Key Clinical Message
This case illustrates the importance and potential of having TCD monitoring in intensive care. This easy‐to‐use, safe, low‐cost, and bedside tool allows evaluation of the safety and feasibility of an alternative treatment of VSP in SCH and demonstrates the potential to avoid the use of angiography, a high cost, invasive procedure.
Keywords: angiography, Doppler ultrasonography, milrinone, subarachnoid hemorrhage, vasospasm
1. BACKGROUND
Cerebral vasospasm (VSP) remains a leading cause of morbidity and mortality following aneurysmal subarachnoid hemorrhage (SAH). We present the case of a 63‐year‐old woman who had a moderate VSP after SAH, treated with venous infusion of milrinone monitored exclusively by bedside transcranial Doppler ultrasound (TCD).
Cerebral vasospasm is a main cause of morbidity and mortality following aneurysmal SAH and contributes to delayed cerebral ischemia (DCI).1 VSP occurs around the fourth day after SAH and usually peaks between seven and ten days.1 Moreover, this condition is a persistent state of vasoconstriction, which is present in 70% of the patients who survive the first 24 hours after SAH.2
New neurological deficit from VSP is clinically difficult to detect in poor‐grade SAH, because of the low level of consciousness of the patient and the frequent use of sedation as part of the treatment, making detection of acute neurological deterioration even more challenging.3 Nevertheless, in these cases it is possible to diagnose VSP by angiography or transcranial Doppler ultrasound (TCD).3
The diagnosis of vasospasm by TCD is based on the velocities in the basal cerebral arteries and Lindegaard's ratio (LR),4 given by the velocities of the internal carotid and the middle cerebral arteries. LR values can indicate mild (3.0‐4.5), moderate (4.5‐6.0), or severe (LR > 6.0) vasospasm.4 Mean cerebral blood flow velocity (CBFV) under 120 cm/s in the MCA practically rules out VSP, while values >200 cm/s indicate a high probability of VSP.4
Angiography is the “gold standard” in VSP diagnosis5 and allows a rescue cerebral angioplasty or intra‐arterial infusion of a vasodilator, such as milrinone.6 The main limitations of angiography in this setting are its high cost and invasiveness, the fact that it requires the use of general anesthesia, the need for contrast administration, and the patient's exposure to radiation.5, 7 In summary, angiography cannot be recommended for repeated assessments, as it is often clinically necessary.
We report our experience in monitoring CBFV by serial TCD in a case of moderate VSP after poor‐grade SAH (Fisher‐IV, Hunt Hess‐V), which was treated by intravenous infusion of milrinone, as an alternative to intra‐arterial milrinone managed by angiography.
2. CASE REPORT
A 63‐year‐old woman, with systemic arterial hypertension, dyslipidemia, coronary arterial disease, a history of acute myocardial infarction, and depression without follow‐up, was admitted to the emergency room with decerebrate posturing and deep coma (Hunt Hess‐V). Family members informed that she was last seen lucid and well oriented at 11 pm on the day prior to admission, being found unconscious in her bathroom at around 7 am on the day of admission. Cranial computerized tomography (CT) showed subarachnoid hemorrhage (Fisher‐IV) with important supratentorial ventricular dilatation and ventricular hemorrhage (Figure 1). External ventricular drainage was inserted.
Figure 1.
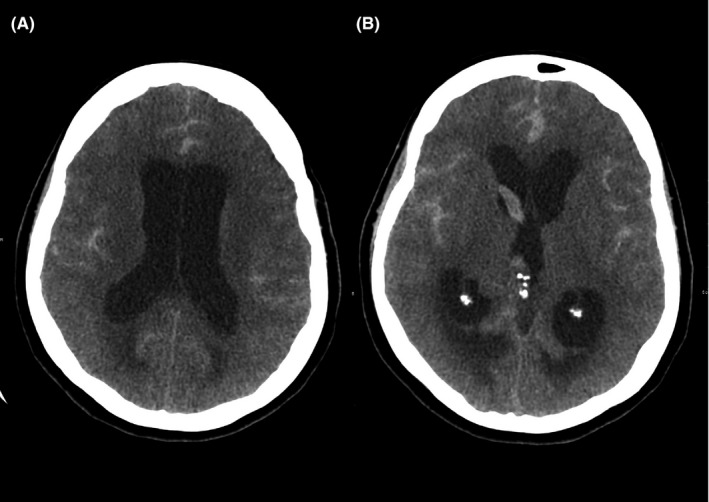
Computed tomography (CT) on hospital day 1 showing subarachnoid hemorrhage and acute hydrocephalus (A) with ventricular involvement suggestive of Fisher grade 4 (B)
The patient was admitted to the intensive care unit (ICU) sedated, intubated, hemodynamically stable, without use of vasoactive drugs. An angiography was performed six hours after admission, and an aneurysm was detected. Subsequently, it was treated with embolization.
