Abstract
Background:
As the adverse clinical outcomes common in patients with chronic kidney disease (CKD) can be prevented or delayed, information on the cost of care across the spectrum of CKD can inform investments in CKD care.
Objectives:
To determine the cost of caring for patients with CKD who are not on dialysis or transplant at baseline.
Design:
Population-based cohort study using administrative health data.
Setting:
Alberta, Canada.
Patients:
Cohort of 219 641 adults with CKD categorized by estimated glomerular filtration rate (eGFR) between April 1, 2012, and March 31, 2014, into Kidney Disease: Improving Global Outcomes (KDIGO) CKD categories, excluding patients on dialysis or transplant at baseline.
Measurements:
The primary outcome was 1-year cumulative unadjusted health care costs, including the cost of drugs, physician visits, emergency department visits, outpatient procedures (including dialysis and other day medicine and surgery procedures), and hospitalizations for the year following each patient’s index date.
Methods:
Mean 1-year direct medical costs were estimated for the cohort as a whole and for patients in the different KDIGO CKD categories as defined at baseline. Costs were further categorized according to baseline demographic and clinical characteristics, and by type of care (ie, kidney care and cardiovascular care).
Results:
In 219 641 adults with CKD, the mean unadjusted cumulative 1-year cost of care was Can$14 634 per patient (median = Can$3672; Q1 = Can$1496, Q3 = Can$10 221). Costs were higher for those with more comorbidity, those with lower eGFR, and those with more severe albuminuria. The cost of kidney and cardiovascular care was Can$230 (1.6% of total costs) and Can$720 (4.9% of total costs), respectively, for the cohort overall. These costs increased substantially for patients with lower eGFR, averaging Can$14 169 (32.3% of total costs) and Can$2395 (5.5% of total costs) for kidney and cardiovascular care, respectively, for people with eGFR<15 mL/min/1.73 m2 at baseline.
Limitations:
We only have estimates of the cost of health care for people with CKD, and not the costs borne by patients or their families. As we have not included costs for people without CKD in this analysis, we are unable to assess the incremental costs associated with CKD.
Conclusions:
We identified that patients with CKD, even when not on dialysis at baseline, had high health care costs (more than twice the cost per person in Canada in 2015), with a graded association between severity of CKD and costs. Our findings can inform current and future cost estimates across the spectrum of CKD, including an estimate of potential savings that might result from interventions that slow or prevent kidney disease.
Keywords: CKD (chronic kidney disease), health care costs, health economics
Abrégé
Contexte:
Les événements cliniques indésirables qui surviennent fréquemment chez les patients atteints d’insuffisance rénale chronique (IRC) peuvent être prévenus ou retardés. Connaître le coût des soins liés à l’ensemble du spectre de la maladie pourrait éclairer les investissements en santé rénale.
Objectifs:
Établir le coût des soins prodigués aux patients atteints d’IRC non dialysés ou transplantés au moment de l’inclusion.
Type d’étude:
Une étude de cohorte représentative de la population réalisée à partir des données administratives en santé.
Cadre:
Alberta, Canada.
Sujets:
Une cohorte de 219 641 adultes atteints d’IRC qui ont été classés entre le 1er avril 2012 et le 31 mars 2014 dans les catégories du KDIGO selon leur DFGe. Les patients dialysés ou transplantés ont été exclus.
Mesures:
Le résultat principal était le coût cumulatif non ajusté des soins de santé sur un an. Pour l’année suivant la date indice de chaque patient, le total incluait les coûts des médicaments, des consultations médicales, des visites aux urgences, des procédures ambulatoires (dialyse et différentes procédures de chirurgie et de médecine d’un jour) et des hospitalisations.
Méthodologie:
La moyenne des coûts médicaux directs sur un an a été estimée à l’inclusion pour l’ensemble de la cohorte et pour chaque catégorie d’IRC du KDIGO. Les coûts ont également été classés selon les caractéristiques démographiques et cliniques des patients à l’inclusion, et par types de soins (soins en néphrologie et en cardiologie).
Résultats:
Dans la cohorte étudiée, la moyenne des coûts cumulatifs non ajustés sur un an s’établissait à 14 634 $ CA par patient (médiane: 3 672 $; Q1: 1 496 $ et Q3: 10 221 $), et davantage pour les patients présentant des comorbidités, un faible DFGe ou une grave albuminurie. Les coûts des soins en santé rénale et cardiovasculaire pour l’ensemble de la cohorte s’élevaient respectivement à 230 $ (1,6 % du montant total) et 720 $ (4,9 % du montant total) par personne. Ces coûts augmentaient considérablement pour les patients présentant un faible DFGe (<15 ml/min/1,73 m2) à l’inclusion, soit en moyenne 14 169 $ (32,3 % du montant total) en santé rénale et 2 395 $ (5,5 % du montant total) en santé cardiovasculaire.
Limites:
L’estimation ne tient compte que du coût des soins prodigués aux patients, et non des coûts assumés par les patients ou leurs proches. L’analyse n’incluant pas les montants pour les patients non atteints d’IRC, nous n’avons pas été en mesure d’évaluer les coûts différentiels associés à la maladie.
Conclusion:
Nous avons constaté que les patients atteints d’IRC, même s’ils n’étaient pas dialysés à l’inclusion, engendraient des coûts de santé plus élevés (plus de deux fois le coût par personne au Canada en 2015) avec une association graduelle de ceux-ci à la gravité de l’IRC. Nos résultats peuvent orienter les évaluations de coût actuelles et futures pour l’ensemble du spectre de l’IRC, notamment l’estimation des économies potentielles qui pourraient résulter d’interventions visant la prévention de l’insuffisance rénale ou le ralentissement de son évolution.
What was known before
While the care of people on dialysis costs nearly Can$100 000 per year, there has been little research to determine the cost of caring for those with nondialysis chronic kidney disease, despite it being 100 times as common as end-stage renal disease.
What this adds
The care of people with nondialysis chronic kidney disease (CKD) averages Can$14 634 per year and is higher for people with more comorbidity, those with lower estimated glomerular filtration rate, and those with more severe albuminuria. Extrapolating our findings to Canada, we estimate that the annual cost of caring for Canadians with CKD (not on dialysis at baseline) approximates Can$32 billion per year—including costs attributable to their CKD and costs attributable to their other medical conditions.
