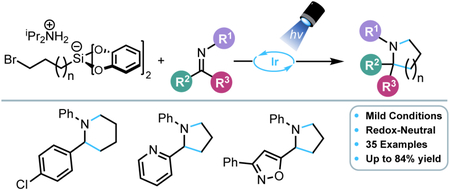
Abstract
Photoredox-mediated radical/polar crossover (RPC) processes offer new avenues for the synthesis of cyclic molecules. This process has been realized for the construction of medium-sized saturated nitrogen heterocycles. Photocatalytically generated alkyl radicals possessing pendant leaving groups engage imines in C–C bond formation, and subsequent reduction of the intermediate nitrogen-centered radical triggers anionic ring closure. With the aid of visible light irradiation, substituted pyrrolidines, piperidines, and azepanes can be prepared under mild, redox-neutral conditions.
Graphical Abstract
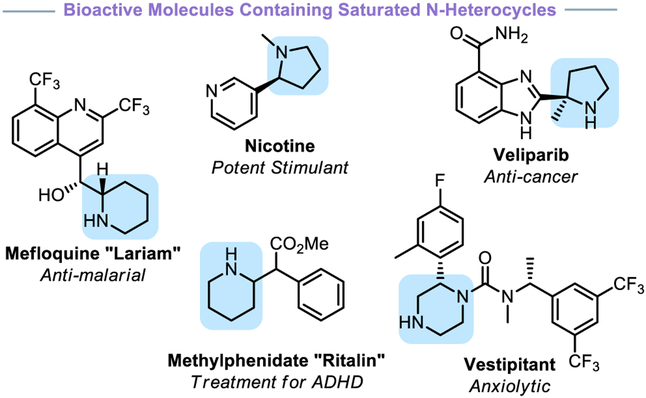
Heterocyclic compounds comprise 87% of known small molecule therapeutics.1 Nitrogen-based heterocycles in particular are among the most common subunit in drug molecules.2 These structures also serve as the backbone of ligands and appear in numerous natural and designed molecules.1,2 In the context of drug development, the improved pharmacological properties of compounds with higher fractions of sp3-hybridized centers3 has sparked an increasing interest in incorporating saturated nitrogen-heterocycles in bioactive molecules.4 Piperidines are the most prominent saturated nitrogen-heterocycles in therapeutic compounds, followed closely by piperazines and pyrrolidines.2 The popularity and utility of these heterocycles motivates the pursuit of novel synthetic methods to prepare these cyclic systems in a mild and modular fashion.
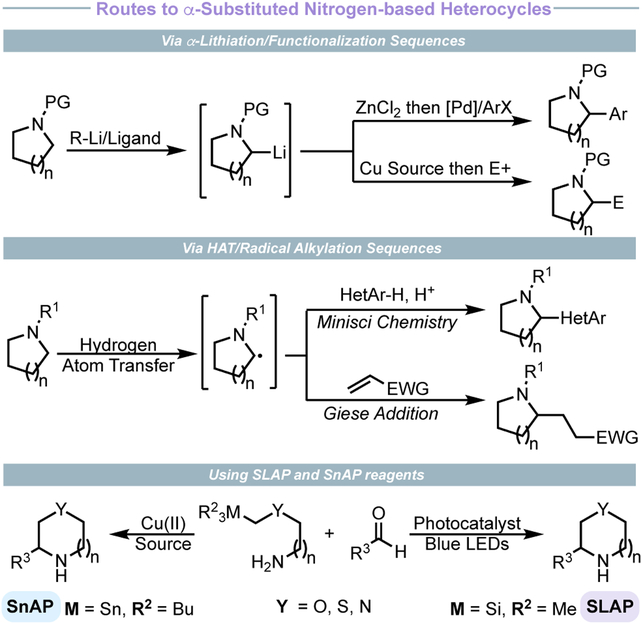
The most common approach to introduce saturated nitrogen-heterocycles is a C–N bond-forming process through SNAr chemistry, cross-coupling strategies such as Buchwald-Hartwig amination, or peptide coupling.1,5 However, many medicinally relevant compounds possess α-substitution (often aryl, Figure 1), which poses challenges to these methods (Figure 2). A classical method for the synthesis of α-arylated nitrogen heterocycles is a lithiation followed by treatment with an electrophile or conversion into a suitable nucleophile (i.e., cuprate or zincate) for cross-coupling.6 Another class of increasingly popular reactions are single-electron variants of the lithiation approach, initiated either through hydrogen-atom transfer (HAT)7 or photoredox-induced oxidation of radical precursors (such as a carboxylic acids or trifluoroborates).8 Although powerful, these strategies are contingent on having a preconstructed saturated N-heterocycle that is subsequently functionalized with the group of interest.