In the fourth day after SAH, her neurological status had not evolved and TCD ultrasound (Box‐X, Compumedics, DWL) was used to assess her cerebral circulation. A TCD probe (2 MHz) was fixed on the temporal window of the patient, and the MCA was insonated at a depth of 50‐55 mm. As a result, moderate VSP (mean CBFV: 131 cm/s; internal carotid artery velocity: 28.4 cm/s; LR = 4.6) was observed on the MCA at 50 mm depth (Figure 2). The patient underwent prophylactic treatment with nimodipine and, after the detection of VSP, treatment with norepinephrine was started, to raise systolic blood pressure (BP) to a target of 180 mm, as well as maintaining normovolemia.
Figure 2.
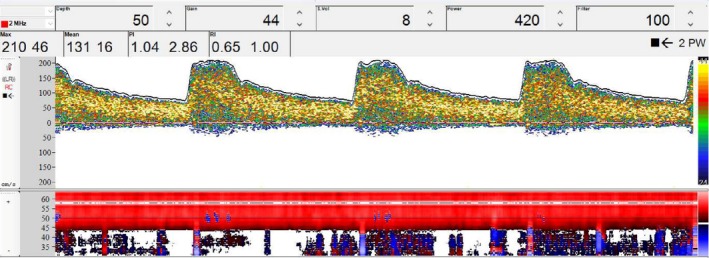
Transcranial Doppler ultrasound of the middle cerebral artery at a depth of 50 mm. The image shows peak systolic velocity of 210 cm/s, mean flow velocity (MFV) of 131 cm/s, pulsatility index of 1.04, and resistance index of 0.65. MFV in the internal carotid artery was 28.4 cm/s, Lindegaard's ratio was 4.6, indicating moderate vasospasm
Due to the lack of response with standard therapy, we attempted the unusual administration of venous milrinone 0.750 mcg/kg/min, after informed consent from her family. Twelve hours later, the patient underwent another evaluation with TCD which showed a surprising reduction in mean CBFV to 78 cm/s and LR = 2.7 compatible with the absence of VSP (Figure 3). Noteworthy, other relevant parameters remained unchanged, with the same conditions of BP, PaCO2, and sedation during these 12 hours. Intravenous milrinone was started on the fourth day after admission and used during 21 days. A CT scan after this VSP event did not show signs of DCI, rebleeding, edema, or other complications. The patient was followed by serial TCD during the ICU stay, and new signs of VSP were not detected. Angiography was not needed to evaluate the occurrence of new VSP.
Figure 3.
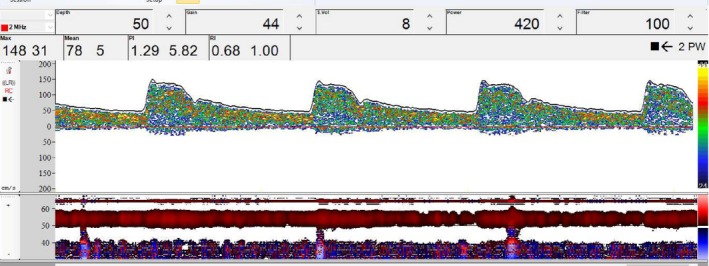
Transcranial Doppler ultrasound of the middle cerebral artery at a depth of 50 mm recording through the temporal bone window. The image shows peak systolic velocity of 148 cm/s, mean flow velocity (MFV) of 78 cm/s, pulsatility index of 1.29, and resistance index of 0.68. MFV in the internal carotid artery was 28.4 cm/s, Lindegaard's ratio was 2.7, compatible with a hyperemic state, but no vasospasm
Finally, three months after symptom onset, the patient was discharged from the hospital. The patient was responsive to verbal stimuli and presented clinical signs such as paresthesia of the right arm as well as small limited movement of the legs. After hospital discharge, rehabilitation involved support from a speech therapist, a neurologist, and a physiotherapist.