Background
Chronic kidney disease (CKD) is defined by estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 or albuminuria >3 mg/mmol. It affects 12.5% of adults in Canada1 and is associated with adverse clinical outcomes and poor quality of life.2 While most CKD patients (90%-95%) are managed in primary care,2 many with lower eGFR have complex medical needs that require specialist nephrology care, often delivered by a multidisciplinary team that includes nurse clinicians, dieticians, pharmacists, and social workers.
While the care of people on dialysis costs nearly Can$100 000 per year,3-5 there has been less research to determine the cost of caring for those with nondialysis CKD, despite it being 100 times as common as end-stage renal disease (ESRD).1,6-8 Because CKD is associated with multimorbidity, use of resource-intensive treatments, and increased risk of many medical complications that often require hospitalization,9 care of patients with nondialysis CKD may also have significant costs.
Given the clinical importance of CKD, its substantial and increasing prevalence, and the fact that progression and complications of CKD may be prevented or delayed by timely care,10 there is interest in optimizing the management of early CKD. However, this would require considerable resource investment by policy makers, which in turn requires a business case that includes the cost of caring for patients across the spectrum of CKD. In this study, we used population-based data from Alberta, Canada, to examine the cost of caring for patients with CKD who were not receiving dialysis nor had a transplant at baseline.
Research Design and Methods
Overview
We used laboratory data and the Kidney Disease: Improving Global Outcomes (KDIGO) CKD staging classification10 to define patients with CKD, and provincial health administrative data to measure their overall health care costs. We assessed costs over time by CKD category at baseline and also determined the proportion of costs attributable to kidney and cardiovascular care using a variety of primary diagnostic codes.
Data Sources
We used population-level data from the Alberta Kidney Disease Network (www.AKDN.info). The AKDN is a provincial network that captures laboratory measurements, including serum creatinine, and measures of urine albumin11 for all residents of Alberta, Canada, who undergo laboratory testing. Use of dialysis or kidney transplantation was assessed using data from the Northern and Southern Alberta Renal programs. Vital statistics data were obtained from Alberta Health, the provincial health ministry.
Data were linked to Alberta Health administrative data, which capture all provincial residents with public health insurance. All residents of Alberta are eligible for public health insurance, and greater than 99% of residents participate in the government-sponsored insurance plan. Alberta Health data capture all health care utilization paid for by the provincial health care plan, including the cost of all medically necessary physician visits, hospitalizations, and ambulatory care visits (including visits in emergency department [ED], noninterventional services, rehabilitation and community-based services, and day surgery), investigations, and procedures. Information on prescription drug use was taken from the Alberta Pharmaceutical Information Network, which has information on all prescription medications dispensed through pharmacies to Albertans, including drug, dose, and amount dispensed.
Cohort
We created a cohort of adults 18 years of age and older with a series of 2 or more eGFR measurement <60 mL/min/1.73 m2 lasting at least 90 days based on an outpatient serum creatinine measurement, or one abnormal measure of albuminuria (consistent with how the studies that informed the KDIGO classification10 considered measures of albuminuria) in those with eGFR >60 mL/min/1.73 m2 between April 1, 2012, and March 31, 2014. The date of each patient’s first eGFR measurement was defined as the “index date.” The CKD category was defined as of the index date. Participants were not analyzed in another CKD category if this changed over time; for example, if a participant transitioned to ESRD requiring dialysis, resource use would be assigned to CKD category at index date. We excluded those who were receiving dialysis or had a kidney transplant at the index date. The cohort was followed for 1 year or censored at death or out migration from the index date.
Baseline Laboratory-Derived Measures
The eGFR and albuminuria at index date were used to categorize patients using the KDIGO CKD criteria. We assessed for moderate and severe albuminuria over 2 years prior to the index eGFR measurement. Moderate albuminuria (KDIGO A2) was defined as random urine albumin-to-creatinine ratio (ACR) 3 to 30 mg/mmol, urine protein-to-creatinine ratio (PCR) 15 to 50 mg/mmol, or random urine dipstick protein result of 1+. Severe albuminuria (KDIGO A3) was defined as ACR >30 mg/mmol, PCR> 50 mg/mmol, or urinalysis dipstick protein result of greater than or equal to 2+. When multiple measurements were available, ACR was used in preference to PCR, which was used in preference to urine dipstick. If multiple albuminuria measurements were available, the measurement closest to the index eGFR was considered.
Other Variables
Age, sex, and First Nations status were determined from the Alberta Health registry file.12,13 Socioeconomic status was determined from the National Household Survey by linkage with residential postal code from the registry and was categorized based on adjusted median neighborhood household income as high-income (household income ≥Can$96 000), middle-income (household income = Can$46 000-Can$95 999), or low-income neighborhood (household income <Can$46 000). Comorbidities including myocardial infarction, stroke, congestive heart failure, hypertension, and diabetes were defined applying validated algorithms11 to administrative data for health care encounters during the 3 years prior to the index date. We also calculated the Charlson comorbidity index,14 a weighted score of 17 comorbid conditions that is associated with adverse outcomes.
Outcomes
The primary outcome was the 1-year cumulative unadjusted health care costs, including the cost of drugs, physician visits, ED visits, and hospitalizations for the year following each patient’s index date (irrespective of whether the patient lived the full 1-year period). Alberta Health uses the Canadian Institute for Health Information (CIHI) case mix grouper methods and ambulatory care costing methods to estimate costs of hospitalization, ED visits, day surgery, and outpatient procedures (including dialysis). Physician claims were based on the amount paid. Drug costs were estimated using the product and amount dispensed, combined with drug list price (from Alberta Blue Cross) and the dispensing fee. We adopted the perspective of the health care payer; therefore, nonmedical costs (ie, patient time and travel costs, as well as costs related to lost productivity) were not included. All costs are reported in 2017 Canadian (Can) dollars, updated to 2017 using health care consumer price index.15
Statistical Analysis
Mean 1-year direct medical costs were estimated for the cohort as a whole and for the KDIGO CKD categories as defined at baseline; attribution of cost was based on initial CKD status even for patients where this changed in follow-up (eg, initiated dialysis). As <1% of patients were lost to follow-up due to outmigration, imputation for missing costs was not required. Costs were further categorized according to baseline demographic and clinical characteristics, including history of comorbid diseases and laboratory measurements—with subgroups chosen based on clinical significance or because previous costing studies had suggested the covariate was associated with higher costs. Given the skewed nature of costing data, the median, as well as first and third quartile, was calculated for each estimate to assess the variability of costs.