Figure 1.
Bioactive nitrogen-containing heterocycles.
Figure 2.
Typical approaches to saturated nitrogen heterocycles.
A less common approach to α-arylated nitrogen heterocycles is the construction of the ring from acyclic precursors through an annulation process. The most obvious way to accomplish such a process, reductive amination, is notoriously challenging, considering that the cyclic imine intermediate is prone to polymerization/dimerization.9 Recently, a resurgence in methods based on the Hofmann–Löffler–Freytag (HLF) reaction have yielded elegant approaches to pyrrolidines from amines with distal olefins.10 Like-wise, PCET has allowed lactams to be prepared from cyclization of an amidyl radical containing a pendant olefin.11
Recently, Bode and co-workers have developed a copper-mediated annulation process using “SnAP” reagents.12 This strategy involves condensation of an organotin-tethered amine with an aldehyde to generate an aldimine. Subsequent oxidative generation of a heteroatom-stabilized primary carbon-centered radical via destannylative fragmentation triggers 6-endo-trig cyclization. Reduction of the aminyl radical provides the desired nitrogen-heterocycle (e.g., thiomorpholine, oxazepane, 3-alkoxypyrrolidine). A silicon-variant of the annulation paradigm has also been developed using “SLAP” reagents and transitioned to continuous flow.13
The identification of various precursors that furnish alkyl radicals under photoredox conditions, such as Bode’s SLAP reagents, has led to the discovery of previously nonviable reactions and allowed new synthetic disconnections to be realized.14 Visible-light photoredox catalysis provides a means to tap the synthetic potential of radicals in a way not possible when using stoichiometric radical generation techniques, while still retaining the benefits associated with odd-electron reactivity. These hallmarks of photoredox processes have precipitated their rapid uptake in both academia and industry, especially for late-stage functionalization.15 Visible-light-mediated approaches have revolutionized an array of different reaction classes, including cross-coupling,16 cycloaddition,17 and, recently, radical/polar crossover (RPC)18 processes.
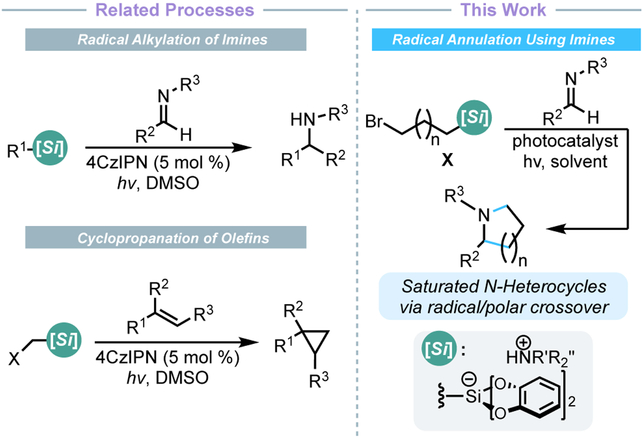
In the context of the latter, our group has initiated a program to apply various radical precursors in RPC processes that lead to annulation reactions, including the cyclopropanation of alkenes (Figure 3).19 Inspired by the successes of these annulation processes and the SLAP/SnAP paradigm,13,14 a novel approach to the synthesis of saturated nitrogen-based heterocycles from acyclic species was considered. The crux of this approach is the radical alkylation of an imine using an oxidizable, bifunctional radical precursor. Subsequent reduction of the resulting nitrogen-centered radical would furnish an amide anion that could engage a distal leaving group in an intramolecular displacement. A series of annulation processes (5-, 6-, or even 7-exo-tet ring closures) can be envisioned using this improved radical/polar crossover approach. Indeed, typical methods to accomplish this type of process require a combination of stoichiometric Mn2(CO)10, InCl3, and UV irradiation.20
Figure 3.
