaling in flow-induced arterial remodeling. Circulation research. 2008;102:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.