We also estimated the 1-year mean cost of kidney care and cardiovascular care in CKD patients. The cost of kidney care was defined as a hospitalization for a kidney-related primary diagnosis (see the appendix for a list of International Classification of Diseases, Ninth Revision/Tenth Revision [ICD-9/10] codes) or ambulatory care visits where kidney care was determined by the location of care (eg, dialysis units or kidney care clinics) or kidney-related physician procedures (see the appendix for further details). The cost of cardiovascular care was defined as hospitalization and ambulatory care visits where cardiovascular disease was coded as the primary diagnosis (see the appendix for further details).
Ethics approval for the study was obtained from the conjoint health ethics review board at the University of Calgary. All analyses used STATA, version 11.2 (College Station, Texas).
Results
Baseline Characteristics
Overall, 219 641 adults with CKD between April 1, 2010, and March 31, 2012, were included in the cohort. Fifty-three percent were female, and 42.9% of patients were younger than the age of 65 years (Table 1). The most common comorbid condition was hypertension, ranging from 45.4% to 93.4% across CKD categories. All comorbidities increased in prevalence with more severe categories of CKD. Compared with patients with eGFR <60 mL/min/1.73 m2, patients with eGFR >60 mL/min/1.73 m2 and moderate or severe albuminuria were more likely to be First Nations, aged <65 years, and have a lower burden of comorbidity measured by a Charlson score of <3 (Table 1). Patients with eGFR <15 mL/min/1.73 m2 were more likely to require dialysis, receive a kidney transplant, or die over the 1-year follow-up (Table 1). Overall, after 1 year of follow-up, 10.5% of people with CKD died, 0.02% of patients received a transplant, and 0.3% started dialysis. Of those with eGFR<15 mL/min/1.73 m2 at baseline, 2.1% of patients received a transplant and 23.0% started dialysis over 1 year.
Table 1.
Baseline Characteristics.
| Overall |
eGFR >60 mL/min/1.73 m2 with moderate albuminuria |
eGFR >60 mL/min/1.73 m2 with severe albuminuria |
eGFR = 45-59.9 mL/min/1.73 m2 |
eGFR = 30-44.9 mL/min/1.73 m2 |
eGFR = 15-29.9 mL/min/1.73 m2 |
eGFR <15a mL/min/1.73 m2 |
|
|---|---|---|---|---|---|---|---|
| % (count) | % (count) | % (count) | % (count) | % (count) | % (count) | % (count) | |
| n | 219 641 | 82 577 | 17 140 | 73 755 | 34 359 | 10 255 | 1555 |
| Age, mean, SD | 65.1 (18.9) | 51.9 (17.6) | 52.1 (18.1) | 75.0 (10.9) | 78.3 (11.2) | 77.8 (13.3) | 71.7 (15.5) |
| Female | 53.4 (117 260) | 50.4 (41 600) | 46.3 (7942) | 55.9 (41 218) | 58.3 (20 017) | 55.7 (5711) | 49.7 (772) |
| Age <65 years | 42.9 (94 176) | 75.7 (62 509) | 75.0 (12 862) | 17.3 (12 750) | 11.5 (3968) | 15.5 (1586) | 32.2 (501) |
| First Nation | 2.5 (5502) | 3.8 (3111) | 5.3 (915) | 1.0 (759) | 1.4 (483) | 2.0 (203) | 3.8 (57) |
| Socioeconomic status | |||||||
| Low-income neighborhood | 26.7 (58 647) | 25.0 (20 606) | 26.2 (4495) | 26.8 (19 773) | 29.5 (10 136) | 30.7 (3148) | 31.4 (489) |
| High-income neighborhood | 15.1 (33 256) | 16.4 (13 525) | 15.1 (2588) | 15.1 (11 197) | 13.2 (4522) | 11.9 (1224) | 12.9 (200) |
| Comorbidities | |||||||
| History of MI | 6.5 (14 337) | 3.1 (2564) | 3.8 (654) | 7.9 (5806) | 11.0 (3780) | 13.1 (1340) | 12.4 (193) |
| History of stroke | 7.1 (15 514) | 3.0 (2511) | 4.2 (723) | 8.8 (6490) | 12.2 (4178) | 14.0 (1438) | 11.2 (174) |
| History of CHF | 16.7 (36 649) | 5.9 (4857) | 8.7 (1468) | 19.8 (14 631) | 31.2 (10 720) | 42.3 (4333) | 39.7 (618) |
| Hypertension | 67.6 (148 571) | 45.4 (37 516) | 51.9 (8895) | 81.4 (60 026) | 90.6 (31 136) | 93.4 (9577) | 91.4 (1421) |
| Diabetes | 32.7 (71 794) | 30.4 (25 119) | 35.0 (5996) | 30.2 (22 260) | 37.4 (12 844) | 46.4 (4753) | 52.9 (822) |
| PVD | 2.8 (6057) | 1.2 (998) | 2.0 (349) | 3.4 (2468) | 4.7 (1597) | 5.3 (548) | 6.2 (97) |
| Dementia | 4.9 (10 785) | 1.7 (1385) | 2.6 (447) | 6.3 (4640) | 9.0 (3102) | 10.5 (1076) | 8.7 (135) |
| Chronic pulmonary disease | 11.8 (25 935) | 8.6 (7118) | 10.3 (1757) | 13.0 (9595) | 15.6 (5351) | 18.1 (1855) | 16.7 (259) |
| Moderate or severe liver disease | 0.3 (539) | 0.1 (103) | 0.2 (36) | 0.3 (201) | 0.4 (143) | 0.5 (52) | 0.3 (4) |
| Metastatic solid tumor | 0.9 (1905) | 0.5 (443) | 0.8 (142) | 0.9 (694) | 1.3 (434) | 1.5 (156) | 2.3 (36) |
| Charlson score ≤1 | 81.9 (179 976) | 91.0 (75 200) | 86.4 (14 801) | 81.1 (59 838) | 70.0 (24 044) | 53.1 (5445) | 41.7 (648) |
| Charlson score 2-3 | 15.4 (33 786) | 8.1 (6712) | 12.2 (2087) | 16.5 (12 169) | 24.5 (8430) | 36.1 (3707) | 43.8 (681) |
| Charlson score >3 | 2.7 (5879) | 0.8 (665) | 1.5 (252) | 2.4 (1748) | 5.5 (1885) | 10.8 (1103) | 14.5 (226) |
| Albuminuria | |||||||
| Severe (KDIGO A3) | 32.6 (71596) | NA | 100.0 (17140) | 4.6 (3397) | 9.5 (3255) | 22.9 (2347) | 46.6 (724) |
| Moderate (KDIGO A2) | 45.6 (100 076) | 100.0 (82 577) | 12.2 (8961) | 17.4 (5965) | 22.6 (2312) | 16.8 (261) | |
| No significant albuminuria (KDIGO A1) | 32.6 (71 596) | NA | NA | 65.8 (48 516) | 55.4 (19 022) | 37.3 (3820) | 15.3 (238) |
| Unmeasured | 9.6 (21 106) | NA | NA | 17.5 (12 881) | 17.8 (6117) | 17.3 (1776) | 21.4 (332) |
| Death without dialysis and/or transplant | 10.4 (22 803) | 4.2 (3440) | 7.7 (1320) | 10.1 (7427) | 19.6 (6732) | 31.6 (3191) | 45.4 (529) |
| Death preceded by dialysis and/or transplant | 0.07 (164) | 0.003 (3) | 0.0 (0) | 0.01 (8) | 0.1 (24) | 0.4 (44) | 5.5 (85) |
| Dialysis only (no death or transplant) during the 1-year follow-up | 0.3 (598) | 0.003 (3) | 0.08 (13) | 0.02 (15) | 0.1 (50) | 1.6 (160) | 23.0 (357) |
| Transplant (irrespective of dialysis) during follow-up | 0.02 (37) | 0.001 (1) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.04 (4) | 2.1 (32) |
Note. eGFR = estimated glomerular filtration rate; MI = myocardial infarction; CHF = congestive heart failure; PVD = peripheral vascular disease; KDIGO = Kidney Disease: Improving Global Outcomes.