Comparison of related developments to this work.
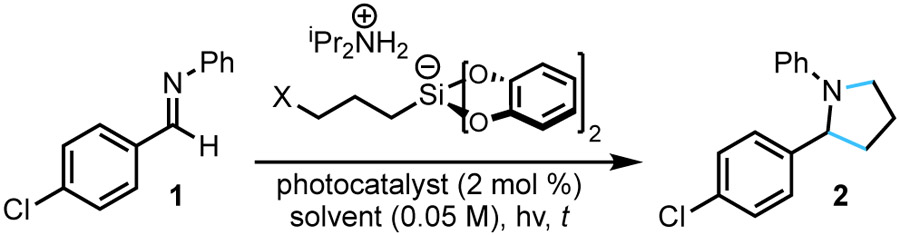
The proposed annulation makes use of a small set of easily accessible bis(catecholato)silicate radical precursors.21 These reagents have previously been effective for the radical alkylation of imines22 and the cyclopropanation of olefins19a (Figure 3). Motivated by these previous successes, a preliminary screen was conducted wherein imine 1 was irradiated with a series of 3-halopropylsilicates (Cl, Br, I) in the presence of a photocatalyst [Ru(bpy)3(PF6)2] in DMF (Table 1 entries 1-3). Although annulation was observed in all cases, the reaction generally proceeded slowly and gave poor conversion to the desired pyrrolidine 2. Interestingly, of this series, the 3-bromopropylsilicate proved best. Speculatively, the 3-bromopropyl radical provides a good compromise of stability (3-iodopropyl radical is prone to SH2 3-exo-tet cydization23) and reactivity (some addition without cyclization was observed when using 3-chloropropylsilicate). Reducing the loading of the 3-bromopropylsilicate and extending the reaction time led to more than doubling the yield of 2 (entry 4). High throughput experimentation (HTE) techniques revealed that using a twofold excess of the imine reagent provided a higher yield (See Supporting Information). However, excess imine could be recovered upon chromatographic purification in cases where the imine is valuable (such as late-stage derivatization scenarios). Scale up of these conditions gave identical yield in a much shorter period (entry 5). Evaluation of alternative photocatalysts revealed that Ir[dF(CF3)ppy]2(bpy)PF6 was superior (entries 6-7). Using this photocatalyst and a more powerful light source, the reaction time was reduced to a mere 30 min (entry 9). Various additives were screened, but gave either similar or diminished yields (see Supporting Information for details). Control studies confirmed irradiation was necessary for reactivity (entry 10), but the reaction could proceed (albeit in poor yield) in the absence of a photocatalyst (entry 11). Given that alkylsilicates are known to fragment when irradiated with UV-A/UV-B light, an electron donor–acceptor complex24 between the highly conjugated imine and the bis(catecholato) moiety may form, facilitating light-mediated radical generation.
Table 1.
Optimization of the annulation process.a
 | |||||
|---|---|---|---|---|---|
| entry a | X | photocat.b | solvent | time (h) | yield (%)c |
| 1 | Cl | [Ru] | DMF | 16 | 8 |
| 2 | Br | [Ru] | DMF | 16 | 20 |
| 3 | I | [Ru] | DMF | 16 | 10 |
| 4d | Br | [Ru] | DMF | 48 | 46 |
| 5 e | Br | [Ru] | DMSO | 16 | 46 |
| 6 e | Br | [Ir] | DMSO | 16 | 54 |
| 7e | Br | 4CzIPN | DMSO | 16 | 35 |
| 8 f | Br | [Ir] | DMSO | 16 | 60 |
| 9 f | Br | [Ir] | DMSO | 0.5 | 59 |
| 10f,g | Br | [Ir] | DMSO | 16 | – |
| 11f | Br | – | DMSO | 16 | 17h |
Conditions unless otherwise noted: silicate (5 equiv, 1.5 mmol), imine (1.0 equiv, 0.3 mmol), photocatalyst (2 mol %), DMSO (0.1 M), irradiating with 21 W CFL.
