This open-label Phase II trial supports the efficacy and safety of sunitinib in Japanese patients with panNETs. Dose reductions and interruptions are critical to maximize the efficacy of sunitinib.
Keywords: efficacy, Japan, pancreatic neuroendocrine tumors, safety, sunitinib
Abstract
Background
In an interim analysis of a Phase II trial in Japanese patients with pancreatic neuroendocrine tumors (panNETs), sunitinib demonstrated antitumor activity with an objective response rate (ORR) of 50% (95% confidence interval [CI], 21–79) and a median progression-free survival (PFS) of 16.8 months (95% CI, 9.3–26.2). Here, we report the final analyses of efficacy and safety, as well as additional analyses, from this Phase II study.
Methods
This was a multicenter, open-label, Phase II trial (NCT01121562) of sunitinib in Japanese patients with panNETs. Patients received oral sunitinib 37.5 mg/day on a continuous daily dosing schedule. Dose modifications were permitted. The primary endpoint was clinical benefit rate (CBR). Secondary endpoints included ORR, PFS, overall survival (OS), safety and pharmacokinetics.
Results
Of 12 patients enrolled and treated, all discontinued treatment—the majority (n = 8) owing to disease progression. Most patients were male (n = 8), <65 years of age (n = 11) and had a non-functional tumor (n = 10). The median (range) number of days on drug was 323.5 (22–727). The CBR (95% CI) was 75.0% (42.8–94.5). ORR (95% CI) was 50.0% (21.1–78.9). Median (95% CI) PFS was 16.8 (9.3–26.2) months; however, median (95% CI) OS was not reached (22.0–not estimable). Most common adverse events (AEs; all-causality) were diarrhea (n = 10; 83.3%), hand-foot syndrome (n = 8; 66.7%) and hypertension (n = 8; 66.7%).
Conclusions
These results support the efficacy and safety of sunitinib in Japanese patients with panNETs. Appropriate AE management through dose reduction and interruption may prolong sunitinib treatment and maximize its efficacy.
Introduction
Pancreatic neuroendocrine tumors (panNETs) are rare and slowly progressing tumors (1,2). The incidence of new-onset panNETs in Japan increased from 1.01 per 100 000 people in 2005 to 1.27 per 100 000 people in 2010 (3). Additionally, a 1.2-fold increase in the number of patients with panNETs was observed between 2005 and 2010: 3379 patients received treatment for panNETs in 2010, a prevalence of 2.69 per 100 000 people, compared with 2845 patients in 2005, a prevalence of 2.23 per 100 000 people (3).
Many patients with panNETs present with unresectable and/or advanced/metastatic disease (4). In Japan, there is a need for additional treatment options for unresectable and/or advanced/metastatic panNETs. Somatostatin analogs (SSAs) such as octreotide, alone or in combination with interferon-alpha, as well as cytotoxic chemotherapy, e.g. streptozocin ± doxorubicin, have been used; however, they have been associated with adverse events (AEs) and limited efficacy (5–8). More recent treatment options available for Japanese patients with panNETs include the long-acting SSA lanreotide, and the mammalian target of rapamycin inhibitor(mTOR) everolimus (9,10). Given the availability of several new and approved treatment options, it will be important to evaluate the efficacy and safety profiles of each agent, as well as the optimal treatment management, in order to select those patients who will benefit the most from the various treatment options available to Japanese patients with panNETs.
The vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) pathways play a critical role in angiogenesis and dysregulation of these pathways has been implicated in panNET growth (11,12). Sunitinib is a multi-targeted tyrosine kinase inhibitor (TKI) of VEGF and PDGF (13). In a Phase III trial in patients with progressive, advanced/metastatic, well-differentiated, unresectable panNETs, sunitinib 37.5 mg once daily (QD) significantly prolonged median progression-free survival (PFS) compared with placebo: 11.4 months versus 5.5 months (hazard ratio 0.42, 95% confidence interval [CI], 0.26–0.66; P < 0.001) (14). Recently, sunitinib has also shown clinical efficacy and tolerability in patients with heavily pre-treated, progressive panNETs and patients with grade 3 gastroenteropancreatic neuroendocrine neoplasms (15,16). Additionally, sunitinib demonstrated antitumor activity in an interim analysis of a Phase II trial in Japanese patients with panNETs (N = 12)—objective response rate (ORR): 50% (95% CI, 21–79); median PFS: 16.8 months (95% CI, 9.3–26.2) (17,18). Sunitinib is a standard therapy for patients with progressive, advanced/metastatic, well-differentiated, unresectable panNETs based on evidence from the worldwide Phase III study (14) and further supported by additional data in Japan (17).