eGFR <15 mL/min/1.73 m2 excludes cohort on dialysis.
One-Year Costs
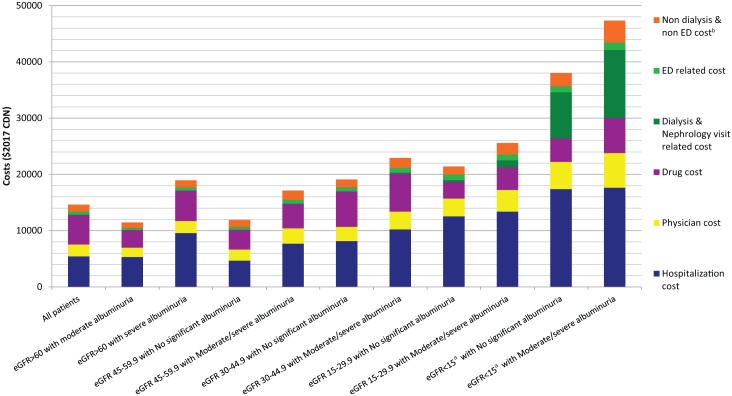
The mean unadjusted 1-year cost of caring for patients with CKD in Alberta was Can$14 634 per patient (interquartile range [IQR] = Can$1496-Can$10 221) (Table 2). Costs were higher for those with lower socioeconomic status, more comorbidity, lower eGFR, and with albuminuria. Hospitalization, drugs, physician, and ambulatory care accounted for 38%, 35%, 14%, and 13% of 1-year total costs, respectively (Figure 1). The mean 1-year unadjusted cost of kidney and cardiovascular care (both excluding drugs) was Can$230 (1.6% of total costs) and Can$720 (4.9% of total costs), respectively (Table 3). However, the cost of kidney and cardiovascular care over 1 year was substantially greater for patients with lower levels of kidney function, averaging Can$14 169 (32.3% of total costs), and Can$2395 (5.4% of total costs) for kidney and cardiovascular care, respectively, for people with eGFR <15 mL/min/1.73 m2 at baseline (Table 3). While kidney care costs per person are much higher at lower eGFR than cardiovascular care costs, the cost of cardiovascular care is about 10-fold higher than kidney care at eGFR above 45 mL/min/1.73 m2, of importance given that the majority of individuals with CKD have eGFR above 45 mL/min/1.73 m2.
Table 2.
Mean 1-Year Unadjusted Cost (2017 Can$) Per Patient With Chronic Kidney Disease, Overall and by Subgroup.
| All patients (Can$) mean (median [Q1, Q3]) | eGFR> 60 mL/min/1.73 m2 with moderate albuminuria (Can$) mean (median [Q1, Q3]) | eGFR> 60 mL/min/1.73 m2 with severe albuminuria (Can$) mean (median [Q1, Q3]) | eGFR = 45-59.9 mL/min/1.73 m2 (Can$) mean (median [Q1, Q3]) | eGFR = 30-44.9 mL/min/1.73 m2
(Can$) mean (median [Q1, Q3]) |
eGFR = 15-29.9 mL/min/1.73 m2
(Can$) mean (median [Q1, Q3]) |
eGFR<15a mL/min/1.73 m2
(Can$) mean (median [Q1, Q3]) |
|
|---|---|---|---|---|---|---|---|
| n | 219 641 | 82 577 | 17 140 | 73 755 | 34 359 | 10 255 | 1555 |
| Overall | 14 634 (3672 [1496, 10 221]) |
11 473 (2408 [919, 6733]) |
18 963 (3529 [1298, 10 252]) |
12 787 (3963 [1814, 10 455]) |
20 125 (5577 [2494, 16 456]) |
23 303 (8941 [3749, 25 662]) |
43 915 (22 098 [8089, 61 503]) |
| Female | 13 237 (3813 [1549, 10 328]) |
9648 (976 [976, 7397]) |
15 790 (3741 [1279, 10 583]) |
11 418 (3914 [1795, 10 080]) |
19 801 (5464 [2435, 15 665]) |
22 199 (8490 [3548, 24 751]) |
40 528 (19 397 [7354, 55 036]) |
| Age <65 years | 12 028 (2594 [923, 7214]) |
9388 (1993 [741, 5686]) |
15 125 (2961 [1069, 8570]) |
11 851 (3547 [696, 9310]) |
30 948 (6029 [2500, 17 295]) |
30 581 (9856 [4142, 38 394]) |
57 781 (38 785 [12 666, 85 373]) |
| Socioeconomic status | |||||||
| Low-income neighborhood | 16 767 (4084 [1674, 11 574]) |
13 465 (2763 [1037, 7792]) |
13 087 (3895 [1418, 11 859]) |
15 432 (4224 [1,940, 11 344]) |
24 550 (5893 [2596, 17 545]) |
22 449 (3662 [3,662, 25 352]) |
45 865 (21 893 [8330, 60 397]) |
| High-income neighborhood | 13 610 (3195 [1290, 8696]) |
11 816 (2100 [786, 5773]) |
10 788 (3076 [1176, 8679]) |
11 291 (3624 [1660, 9261]) |
22 713 (5234 [2391, 15 366]) |
22 801 (8209 [3663, 24 685]) |
39 202 (22 462 [7723, 60 785]) |
| Comorbidities | |||||||
| History of MI | 21 621 (6758 [3008, 19 317]) |
26 987 (4887 [2369, 13 287]) |
36 116 (7274 [3000, 22 373]) |
16 507 (5924 [2812, 16 357]) |
20 463 (8118 [3468, 22 590]) |
26 150 (5126 [5126, 32 214]) |
46 255 (26 525 [10 959, 67 322]) |
| History of stroke | 18 564 (6940 [3087, 19 013]) |
16 355 (5769 [2582, 14 572]) |
21 092 (7546 [3155, 20 007]) |
16 548 (6320 [2909, 16 614]) |
19 680 (7614 [3302, 20 961]) |
24 849 (10 448 [4386, 29 307]) |
36 429 (20 237 [6954, 54 028]) |
| History of CHF | 24 501 (8341 [3633, 23 108]) |