Photocatalyst: [Ru] = [Ru(bpy)3](PF6)2 and [Ir] = [Ir{dF(CF3)2ppy}2(bpy)]PF6.
Isolated yield after purification.
2 equiv of silicate used.
A 1:2 silicate/imine ratio used along with 4 W blue LEDs.
A 1:2 silicate/imine ratio used along with 30 W blue LEDs.
Reaction conducted in the dark.
NMR yield of crude reaction mixture.
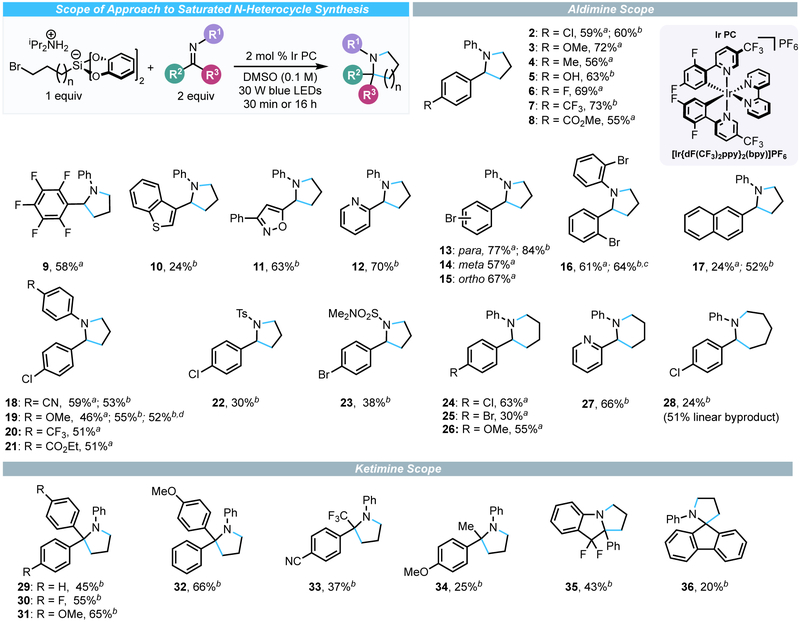
With suitable conditions established, the scope of the annulation process was examined (Scheme 1). In general, when using 3-bromopropylsilicate, a variety of N-phenyl aldimines were amenable to the RPC cyclization process to provide the corresponding pyrrolidines. In some cases, extending the reaction time to 16 h was necessary to give improved yield. The reaction tolerated imines derived from both electron-rich and electron-poor benzaldehydes, including a free phenol (5). The cyclization of benzothiophene, isoxazole, and pyridine-containing imines successfully provided pyrrolidines 10-12. Substituents with a variety of steric and electronic characteristics were tolerated on either of the aryl rings on the imine substrate. In particular, replacement of the N-phenyl group with a p-methoxyphenyl (PMP) group gave comparable yield of pyrrolidine product (2 vs. 19). Given the ability to oxidatively cleave PMP groups, these species would be suitable for further derivatization. Additionally, other cleavable groups were compatible with the RPC annulation process. Both p-toluenesulfonyl and N,N-dimethylsulfamidyl aldimines underwent the cyclization process to provide 22 and 23, respectively, but in diminished yields. Unfortunately, the process could not be extended to unprotected (NH) imines, N-Boc-imines, or hydrazones. Using a 4-bromobutylsilicate and identical reaction conditions, piperidines were accessible from aldimines in comparable yields (24-27). Attempts using either a 5-bromopentylsilicate or 5-iodopentylsilicate reagent did provide azepane 28, although in low yield. The major product observed was the uncyclized alkyl halide. In these cases, the rate of protonation by the acidic ammonium counterion of the alkylsilicate likely exceeds the rate of 7-exo-tet cyclization. Smaller rings could not be obtained through this method; only traces of the corresponding aziridine were observed. The inability to access the requisite 2-bromoethylsilicate (likely because of facile β-silyl elimination) prevented any attempts at preparing azetidines.