3. DISCUSSION
To our knowledge, this is the first report of the use of TCD monitoring in venous administration of milrinone as a treatment for VSP. This approach allowed the simultaneous evaluation of safety and effectiveness of an alternative treatment, which was particularly relevant with the limitations of other forms of clinical examination in cases of poor‐grade SAH, with the ensuing decrease in the level of consciousness.
According to the American Heart Association, the Neurocritical Care Society, and the European guidelines,8 nimodipine is the only medication proven to improve outcomes after SAH and to reduce the rate of angiographic vasospasm.8 Hemodynamic manipulation, known as the triple‐H therapy, has for decades been the cornerstone of VSP management.9 However, the literature supporting its safety and efficacy is scarce and its use may be associated with adverse events.10 The current main goal is to maintain normovolemia and BP augmented in a stepwise fashion by the use of a vasopressor.11
In our case, the VSP was not resolved after standard clinical treatment, involving normovolemia and augmented BP with vasopressors and fluid. This prompted us to use intravenous milrinone, and it provided a fast, safe, and effective resolution of VSP manifestations monitored by TCD.
Recent retrospective series reported that an intra‐arterial milrinone treatment with angiography is safe and effective to reduce refractory VSP after SAH.12, 13 Important to notice though, that in these studies, patients who did not recover from the coma status were excluded. Furthermore, evidence about treatment of DCI with milrinone and its use to improve outcomes in patients with SAH, come from studies regarded of very low quality.14
Milrinone is an inhibitor of phosphodiesterase III, with positive inotropic and vasodilatory effects.1, 2 Although intra‐arterial milrinone contributes to the reduction of VSP, this usually requires concomitant use of angiography.12, 13 On the other hand, its intravenous use is not well known and further studies are needed to assess this option, supported by bedside monitoring to guarantee the safety and effectiveness of the treatment.
It is well known that TCD has been proposed as a VSP detection method since 1980s,15 given that it is a non‐invasive, bedside, low‐cost, repeatable, quick, and reliable examination.4, 7 In the case we described here though, its use has been extended to the monitoring of intravenous infusion of milrinone in a poor‐grade SAH thus precluding the need to use an arterial route for infusion during angiography.
There are three hemodynamic phases associated with SAH: oligemia, hyperemia, and VSP.16 In all of these, TCD can be helpful. The differential diagnosis of VSP has a hyperemic pattern, due to brain tissue acidosis and microvascular bed dilatation.16 However, the Lindegaard index can confirm the VSP.16
The gold standard for diagnosis and treatment of VSP is still angiography.5 However, it is an invasive procedure, besides giving a static measurement of cerebral blood flow.5 Furthermore, this method involves certain risks, such as a new cerebrovascular accident during the procedure, rupture of cerebral vessels, and embolism.7
In presenting this case study, our main aim was to report on the feasibility of an alternative approach to treatment of vasospasm, using TCD as a bedside monitoring tool. Further clinical trials are needed to assess the feasibility of this approach to be extended to a larger number of patients with SAH and to compare its cost‐effectiveness with the classical approach involving infusion of milrinone during angiography.
4. CONCLUSION
In a patient with moderate VSP, we demonstrated the possibility of treatment with intravenous milrinone, in association with standard clinical treatment, monitored by TCD, without the need of resorting to intra‐arterial milrinone infusion during angiography.
Our results demonstrate the benefits of having TCD available in ICU as an easy‐to‐use, safe, and low‐cost bedside tool that has the potential to prevent repeated angiographic examinations in many patients. Clearly, formal clinical trials are needed to produce guidelines that will allow extending this approach to routine clinical management of patients with SAH.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTION
AGS‐T: performed measurements and drafted manuscript. RHP and CJR: performed data analysis and interpretation. RBP and CJR: supervised data analysis and interpretation. CR and SF: designed the figures and drafted manuscript. JGR, AG and PB: revised final version of manuscript. All authors checked manuscript and approved final version.