24 517 (6335 [2896, 16 805]) |
32 361 (8263 [3608, 23 615]) |
19 048 (7297 [3351, 19 584]) |
26 445 (9335 [3966, 25 746]) |
28 540 (13 030 [5079, 34 132]) |
48 387 (24 265 [8586, 69 660]) |
| Hypertension | 16 106 (4528 [2055, 12 616]) |
13 812 (3364 [1579, 8423]) |
19 941 (4570 [2034, 12 532]) |
13 378 (4271 [2008, 11 252]) |
19 683 (5676 [2562, 16 601]) |
22 952 (8990 [3783, 25 688]) |
43 401 (21 769 [8049, 60 616]) |
| Diabetes | 17 708 (5041 [2397, 13 317]) |
14 351 (3661 [1821, 8509]) |
16 593 (5181 [2413, 12 755]) |
14 663 (5117 [2531, 13 034]) |
24 908 (6766 [3182, 19 079]) |
26 163 (10 561 [4722, 29 078]) |
49 520 (26 301 [9564, 70 752]) |
| PVD | 25 066 (9978 [4093, 28 590]) |
23 767 (9582 [3433, 25 964]) |
32 622 (14 547 [5504, 33 480]) |
21 983 (8374 [3724, 24 005]) |
24 785 (10 561 [4466, 30 383]) |
32 604 (45 453 [5580, 38 768]) |
51 743 (21 023 [7245, 66 826]) |
| Dementia | 22 842 (7950 [3803, 21 799]) |
37 635 (7533 [3534, 22 562]) |
54 867 (7682 [3245, 23 471]) |
17 603 (7318 [3651, 18 845]) |
19 455 (8297 [4136, 22 907]) |
22 659 (10 671 [4409, 29 154]) |
24 405 (10 285 [2535, 33 630]) |
| Chronic pulmonary disease | 26 705 (7541 [3256, 20 677]) |
32 587 (5168 [2070, 13 362]) |
18 650 (6858 [2849, 18 042]) |
19 395 (7302 [3437, 19 129]) |
31 675 (10 610 [4517, 27 977]) |
32 283 (16 264 [6012, 38 930]) |
47 145 (27 763 [8796, 63 269]) |
| Moderate or severe liver disease | 47 794 (20 701 [6123, 51 973]) |
45 678 (11 243 [4033, 39 502]) |
61 370 (29 134 [7242, 56 713]) |
41 794 (16 819 [6468, 44 003]) |
47 344 (25 804 [7099, 59 470]) |
65 061 (40 902 [12 948, 98 130]) |
73 250 (74 607 [20 126, 126 373]) |
| Metastatic solid tumor | 85 184 (16 623 [5709, 39 391]) |
152 799 (16 171 [5513, 41 279]) |
36 561 (18 170 [6921, 45 981]) |
45 944 (13 564 [5067, 32 198]) |
118 269 (20 568 [7005, 43 259]) |
30 440 (19 652 [7210, 44 116]) |
39 750 (26 591 [9644, 54 310]) |
| Charlson score ≤1 | 11 148 (2957 [1257, [7756]) |
8788 (2148 [832, 5853]) |
16 258 (2979 [1123, 8364]) |
10 005 (3305 [1571, 8098]) |
15 933 (4321 [2043, 11 777]) |
17 967 (6025 [2779, 17 829]) |
39 098 (17 100 [6523, 53 353]) |
| Charlson score 2-3 | 27 504 (8611 [4040, 23 028]) |
35 078 (6888 [3288, 17 872]) |
35 272 (9068 [4070, 24 613]) |
23 259 (7992 [3944, 21 019]) |
24 597 (9412 [4273, 24 865]) |
26 702 (11 776 [5304, 30 590]) |
43 866 (24 525 [8985, 65 638]) |
| Charlson score >3 | 47 385 (17 512 [7174, 42 916]) |
76 824 (16 153 [6310, 38 068]) |
39 015 (17 007 [7640, 44 122]) |
35 120 (15 223 [6785, 35 122]) |
53 600 (17 197 [7265, 44 537]) |
38 217 (20 984 [8349, 50 071]) |
57874 (32 916 [12 023, 79 771]) |
| Albuminuria | |||||||
| Moderate (KDIGO A2) | 12 859 (2882 [1099, 8093]) |
11 473 (2408 [919, 6733]) |
NA | 15 594 (5202 [2434, 14 126]) |
23 019 (6446 [1505, 19 110]) |
23 045 (9589 [3943, 25 500]) |
35 209 (17 641 [6503, 48 736]) |
| Severe (KDIGO A3) | 21 387 (4969 [1861, 14 718]) |
NA | 18 963 (3529 [1298, 10 252]) |
21 213 (6265 [2769, 17 635]) |
22 769 (7438 [3274, 22 557]) |
28 066 (10 929 [4711, 32 384]) |
51 707 (30 891 [10 972, 76 780]) |
| No significant (KDIGO A1) | 13 305 (4225 [1944, 11 284]) |
NA | NA | 11 504 (3711 [1731, 9316]) |
16 243 (7624 [2374, 14 600]) |
20 564 (7624 [3409, 22 610]) |
29 199 (13, 916 [5696, 35 768]) |
| Unmeasured | 18 964 (4496 [1867, 14 160]) |
NA | NA | 13 445 (3695 [1603, 10 791]) |
23 235 (8283 [3279, 25 018]) |
27 968 (5386 [2265, 16 946]) |
44 317 (20 173 [7035, 61 014]) |
Note. eGFR = estimated glomerular filtration rate; MI = myocardial infarction; CHF = congestive heart failure; PVD = peripheral vascular disease; KDIGO = Kidney Disease: Improving Global Outcomes.
eGFR< 15 mL/min/1.73 m2 excludes cohort on dialysis.