In order to characterize the efficacy and safety of sunitinib in Japanese patients with panNETs, we report the final analyses of efficacy and safety, as well as additional analyses, from the Phase II study. The study evaluated the clinical benefit rate (CBR, also known as disease control rate) of continuous sunitinib 37.5 mg/day, as well as ORR, PFS, pharmacokinetics, safety and tolerability, in this patient population.
Methods
Study design
This was a multicenter, open-label, Phase II trial (ClinicalTrials.gov NCT01121562; Pfizer study number A6181193) of sunitinib in Japanese patients with panNETs (17). The study was conducted at four centers in Japan between 28 July 2010 and 5 November 2013. The cut-off date for these final analyses is 27 December 2013. The trial, protocol, amendments and informed consent forms were approved by the institutional review board or ethics committee at every center and complied with Good Clinical Practice guidelines, the Declaration of Helsinki and applicable local laws. All patients provided written informed consent.
Patients
Eligibility criteria have been reported previously (17). Briefly, Japanese patients were aged ≥20 years and had histologically or cytologically confirmed well-differentiated panNETs (according to World Health Organization 2004 classification), as well as unresectable advanced or metastatic disease with documented radiologic progression per Response Evaluation Criteria in Solid Tumors v1.0 (RECIST), ≤12 months prior to study enrollment. Additional inclusion criteria were: ≥1 measurable target lesion; Eastern Cooperative Oncology Group performance status (ECOG PS) 0 or 1; and adequate hepatic, hematologic and renal function.
Patients were excluded if they had any of the following: brain metastases; prior treatment with any TKIs or anti-VEGF angiogenic inhibitors; uncontrolled hypertension (despite therapy); myocardial infarction, severe/unstable angina, congestive heart failure or pulmonary embolism in the previous 12 months.
Treatments and assessments
Treatments and assessments for the Phase II trial have been reported previously (17). Patients received oral sunitinib 37.5 mg/day on a continuous daily dosing (CDD) schedule, and each treatment cycle lasted 4 weeks. Dose could be temporarily interrupted or reduced to 25 mg/day to manage toxicity. The sunitinib dose could also be increased to 50 mg/day (if no response was observed in the first 8 weeks and if individual tolerability permitted). Patients were treated until within 3 months of achieving median PFS or 2 years after the last patient enrolled started treatment, whichever was longer.
SSAs were permitted for symptomatic control. No other approved or investigational anticancer treatment was permitted during the study, including chemotherapy, chemoembolization therapy or immunotherapy. Prior treatment with non-VEGF-targeted angiogenic inhibitors was permitted.
Investigator-assessed tumor imaging by computed tomography (CT), spiral CT or magnetic resonance imaging was performed at screening and Weeks 5 and 9, and then at 8-week intervals during the study. Tumor responses were evaluated per RECIST criteria. Imaging studies at screening included, at a minimum, a CT scan of the chest, abdomen and pelvis. Brain CT and bone scan were performed at screening and repeated if metastases were present or suspected. Additional scans were performed when disease progression was suspected or to confirm a complete response (CR) or partial response (PR) based on RECIST.
AEs were monitored throughout the study. Severity was graded by the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0. Other safety assessments included physical examination, vital signs, 12-lead electrocardiogram and laboratory evaluations.
Blood samples were collected predose on Day 15 (±1) of cycle 1 and on Day 1 of cycles 2–4 to evaluate trough concentrations (Ctrough) of sunitinib and its active metabolite using a validated high-performance liquid chromatography−tandem mass spectrometry method (Bioanalytical Systems Inc., West Lafayette, Indiana, USA).