Santos‐Teles AG, Passos RH, Panerai RB, et al. Intravenous administration of Milrinone, as an alternative approach to treat vasospasm in subarachnoid hemorrhage: A case report of transcranial Doppler monitoring. Clin Case Rep. 2019;7:648–652. 10.1002/ccr3.2034
REFERENCES
- 1. Romero CM, Morales D, Reccius A, et al. Milrinone as a rescue therapy for symptomatic refractory cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2009;11:165‐171. [DOI] [PubMed] [Google Scholar]
- 2. Fraticelli AT, Cholley BP, Losser MR. Milrinone for the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2008;39:893‐898. [DOI] [PubMed] [Google Scholar]
- 3. D’Andrea A, Conte M, Scarafile R, et al. Transcranial Doppler ultrasound: physical principles and principal applications in neurocritical care unit. J Cardiovasc Echogr. 2016;26:28‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirsch JD, Mathur M, Johnson MH, et al. Advances in transcranial Doppler US: imaging ahead. Radiographics. 2013;33:E1‐E14. [DOI] [PubMed] [Google Scholar]
- 5. Wang HC, Lin WC, Yang TM, et al. Time course of cerebral hemodynamics in aneurysmal subarachnoid hemorrhage. J Clin Ultrasound. 2012;40:91‐98. [DOI] [PubMed] [Google Scholar]
- 6. Kimball MM, Velat GJ, Hoh BL. Participants in the international multidisciplinary consensus conference on the critical care management of subarachnoid hemorrhage. Critical care guidelines on the endovascular management of cerebral vasospasm. Neurocrit Care. 2011;15:336‐341. [DOI] [PubMed] [Google Scholar]
- 7. Wintermark M, Ko N, Smith WS, et al. Vasospasm after subarachnoid hemorrhage: utility of perfusion CT and CT angiography on diagnosis and management. Am J Neuroradiol. 2006;27:26‐34. [PMC free article] [PubMed] [Google Scholar]
- 8. Connolly ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:1711‐1737. [DOI] [PubMed] [Google Scholar]
- 9. Oliveira M, Turkel‐Parrella D, Duggal A, et al. Managing aneurysmal subarachnoid hemorrhage: it takes a team. Cleve Clin J Med. 2015;82:177‐192. [DOI] [PubMed] [Google Scholar]
- 10. Egge A, Waterloo K, Sjøholm H, et al. Prophylactic hyperdynamic postoperative fluid therapy after aneurysmal subarachnoid hemorrhage: a clinical, prospective, randomized, controlled study. Neurosurgery. 2001;49:593‐605. [DOI] [PubMed] [Google Scholar]
- 11. Kassell NF, Peerless SJ, Durward QJ, et al. Treatment of ischemic deficits from vasospasm with intravascular volume expansion and induced arterial hypertension. Neurosurgery. 1982;11:337‐343. [DOI] [PubMed] [Google Scholar]
- 12. Alamri AS, Alturki A, Tampeiri D, et al. Use of intra‐arterial milrinone rescue therapy in patients with refractory and super refractory vasospasm after aneurysmal subarachnoid hemorrhage. Can J Neurol Sci. 2016;43:S2. [Google Scholar]
- 13. Lannes M, Teitelbaum J, del Pilar Cortés M, Cardoso M, Angle M. Milrinone and homeostasis to treat cerebral vasospasm associated with subarachnoid hemorrhage: the montreal neurological hospital protocol. Neurocrit Care. 2012;16:354‐362. [DOI] [PubMed] [Google Scholar]
- 14. Lannes M, Zeiler F, Guichon C, Teitelbaum J. The use of milrinone in patients with delayed cerebral ischemia following subarachnoid hemorrhage: a systematic review. Can J Neurol Sci. 2017;44:152‐160. [DOI] [PubMed] [Google Scholar]
- 15. Panerai RB. Transcranial Doppler for evaluation of cerebral autoregulation. Clin Auton Res. 2015;19:197‐211. [DOI] [PubMed] [Google Scholar]
- 16. Oliveira ML, Kairalla AC, Fonoff ET, et al. Cerebral microdialysis in traumatic brain injury and subarachnoid hemorrhage: state of the art. Neurocrit Care. 2014;21:152‐162. [DOI] [PubMed] [Google Scholar]