Figure 1.
Mean 1-year costs of care for patients across KDIGO chronic kidney disease categories, by category of cost.
Note. KDIGO = Kidney Disease: Improving Global Outcomes; eGFR = estimated glomerular filtration rate; ED = emergency department.
aeGFR<15 mL/min/1.73 m2 excludes those on dialysis at baseline.
bNondialysis and non-ED costs represent ambulatory care costc other than ED cost and dialysis and nephrologist visit–related costs.
cIncluding visits in ED, noninterventional services (ie, clinical assessments, diagnostic services such as imaging and treatment, and education), rehabilitation services and community-based services, as well as procedures such as day surgery.
Table 3.
Mean 1-Year Costs (2017 Can$) of Kidney and Cardiovascular Care for Patients With CKD.
| All patients | eGFR> 60 mL/min/1.73 m2 with moderate albuminuria | eGFR> 60 mL/min/1.73 m2 with severe albuminuria | eGFR = 45-59.9 mL/min/1.73 m2 | eGFR = 30-44.9 mL/min/1.73 m2 | eGFR = 15-29.9 mL/min/1.73 m2 | eGFR< 15a mL/min/1.73 m2 | |
|---|---|---|---|---|---|---|---|
| n | 219 641 | 82 577 | 17 140 | 73 755 | 34 359 | 10 255 | 1555 |
| Cost of cardiovascular care | |||||||
| Hospitalization cost, Can$ mean | 570 | 206 | 434 | 519 | 1140 | 1825 | 2007 |
| Physician cost, Can$ mean | 70 | 31 | 56 | 73 | 126 | 190 | 186 |
| Ambulatory care cost,b Can$ mean | 78 | 32 | 61 | 84 | 146 | 141 | 201 |
| Total cost of cardiovascular care, Can$ mean | 720 | 270 | 552 | 646 | 1413 | 2157 | 2395 |
| Cost of kidney care | |||||||
| Hospitalization cost, Can$ mean | 100 | 13 | 32 | 46 | 174 | 665 | 2571 |
| Physician cost, Can$ mean | 13 | 1 | 5 | 2 | 7 | 58 | 1119 |
| Ambulatory care cost,b Can$ mean | 118 | 4 | 27 | 11 | 54 | 599 | 10 478 |
| Total cost of kidney care, Can$ mean | 230 | 18 | 64 | 59 | 234 | 1322 | 14 169 |
Note. CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate.
eGFR<15 mL/min/1.73 m2 excludes those on dialysis at baseline.
Including visits in emergency department, noninterventional services (ie, clinical assessments, diagnostic services such as imaging and treatment, and education), rehabilitation services and community-based services, as well as procedures such as day surgery.
As expected, receiving dialysis during follow-up was a significant driver of costs. For instance, the cost of caring for people with eGFR <15 mL/min/1.73 m2 at baseline was Can$91 112 per patient (IQR = Can$45 941-Can$116 186) and Can$28 550 per patient (IQR = Can$6102-Can$33 175) for those who did and did not receive dialysis during the 1-year follow-up period, respectively (P < .001).
Population-Based Estimates of the Cost of Caring for People With CKD
Extrapolating our findings to all adults in Canada16 using CKD prevalence estimates from a recent Canadian population-based survey,1 we estimate that the annual cost of caring for Canadians with CKD (not on dialysis at baseline) approximates Can$32 billion. Of these costing estimates, 4.8% and 12.4% of these costs are attributable to kidney care (including dialysis) and cardiovascular disease, respectively. Given the size of the population with earlier categories of kidney disease, 63%, 27%, and 7% of costs relate to the care of individuals with eGFR> 60 mL/min/1.73 m2 and moderate or severe albuminuria, those with eGFR = 45-59 mL/min/1.73 m2, and those with eGFR = 30-44 mL/min/1.73 m2, respectively.
Discussion
We observed a strong graded association of the 1-year unadjusted mean cost of care for patients by CKD category, varying from Can$14 634 (for patients with eGFR> 60 mL/min/1.73 m2 and moderate albuminuria) to Can$51 707 (for patients with eGFR< 15 mL/min/1.73 m2 and severe albuminuria). Not surprisingly, we noticed that the majority of costs for patients with more advanced CKD related to costs for kidney care (including dialysis which was required by just over one-third of those with eGFR< 15 mL/min/1.73 m2 during the 1-year follow-up period), while cardiovascular costs were smaller but relatively consistent across CKD categories. Extrapolating our findings to Canada, we estimate that the annual cost of caring for Canadians with CKD (not on dialysis at baseline) approximates Can$32 billion per year—including costs attributable to their CKD and costs attributable to their other medical conditions.
Most previous costing studies in CKD have focused on patients with ESRD treated with dialysis or transplantation. In a recent review that estimated costs in 2013 Can$, the total annual health care cost of treating a patient with ESRD using in-center hemodialysis (HD) (at hospitals and satellite centers), home HD, and peritoneal dialysis (PD) was approximately Can$95 000 to Can$107 000, Can$71 000 to Can$90 000, and Can$56 000, respectively.17 However, only 1% of CKD patients have ESRD,1,6,7 and few studies have examined the costs of earlier categories of CKD. Small studies of patients with advanced CKD (GFR< 30 mL/min/1.73 m2) in Sweden, the United Kingdom, and the United States have noted that the cost for adults with advanced nondialysis CKD (while lower than for individuals on dialysis) is 2- to 4-fold higher than age and sex-matched controls without CKD.18-23 In addition, it has been shown that patients with diabetes, who have more severe CKD, and those who progress from less advanced stages of diabetic nephropathy to more advanced stages of diabetic nephropathy incur higher health care costs.8,24 Finally, Honeycutt et al25 linked data from a cohort of 1609 patients with CKD within the National Health and Nutrition Examination Survey (1988-1994) to US Medicare costing data to assess the attributable cost that was related to having CKD, noting that the attributable cost of having CKD (in comparison to similar patients without CKD) was Can$1500, Can$3000, and Can$12 300 each year for patients with KDIGO Category 2, 3 and 4 CKD, respectively.