Statistical analyses
A sample size of at least 10 patients was determined based on feasibility of study conduct rather than statistical requirements. The full analysis set (FAS) included all enrolled patients who received at least one dose of study treatment. Efficacy and safety analyses were based on the FAS.
The primary endpoint was CBR—the percentage of patients with a confirmed CR, confirmed PR or stable disease for ≥24 weeks according to RECIST criteria. Secondary endpoints included: ORR; tumor shrinkage (the percentage change from baseline in the sum of the longest diameter of target lesions); PFS; time-to-tumor-progression (TTP); overall survival (OS); safety and pharmacokinetics. OS data are updated with an additional 21 months of follow-up.
For binary data (CBR and ORR), point estimates of the rates and two-sided exact 95% CIs were calculated. Time-to-event endpoints (PFS, TTP and OS) were summarized using Kaplan–Meier methods. Descriptive statistics were used to summarize other parameters. Statistical analyses were performed using SAS version 9.2.
Results
Patients and treatments
Of 12 patients enrolled, 12 patients received treatment and were included in these final analyses. As of the 27 December 2013 cut-off date, all 12 patients had discontinued treatment, the majority (n = 8) owing to disease progression. Most patients were male (n = 8), <65 years of age (n = 11), had a non-functional tumor (n = 10) and had an ECOG PS of 0 (n = 11). The mean (standard deviation [SD]) sum of longest diameter (target lesion) was 119.3 (93.2) mm. Six (50.0%) patients overall had prior anticancer therapy: four (33.3%) patients had one and two (16.7%) patients had at least three prior treatment regimens.
The median (range) months on treatment was 10.6 (0.7–23.9); the median (range) number of treatment cycles started was 16 (3–29). The mean relative dose intensity was 55.8%. The sunitinib dose was interrupted in 11 patients and reduced in nine patients. The most frequently reported cause of dosing interruptions and dose reductions was AEs (Table 1).
Table 1.
Sunitinib treatment
| Sunitinib (N = 12) | |
|---|---|
| Treatment cycles started, median (range) | 16 (3–29) |
| Months on treatment, median (range) | 10.6 (0.7–23.9) |
| Months on study, median (range) | 14.0 (0.7–26.2) |
| Number of patients with ≥1 dose interruption, n (%) | 11 (91.7) |
| Number of patients with ≥1 dose reduction, n (%) | 9 (75.0) |
| Relative dose intensity, % | |
| Median (range) | 49.7 (26.5–92.9) |
| Mean (SD) | 55.8 (24.5) |