Scheme 1.
Scope of the Radical Alkylation/Cyclization Processes with Imines.a b
aReaction conditions unless otherwise noted: alkylsilicate (1 equiv, 0.3 mmol), imine (2.0 equiv, 0.6 mmol), photocatalyst (2 mol %), DMSO (0.1 M), irradiating with 30 W blue LEDs at 35 °C for 30 min; all yields are isolated yields after purification. bReaction run for 16 h. cReaction conducted on 1 mmol scale with 1 mol % photocatalyst. dReaction conducted on 2 mmol scale with 1 mol % of photocatalyst.
Ketimines were also amenable to the reaction, allowing pyrrolidines with α-quaternary centers to be assembled rapidly. In general, ketimines derived from benzophenones fared best. Various aryl ring substituents had minimal effect on yield (29-32), an observation consistent with aldimines. Mixed aryl-alkyl ketimines could be used, albeit with diminished yields. Subjecting a ketimine derived from an α-CF3 ketone enabled synthesis of α-CF3-substituted pyrrolidine 33. Similarly, an acetophenone-derived ketimine was competent in the annulation process (34). Ketimines also provide opportunities for bicyclic ring construction, as demonstrated by the preparation of benzopyrrolizidine 35. This bicyclic structure bears resemblance to the core of recently reported anticancer candidates.25 Piperidines bearing α-quaternary centers are also accessible using this RPC approach. Aryl ketiminoacetates and ketimidates, however, were not amenable to this process. The formation of piperidines from ketimines using the analogous 4-bromobutylsilicate reagent was not as effective as piperidine formation, and in some cases unexpected side products were observed.
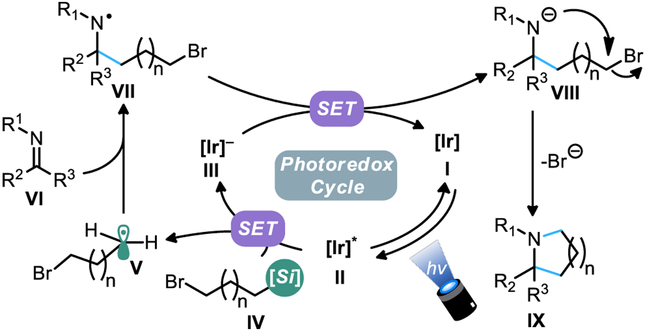
Based on the prior art18-21 and our own observations during the course of this study, the following sequence of events constitute a plausible mechanistic path for the annulation process: (1) Visible light-mediated photoexcitation of I to its excited state II; (2) Reductive quenching of II (E1/2 [P*/P]: 1.35 V vs SCE) by the bifunctional alkylsilicate (E1/2 = +0.4–0.7 vs SCE for most alkylsilicates); (3) Homolytic fragmentation of oxidized IV to furnish alkyl radical V, which readily adds to imine VI, giving the N-centered radical VII; (4) SET reduction ofVII by the reduced state of III to provide amide anion VIII; (5) C-N bond formation and ring closure by way of anionic cyclization, giving IX (Fig. 4).
Figure 4.
Plausible mechanism for the annulation process.
The radical/polar annulation process described herein enables the rapid construction of saturated nitrogen heterocycles from acyclic precursors. This RPC reaction accommodates numerous functional groups, occurs under mild conditions, and can be completed in as little as 30 minutes. Furthermore, its modular nature allows various medium-sized rings to be assembled from a single imine by adjusting the alkylsilicate used, and it also permits quaternary centers to be established α to nitrogen with ease. The inherent flexibility and benign conditions of this strategy make it ideal for the late-stage introduction of saturated N-containing heterocycles.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful for the financial support provided by NIGMS (R01 GM 113878 to G.M.). C.B.K. is grateful for an NIH NRSA fellowship (F32 GM117634) and acknowledges start-up funds from Virginia Commonwealth University. J.K.M. is grateful for a BMS graduate Fellowship in Synthetic Organic Chemistry. We thank Dr. Charles W. Ross, III (University of Pennsylvania) for obtaining HRMS data. We acknowledge Johnson Matthey for fine chemicals donations and Kessil Lighting for donation of a prototype PR160 Rig.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures, details of high-throughput screening, and 1H, 13C, and 19F NMR spectra for all compounds. (PDF)
Notes
The authors declare no competing financial interest.