Our analysis has strengths and limitations. We used laboratory data collected during routine clinical care across an entire province of more than 4 million people, linking this to an administrative data system with full patient capture, including health care costs, and we were able to stratify our costing estimates by measures of eGFR and albuminuria. Despite this comprehensive data system, we only have estimates of the cost of health care for people with CKD, and not the costs borne by patients or their families. Recent studies have shown that the societal costs of CKD are also high, in part because patients with kidney failure are often unable to work, leading to productivity losses and estimated costs to disability insurance and the Canadian Pension Plan of well over Can$200 million per year in Canada.26 We have not included costs for people without CKD in this analysis given the additional complexities of adjusting costs for differences in other patient characteristics, and as such, we are unable to assess the incremental costs associated with CKD. However, we do know that current health care spending per person is Can$6299 per year in 2015 (including people with and without chronic health conditions like CKD),27 less than half the mean costs of our overall CKD cohort. The higher mean costs of patients with CKD compared with the overall population are consistent with prior studies that have had a non-CKD comparator group.18-23 Future work will assess the population-based costs for those with and without CKD in Alberta.
In summary, the cost of care for people with nondialysis CKD is high, particularly for those with albuminuria and those with lower eGFR where a graded association between costs and severity was observed. Our estimates can be used by health care planners and kidney care programs to estimate the cost of CKD care across the spectrum of CKD as well as predict future costs that will be required based on expected increases in the prevalence of CKD. Moreover, health care planners and kidney care programs can use our results to estimate how much money could be saved through interventions that slow or prevent kidney disease.
Appendix
Appendix.
Definition of Kidney and Cardiovascular Care.
| Category | Diagnostic codes defining cardiovascular-related care19-30 | Codes defining kidney-related care | ||||
|---|---|---|---|---|---|---|
| Ambulatory care claims | Codes description | ICD-9-CM | ICD-10 | Facility description | Location codes a | |
| Myocardial infarction | 410 | I21.xI22.x | HD specialty day/night care | 713408510 | ||
| Heart failure | 428 | I09.9 I11.0 I13.0 I13.2 I25.5 I42.0 I42.5 I42.6 I42.7 I42.8 I42.9 I43 I50.x P29.0 |
Home dialysis (teaching) | 713408520 | ||
| HHD (teaching) | 713408530 | |||||
| Home PD (teaching) | 713408540 | |||||
| Nephrology specialty clinic | 713501095 | |||||
| PD specialty day/night | 713408550 | |||||
| Renal dialysis specialty day/night care | 713408500 | |||||
| Cerebrovascular | 362.3 430 431 433 434 435 436 |
H34.1 I60.x I61.x I63.x I64.x G45.x |
Self-care HD specialty day/night | 713408560 | ||
| Hospitalization | Codes description | ICD-9 | ICD-10 | Codes description | ICD-9 | ICD-10 |
| Myocardial infarction | 410 | I21.x I22.x |
Type 1 diabetes mellitus with kidney complications | E102.1 E102.2 E102.9 |
||
| Heart failure | 428 | I09.9 I11.0 I13.0 I13.2 I25.5 I42.0 I42.5 I42.6 I42.7 I42.8 I42.9 I43 I50.x P29.0 |
Acute kidney failure | N17.0 N17.1 N17.2 N17.8 N17.9 |
||
| Chronic kidney disease | N18.0 N18.1 N18.2 N18.3 N18.5 N18.6 N18.9 |
|||||
| Cerebrovascular | 362.3 430 431 433 434 435 436 |
H34.1 I60.x I61.x I63.x I64.x G45.x |
Unspecified kidney failure | N19 | ||
| Postprocedural (acute) (chronic) kidney failure | N99.0 | |||||
| Extrarenal uremia | R39.2 | |||||
| Encounter for care involving renal dialysis | Z49.0 Z49.01 Z49.02 Z49.3 Z49.31 Z49.32 |
|||||
| Physician cost | Codes description | ICD-9 | ICD-10 | Claims description | Claims procedure code | |
| Myocardial infarction | 410 | I21.x I22.x |
PD catheter removal | 11.81A | ||
| Acute HD | 13.99A | |||||
| Heart failure | 428 | I09.9 I11.0 I13.0 I13.2 I25.5 I42.0 I42.5 I42.6 I42.7 I42.8 I42.9 I43 I50.x P29.0 |
Chronic HD | 13.99B | ||
| Assessment and management of an unstable patient with acute/chronic renal failure treated by PD | 13.99C | |||||
| Assessment and management of a stable patient with chronic renal failure treated by PD | 13.99D | |||||
| HD in ICD | 13.99AB | |||||
| Cerebrovascular | 362.3 430 431 433 434 435 436 |
H34.1 I60.x I61.x I63.x I64.x G45.x |
Weekly satellite/PD | 13.99OA | ||
| HHD | 13.99O | |||||
| Dialysis line insertion | 50.93A | |||||
| AVF creation | 51.27 | |||||
| PD catheter insertion | 66.98 | |||||
| AVF declot | 51.49A | |||||
| Fistulogram | 46.88 | |||||
Note. ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10 = International Classification of Diseases, Tenth Revision; PD = peritoneal dialysis; HD = hemodialysis; HHD = home hemodialysis; AVF = arteriovenous fistula.
Location codes (rather than ICD-9/10 codes) were used to define kidney-related care; results using kidney-relevant ICD-9/10 codes revealed similar costing estimates.
Footnotes
Ethics Approval and Consent to Participate: Ethics approval for the study was obtained from the conjoint health ethics review board at the University of Calgary. As this study uses secondary data, individual patient consent was not required.
Consent for Publication: All authors reviewed the final manuscript and provided consent for publication.