SD, standard deviation.
Efficacy and pharmacokinetics
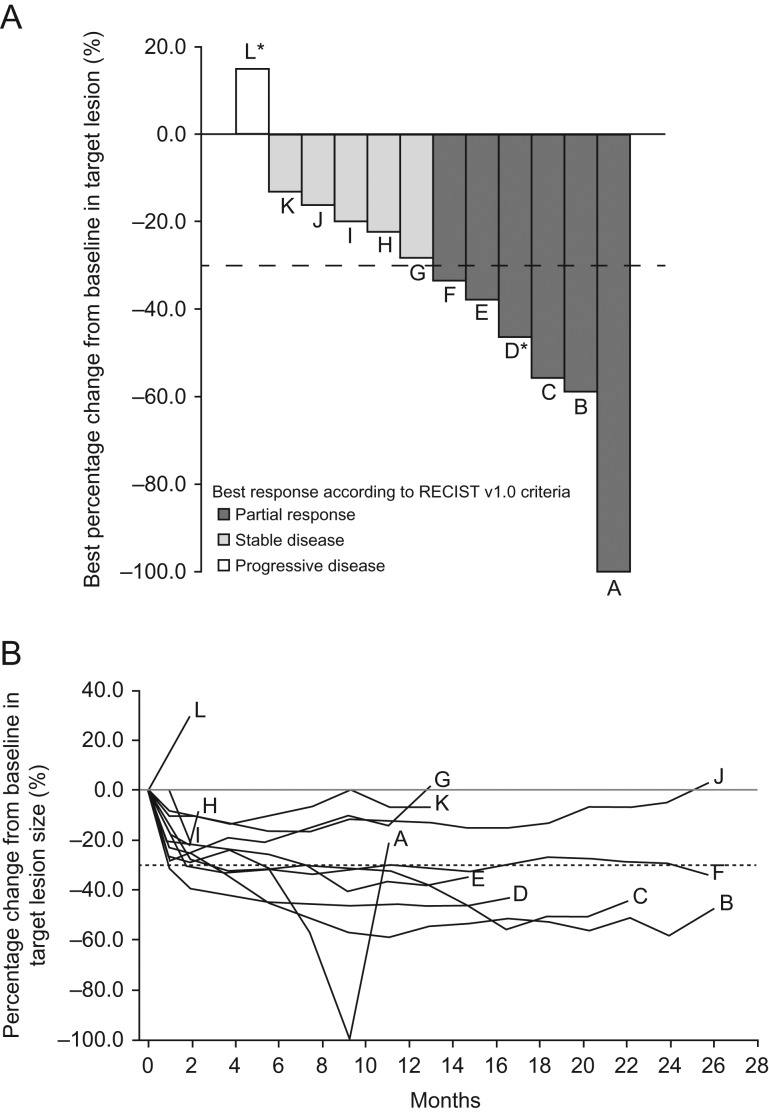
Six patients (50.0%) had a PR and three patients (25.0%) had stable disease for ≥24 weeks, for a CBR (95% CI) of 75.0% (42.8–94.5) (Fig. 1A). Of the two patients with a functioning tumor, one had a PR and the other had progressive disease. ORR (95% CI) was 50.0% (21.1–78.9). Mean (SD) tumor shrinkage was –34.8% (28.8) (Table 2 and Fig. 1B).
Figure 1.
(A) Maximum reduction from baseline in target lesion size by patient. (B) Change from baseline in target lesion size over time. RECIST, Response Evaluation Criteria in Solid Tumors. *Functioning tumor (gastrinoma).
Table 2.
Summary of individual patient response
| Patient | Age/sex | Tumor functionality | Prior treatment | Maximum reduction in target lesion size, % | Best overall response | PFS, months | Reason for discontinuation |
|---|---|---|---|---|---|---|---|
| A | 62/F | Non-functioning | Gemcitabine, SSA | –100.000 | PR | 11.14 | Objective progression |
| B | 44/M | Non-functioning | Epirubicin, mitomycin-C | –58.904 | PR | 26.18 | Objective progression |
| C | 64/F | Non-functioning | Epirubicin, SSA | –55.696 | PR | 22.14 | Objective progression |
| D | 40/F | Functioning (gastrinoma) | SSA | –46.341 | PR | 16.82 | Objective progression |
| E | 64/M | Non-functioning | None | –37.895 | PR | 14.75 | Objective progression |
| F | 51/M | Non-functioning | Cisplatin, etoposide, SSA | –33.508 | PR | 25.82 | Study terminated |
| G | 46/M | Non-functioning | Fluorouracil, cisplatin, gemcitabine, streptocozin, tegafururacil, SSA | –28.161 | Stable disease and time to failure ≥168 days | 9.33 | Objective progression |
| H | 57/M | Non-functioning | SSA | –22.353 | Stable disease and time to failure <168 days | 2.43 | Other—need for treatment rest >4 weeks |
| I | 64/F | Non-functioning | None | –20.000 | Stable disease and time to failure <168 days | 2.07 | Refused to continue treatment for reason other than AE |
| J | 44/M | Non-functioning | Epirubicin, cisplatin, cancer vaccinations, cyclophosphamide | –16.393 | Stable disease and time to failure ≥168 days | 25.89 | Objective progression |
| K | 79/M | Non-functioning | None | –13.333 | Stable disease and time to failure ≥168 days | 13.01 | AE—enterocolitis |
| L | 34/M | Functioning (gastrinoma) | None | 14.894 | Objective progression | 1.97 | Objective progression |
AE, adverse event; F, female; M, male; PFS, progression-free survival; PR, partial response; SSA, somatostatin analogs.