REFERENCES
- (1) (a).Zhang TY The Evolving Landscape of Heterocycles in Drugs and Drug Candidates Adv. Heterocyclic Chem 2017, 121, 1–12. [Google Scholar]; (b) Taylor RD; MacCoss M; Lawson ADG Rings in Drugs. J. Med. Chem 2014, 57, 5845–5859. [DOI] [PubMed] [Google Scholar]; (c) Baumann M; Baxendale IR An overview of the synthetic routes to the best-selling drugs containing 6-membered heterocycles Beilstein J. Org. Chem 2013, 9, 2265–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Baumann M; Baxendale IR; Ley SV; Nikbin N An overview of the key routes to the best-selling 5-membered ring heterocyclic pharmaceuticals Beilstein J. Org. Chem 2011, 7, 442–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals J. Med. Chem 2014, 57, 10257–10274. [DOI] [PubMed] [Google Scholar]
- (3) (a).Lovering F; Bikker J; Humble C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success J. Med. Chem 2009, 52, 6752–6756. [DOI] [PubMed] [Google Scholar]; (b) Dandapani S; Marcaurelle LA Accessing New Chemical Space for ‘Undruggable’ Targets Nature Chem. Biol 2010, 6, 861–863. [DOI] [PubMed] [Google Scholar]; (c) López-Vallejo F; Giulianotti MA; Houghten RA; Medina-Franco JL Expanding the Medicinally Relevant Chemical Space with Compound Libraries Drug Discov. Today 2012, 17, 718–726. [DOI] [PubMed] [Google Scholar]
- (4).Blakemore DC; Castro L; Churcher I; Rees DC; Thomas AW; Wilson DM; Wood A Organic synthesis provides opportunities to transform drug discovery. Nat. Chem 2018, 10, 383–394. [DOI] [PubMed] [Google Scholar]
- (5).Schneider N; Lowe DM; Sayle RA; Tarselli MA; Landrum GA Big Data from Pharmaceutical Patents: A Computational Analysis of Medicinal Chemists’ Bread and Butter J. Med. Chem 2016, 59, 4385–4402. [DOI] [PubMed] [Google Scholar]
- (6).Vo C-VT; Bode JW Synthesis of Saturated N-Heterocycles J. Org. Chem 2014, 79, 2809–2815 [DOI] [PubMed] [Google Scholar]
- (7) (a).Capaldo L; Ravelli D Hydrogen Atom Transfer (HAT): A Versatile Strategy for Substrate Activation in Photocatalyzed Organic Synthesis Eur. J. Org. Chem 2017, 2056–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ahneman DT; Doyle AG C-H functionalization of amines with aryl halides by nickel-photoredox catalysis Chem. Sci 2016, 7, 7002–7006. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Heitz DR; Tellis JC; Molander GA Photochemical Nickel-Catalyzed C-H Arylation: Synthetic Scope and Mechanistic Investigations J. Am. Chem. Soc 2016, 138, 12715–12718. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Vega JA; Alonso JM; Mendez G; Ciordia M; Delgado F; Trabanco AA Continuous Flow α-Arylation of N,N-Dialkylhydrazones under Visible-Light Photoredox Catalysis Org. Lett 2017, 19, 938–941 [DOI] [PubMed] [Google Scholar]