Availability of Data and Materials: Study data are held by the Interdisciplinary Chronic Disease Collaboration (ICDC), a research team at the University of Calgary, under contract with Alberta Health. The contract does not permit sharing of data with researchers outside the ICDC.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This study is based in part on data provided by Alberta health and Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta or Alberta Health Services. Neither the Government of Alberta nor Alberta Health or Alberta Health Services express any opinion in relation to this study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded through an AI-HS Collaborative Research & Innovation Opportunity Team Grant, by a Canadian Institutes of Health Research (CIHR) Strategy for Patient-Oriented Research Network grant, and by a CIHR Foundation Award (Manns). Dr Manns is supported by the Svare Chair in Health Economics. Dr Tonelli is supported by the David Freeze Chair in Health Services Research, and Dr Hemmelgarn is supported by the Roy and Vi Baay Chair in Kidney Research. Dr Klarenbach is supported by the Kidney Health Research Chair and the Division of Nephrology at the University of Alberta.
ORCID iD: Braden Manns  https://orcid.org/0000-0002-8823-6127
https://orcid.org/0000-0002-8823-6127
References
- 1. Arora P, Vasa P, Brenner D, Iglar K, McFarlane P, Morrison H, Badawi A. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ. 2013;185(9):E417-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manns B, Tonelli M, Culleton B, et al. ; and Alberta Kidney Disease Network. A cluster randomized trial of an enhanced eGFR prompt in chronic kidney disease. Clin J Am Soc Nephrol. 2012;7:565-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee H, Manns B, Taub K, et al. Cost analysis of ongoing care of patients with end-stage renal disease: the impact of dialysis modality and dialysis access. Am J Kidney Dis. 2002;40(3):611-622. [DOI] [PubMed] [Google Scholar]
- 4. Beaudry A, Ferguson TW, Rigatto C, Tangri N, Dumanski S, Komenda P. Cost of dialysis therapy by modality in Manitoba. Clin J Am Soc Nephrol. 2018;13(8):1197-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dialysis modalities for the treatment of end-stage kidney disease: a review. In: CADTH Report/Project in Briefs. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health, 2011. [PubMed] [Google Scholar]
- 6. Canadian Institute for Health Information (CIHI). Treatment of End-Stage Organ Failure in Canada, Canadian Organ Replacement Register, 2005 to 2014. Ottawa, ON: Canadian Institute for Health Information, 2015. [Google Scholar]
- 7. Interdisciplinary Chronic Disease Collaboration, Alberta Kidney Disease Network. Quality of care in early stage chronic kidney disease 2012-2013.Alberta, Canada: Alberta Health Services, 2015. [Google Scholar]
- 8. Vupputuri S, Kimes TM, Calloway MO, et al. The economic burden of progressive chronic kidney disease among patients with type 2 diabetes. J Diabetes Complications. 2014;28(1):10-16. [DOI] [PubMed] [Google Scholar]
- 9. James MT, Quan H, Tonelli M, et al. ; and Alberta Kidney Disease Network. CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis. 2009;54:24-32. [DOI] [PubMed] [Google Scholar]
- 10. Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1-50. [Google Scholar]
- 11. Hemmelgarn BR, Clement F, Manns BJ, et al. Overview of the Alberta kidney disease network. BMC Nephrol. 2009;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson D, Jin Y, Truman C. Influence of aboriginal and socioeconomic status on birth outcome and maternal morbidity. J Obstet Gynaecol Can. 2002;24(8):633-640. [DOI] [PubMed] [Google Scholar]
- 13. Pohar SL, Johnson JA. Health care utilization and costs in Saskatchewan’s registered Indian population with diabetes. BMC Health Serv Res. 2007;7:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 15. Statistics Canada. The Canadian consumer price index reference paper. Ottawa, ON: The Minister responsible for Statistics Canada, 2015. [Google Scholar]
- 16. Statistics Canada. 2016. population by sex and age group table, 2016. http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo10a-eng.htm. Accessed February 23, 2019.
- 17. Klarenbach SW, Tonelli M, Chui B, Manns BJ. Economic evaluation of dialysis therapies. Nat Rev Nephrol. 2014;10:644-652. [DOI] [PubMed] [Google Scholar]
- 18. Eriksson JK, Neovius M, Jacobson SH, Elinder CG, Hylander B. Healthcare costs in chronic kidney disease and renal replacement therapy: a population-based cohort study in Sweden. BMJ Open. 2016;6(10):e012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kent S, Schlackow I, Lozano-Kuhne J, et al. What is the impact of chronic kidney disease stage and cardiovascular disease on the annual cost of hospital care in moderate-to-severe kidney disease. BMC Nephrol. 2015;16:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vekeman F, Yameogo ND, Lefebvre P, Bailey RA, McKenzie RS, Piech CT. Healthcare costs associated with nephrology care in pre-dialysis chronic kidney disease patients. J Med Econ. 2010;13(4):673-680. [DOI] [PubMed] [Google Scholar]
- 21. Wyld ML, Lee CM, Zhuo X, et al. Cost to government and society of chronic kidney disease stage 1-5: a national cohort study. Intern Med J. 2015;45(7):741-747. [DOI] [PubMed] [Google Scholar]
- 22. Baumeister SE, Boger CA, Kramer BK, et al. Effect of chronic kidney disease and comorbid conditions on health care costs: a 10-year observational study in a general population. Am J Nephrol. 2010;31(3):222-229. [DOI] [PubMed] [Google Scholar]
- 23. Smith DH, Gullion CM, Nichols G, Keith DS, Brown JB. Cost of medical care for chronic kidney disease and comorbidity among enrollees in a large HMO population. J Am Soc Nephrol. 2004;15(5):1300-1306. [DOI] [PubMed] [Google Scholar]
- 24. Nichols GA, Vupputuri S, Lau H. Medical care costs associated with progression of diabetic nephropathy. Diabetes Care. 2011;34(11):2374-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D. Medical costs of CKD in the Medicare population. J Am Soc Nephrol. 2013;24(9):1478-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manns B, McKenzie SQ, Au F, Gignac PM, Geller LI; Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (Can-SOLVE CKD) Network. the financial impact of advanced kidney disease on Canada pension plan and private disability insurance costs. Can J Kidney Health Dis. 2017;4:2054358117703986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Canadian Institute for Health Information (CIHI). National health expenditure trends, 1975 to 2016. Ottawa, ON: Canadian Institute for Health Information, 2016. [Google Scholar]
- 28. Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144(2):290-296. [DOI] [PubMed] [Google Scholar]
- 29. Lee DS, Donovan L, Austin PC, et al. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43(2):182-188. [DOI] [PubMed] [Google Scholar]
- 30. Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36(8):1776-1781. [DOI] [PubMed] [Google Scholar]