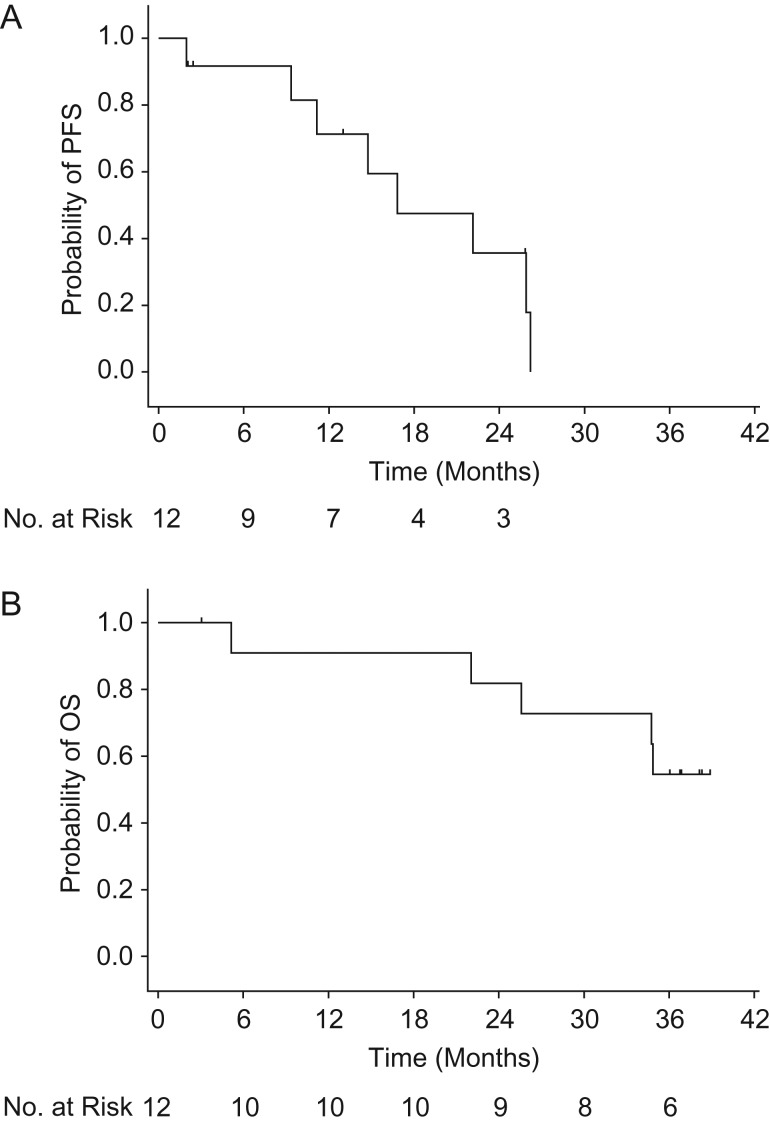
Median (95% CI) PFS was 16.8 (9.3–26.2) months (Fig. 2A). Patients were treated for 2 years after the last patient enrolled started treatment. With a median follow-up period of 35.5 months (range 3.1–38.9), median OS (95% CI) was not reached (22.0 months–not estimable) (Fig. 2B).
Figure 2.
Kaplan–Meier plot of (A) PFS and (B) OS. OS, overall survival; PFS, progression-free survival.
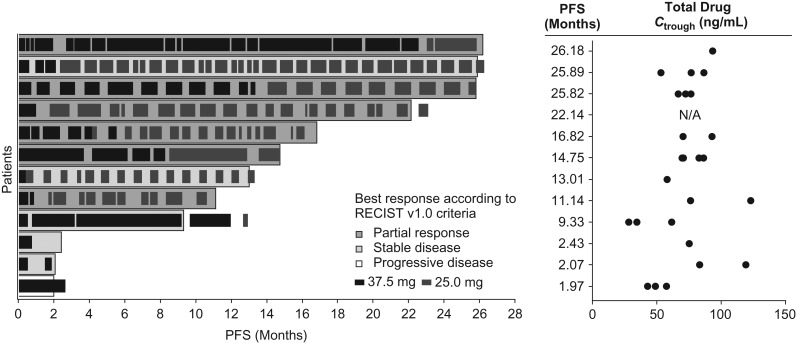
Dose reduction and interruption enabled continuous dosing of sunitinib (Fig. 3). The Ctrough values of total drug (which is the summation of sunitinib and its active metabolite) observed at Cycle 1–4 in most patients were above 50 ng/ml, which is the estimated minimum concentration to achieve target inhibition based on the preclinical data (19); the steady-state Ctrough values of total drug after Cycle 5 were not monitored.