- (8).Zuo Z; Ahneman DT; Chu L; Terrett JA; Doyle AG; MacMillan DWC Merging photoredox with nickel catalysis: Coupling α-carboxyl sp3 carbons with aryl halides. Science 2014, 345, 437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Iwanejko J; Wojaczyńska E Cyclic imines – preparation and application in synthesis Org. Biomol. Chem 2018, 16, 7296–7314. [DOI] [PubMed] [Google Scholar]
- (10) (a).Musacchio AJ; Nguyen LQ; Beard H; Knowles RR Catalytic Olefin Hydroamination with Aminium Radical Cations: A Photoredox Method for Direct C-N Bond Formation J. Am. Chem. Soc 2014, 136, 12217–12220 [DOI] [PubMed] [Google Scholar]; (b) Wappes A; Fosu SC; Chopko TC; Nagib DA Triiodide-Mediated δ-Amination of Secondary C–H Bonds Angew. Chem. Int. Ed 2016, 55, 9974–9978. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Martinez C; Muniz K An Iodine-Catalyzed Hofmann-Loffler Reaction Angew. Chem. Int Ed 2015, 54, 8287–8291. [DOI] [PubMed] [Google Scholar]
- (11) (a).Miller DC; Choi GJ; Orbe HS; Knowles RR Catalytic Olefin Hydroamidation Enabled by Proton-Coupled Electron Transfer J. Am. Chem. Soc 2015, 137, 13492–13495. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zheng S; Gutiérrez-Bonet A; Molander GA Merging Photoredox PCET with Ni-Catalyzed Cross-Coupling: Cascade Amidoarylation of Unactivated Olefins Chem, 2018, in press. DOI: 10.1016/j.chempr.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12) (a).Luescher MU; Vo C-VT; Bode JW SnAP Reagents for the Synthesis of Piperazines and Morpholines Org. Lett 2014, 16, 1236–1239. [DOI] [PubMed] [Google Scholar]; (b) Luescher MU; Bode JW SnAP-eX Reagents for the Synthesis of Exocyclic 3-Amino- and 3-Alkoxypyrrolidines and Piperidines from Aldehydes Org. Lett 2016, 18, 2652–2655. [DOI] [PubMed] [Google Scholar]
- (13) (a).Hsieh S-Y; Bode JW Silicon Amine Reagents for the Photocatalytic Synthesis of Piperazines from Aldehydes and Ketones Org. Lett 2016, 18, 2098–2101. [DOI] [PubMed] [Google Scholar]; (b) Hsieh S-Y; Bode JW Lewis Acid Induced Toggle from Ir(II) to Ir(IV) Pathways in Photocatalytic Reactions: Synthesis of Thiomorpholines and Thiazepanes from Aldehydes and SLAP Reagents ACS Cent. Sci 2017, 3, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jackl MK; Legnani L; Morandi B; Bode JW Org. Lett 2017, 19, 4696–4699. [DOI] [PubMed] [Google Scholar]
- (14).For reviews on photoredox catalysis, see:Prier CK; Rankic DA; MacMillan DWC Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev 2013, 113, 5322–5363.Romero NA; Nicewicz DA Organic Photoredox Catalysis. Chem. Rev 2016, 116, 10075–10166.Matsui JK; Lang SB; Heitz DR; Molander GA Photoredox-Mediated Routes to Radicals: The Value of Catalytic Radical Generation in Synthetic Methods Development. ACS Catal. 2017, 7, 2563–2575.Shaw MH; Twilton J; MacMillan DWC Photoredox Catalysis in Organic Chemistry. J. Org. Chem 2016, 81, 6898–6926.