Figure 3.
Individual PFS with Ctrough. Ctrough, trough concentration of total drug (the summation of sunitinib and its active metabolite); N/A, not available; PFS, progression-free survival.
Safety
A total of 198 AEs were reported in 12 patients; 169 AEs were considered treatment related (Table 3). The most common all-causality and treatment-related AEs were diarrhea (n = 10; 83.3%), hand-foot syndrome (n = 8; 66.7%) and hypertension (n = 8; 66.7%) (Table 4). The most common all-causality and treatment-related Grade 3–4 AEs were neutrophil count decreased (n = 4; 33.3%), lipase increased (n = 2; 16.7%), white blood cell count decreased (n = 2; 16.7%) and neutropenia (n = 2; 16.7%).
Table 3.
Summary of AEs
| Sunitinib (N = 12), n (%) | |
|---|---|
| Patients evaluable for AEs | 12 |
| Number of AEs | 198 |
| Patients with AEs | 12 (100.0) |
| Patients with serious AEs | 3 (25.0) |
| Patients with Grade 3 or 4 AEs | 12 (100.0) |
| Patients with Grade 5 AEs | 0 |
| Patients discontinued owing to AEs | 1 (8.3) |
| Patients with dose reduced owing to AEsa | 1 (8.3) |
| Patients with temporary discontinuation owing to AEsb | 12 (100.0) |
AE, adverse event.
aPatients who required only dose reduction, but not temporary discontinuation, owing to AEs.
bPatients who required only temporary discontinuation, but not dose reduction, OR patients who required both temporary discontinuation and dose reduction owing to AEs.
Table 4.
AEs (all-causality) reported in ≥10% of all patients
| AE, n (%) | Grade 1 | Grade 2 | Grade 3a | Total (N = 12) |
|---|---|---|---|---|
| Diarrhea | 4 (33.3) | 5 (41.7) | 1 (8.3) | 10 (83.3) |
| Hand-foot syndrome | 1 (8.3) | 7 (58.3) | 0 (0.0) | 8 (66.7) |
| Hypertension | 1 (8.3) | 7 (58.3) | 0 (0.0) | 8 (66.7) |
| Fatigue | 1 (8.3) | 6 (50.0) | 0 (0.0) | 7 (58.3) |
| Headache | 3 (25.0) | 4 (33.3) | 0 (0.0) | 7 (58.3) |
| Dysgeusia | 5 (41.7) | 0 (0.0) | 0 (0.0) | 5 (41.7) |
| Pyrexia | 1 (8.3) | 3 (25.0) | 1 (8.3) | 5 (41.7) |
| Nasopharyngitis | 4 (33.3) | 1 (8.3) | 0 (0.0) | 5 (41.7) |
| Nausea | 4 (33.3) | 1 (8.3) | 0 (0.0) | 5 (41.7) |
| Vomiting | 5 (41.7) | 0 (0.0) | 0 (0.0) | 5 (41.7) |
| Hypothyroidism | 0 (0.0) | 4 (33.3) | 0 (0.0) | 4 (33.3) |
| Edema | 3 (25.0) | 1 (8.3) | 0 (0.0) | 4 (33.3) |
| Neutrophil count decreased | 0 (0.0) | 0 (0.0) | 4 (33.3) | 4 (33.3) |
| Decreased appetite | 4 (33.3) | 0 (0.0) | 0 (0.0) | 4 (33.3) |
| Electrocardiogram QT prolonged | 1 (8.3) | 1 (8.3) | 1 (8.3) | 3 (25.0) |
| Mucosal inflammation | 2 (16.7) | 1 (8.3) | 0 (0.0) | 3 (25.0) |
| White blood cell count decreased | 0 (0.0) | 1 (8.3) | 2 (16.7) | 3 (25.0) |
| Muscle spasms | 3 (25.0) | 0 (0.0) | 0 (0.0) | 3 (25.0) |
| Anemia | 0 (0.0) | 1 (8.3) | 1 (8.3) | 2 (16.7) |
| Neutropenia | 0 (0.0) | 0 (0.0) | 2 (16.7) | 2 (16.7) |
| Left ventricular dysfunction | 2 (16.7) | 0 (0.0) | 0 (0.0) | 2 (16.7) |
| Ascites | 0 (0.0) | 2 (16.7) | 0 (0.0) | 2 (16.7) |
| Gingivitis | 1 (8.3) | 1 (8.3) | 0 (0.0) | 2 (16.7) |
| Oral dysesthesia | 1 (8.3) | 1 (8.3) | 0 (0.0) | 2 (16.7) |