- (15) (a).Zhang R; Li G; Wismer M; Vachal P; Colletti SL; Shi Z-C Profiling and Application of Photoredox C(sp3)–C(sp2) Cross-Coupling in Medicinal Chemistry. ACS Med. Chem. Lett 2018, 9, 773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Douglas JJ; Sevrin MJ; Stephenson CRJ Visible Light Photocatalysis: Applications and New Disconnections in the Synthesis of Pharmaceutical Agents. Org. Process Res. Dev 2016, 20, 1134–1147. [Google Scholar]
- (16) (a).Tellis JC; Kelly CB; Primer DN; Jouffroy M; Patel NR; Molander GA Single-Electron Transmetalation via Photoredox/Nickel Dual Catalysis: Unlocking a New Paradigm for sp3–sp2 Cross-Coupling. Acc. Chem. Res 2016, 49, 1429–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Skubi KL; Blum TR; Yoon TP Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev 2016, 116, 10035–10074. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gui Y-Y; Sun L; Lu Z-P, Yu D-G Photoredox sheds new light on nickel catalysis: from carbon–carbon to carbon–heteroatom bond formation Org. Chem. Front 2016, 3, 522–526. [Google Scholar]
- (17).Yoon TP Visible Light Photocatalysis: The Development of Photocatalytic Radical Ion Cycloadditions, ACS Catal. 2013, 3, 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).For examples see:Xiao T; Li L; Zhou L Synthesis of Functionalized gem-Difluoroalkenes via a Photocatalytic Decarboxylative/Defluorinative Reaction J. Org. Chem 2016, 81, 7908–7916.Lang SB; Wiles RJ; Kelly CB; Molander GA Photoredox Generation of Carbon-Centered Radicals Enables the Construction of 1,1-Difluoroalkene Carbonyl Mimics Angew. Chem., Int. Ed 2017, 56, 15073–15077.Grandjean J-MM; Nicewicz DA Synthesis of Highly Substituted Tetrahydrofurans by Catalytic Polar-Radical-Crossover Cycloadditions of Alkenes and Alkenols Angew. Chem., Int. Ed 2013, 52, 3967–3971.Perkowski AJ; Nicewicz DA Direct Catalytic Anti-Markovnikov Addition of Carboxylic Acids to Alkenes. J. Am. Chem. Soc 2013, 135, 10334–10337.
- (19) (a).Phelan JP; Lang SB; Compton JS; Kelly CB; Dykstra R; Gutierrez O; Molander GA Redox-Neutral Photocatalytic Cyclopropanation via Radical/Polar Crossover. J. Am. Chem. Soc 2018, 140, 8037–8047. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Milligan JA; Phelan JP; Polites VC; Kelly CB; Molander GA Radical/Polar Annulation Reactions (RPARs) Enable the Modular Construction of Cyclopropanes Org. Lett 2018, 20, 6840–6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Slater KA; Friestad GK Mn-Mediated Radical-Ionic Annulations of Chiral N-Acylhydrazones J. Org. Chem 2015, 80, 6432–6440. [DOI] [PubMed] [Google Scholar]
- (21) (a).Jouffiroy M; Primer DN; Molander GA Base-Free Photoredox/Nickel Dual-Catalytic Cross-Coupling of Ammonium Alkylsilicates. J. Am. Chem. Soc 2016, 138, 475–478 [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lin K; Kelly CB; Jouffroy M; Molander GA Preparation of Diisopropylammonium Bis(catecholato) cyclohexylsilicate Org. Synth 2017, 94, 16–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Patel NR; Kelly CB; Siegenfeld AP; Molander GA Mild, Redox-Neutral Alkylation of Imines Enabled by an Organic Photocatalyst ACS Catal. 2017, 7, 1766–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23) (a).Otteson D; Michl J A Procedure fo Gas-Phas Dehalogenation of Organic Dihalides with Alkali Metal Vapors using Micro-wave and/or Ultrsound Excitation and Matrix Isolation of Products J. Org. Chem 1984, 49, 866–873. [Google Scholar]; (b) Ohkita T; Tsuchiya Y; Togo H Radical 3-exo-tet Cyclization of 1,3-Dihalopropanes with SmI2 to form Cyclopropanes Tetrahedron 2008, 64, 7247–7251. [Google Scholar]
- (24).Kandukuri SR; Bahamonde A; Chatterjee I; Jurberg ID; Escudero-Adán EC; Melchiorre P X-Ray Characterization of an Electron Donor–Acceptor Complex that Drives the Photochemical Alkylation of Indoles Angew. Chem., Int. Ed 2015, 54, 1485–1489. [DOI] [PubMed] [Google Scholar]
- (25).Yunlin Y; Xiangyu M; Xiaotong L; Jinxi X; Guoshu C A sixring spiro porphyrin compound and preparation method thereof. CN Patent 108329324 A, July 27, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.