| Reflux esophagitis | 1 (8.3) | 1 (8.3) | 0 (0.0) | 2 (16.7) |
| Stomatitis | 1 (8.3) | 1 (8.3) | 0 (0.0) | 2 (16.7) |
| Malaise | 1 (8.3) | 1 (8.3) | 0 (0.0) | 2 (16.7) |
| Edema peripheral | 2 (16.7) | 0 (0.0) | 0 (0.0) | 2 (16.7) |
| Trichophytosis | 2 (16.7) | 0 (0.0) | 0 (0.0) | 2 (16.7) |
| Contusion | 2 (16.7) | 0 (0.0) | 0 (0.0) | 2 (16.7) |
| Lipase increased | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (16.7) |
| Platelet count decreased | 1 (8.3) | 0 (0.0) | 1 (8.3) | 2 (16.7) |
| Protein urine present | 0 (0.0) | 1 (8.3) | 1 (8.3) | 2 (16.7) |
| Back pain | 2 (16.7) | 0 (0.0) | 0 (0.0) | 2 (16.7) |
| Proteinuria | 0 (0.0) | 2 (16.7) | 0 (0.0) | 2 (16.7) |
| Epistaxis | 2 (16.7) | 0 (0.0) | 0 (0.0) | 2 (16.7) |
| Pruritus | 2 (16.7) | 0 (0.0) | 0 (0.0) | 2 (16.7) |
| Rash | 1 (8.3) | 1 (8.3) | 0 (0.0) | 2 (16.7) |
aThere were no Grade 5 AEs; Grade 4 AEs occurred in four patients: lipase increased (n = 2), enterocolitis (n = 1) and encephalitis herpes (n = 1).
AE, adverse event.
One patient permanently discontinued treatment owing to an AE of enterocolitis. Three patients (25.0%) experienced serious AEs during the study: one patient had enterocolitis; a second patient had acute cholecystitis and cholangitis; and a third patient had convulsion and loss of consciousness. These were considered treatment related for the cases of enterocolitis, and convulsion and loss of consciousness. One patient died from multi-organ failure due to enteritis infection post treatment.
Of laboratory results, five (41.7%) and six (50.0%) patients had post baseline thyroid stimulating hormone levels > upper limit of normal and < lower limit of normal, respectively. The maximum AE grade for hematology parameters reported was Grade 3: n = 1 (8.3%) for hemoglobin; n = 6 (50.0%) for absolute neutrophils; n = 1 (8.3%) for platelets and n = 3 (25.0%) for white blood cells. Of chemistry parameters, n = 1 (8.3%) Grade 3 AE was reported for alkaline phosphatase, amylase, hyperglycemia, hypoalbuminemia, hyponatremia and hypophosphatemia; n = 2 (16.7%) Grade 4 AEs were reported for lipase.
Discussion
In this updated and final analysis of the Phase II study in Japanese patients with panNETs, the CBR was 75.0% and the ORR was 50.0%, thus supporting the data from the interim analyses (17). Furthermore, the ORR of 50.0% was higher than the ORR reported in the sunitinib Phase III study in predominantly non-Asian patients (14). Although comparisons between studies are limited, the median PFS in the current study in Japanese patients was equivalent to that observed in the global Phase III study (14). Median OS was not reached in this study; this was similar to the initial global Phase III study results (14), although updated analyses of OS in the Phase III study have reported a median OS of 38.6 months (20).
Possible reasons for the difference in efficacy between the Phase II study in Japanese patients and the global Phase III study could be differences in patient background, AE management, and the medical environment, rather than ethnic differences. For example, a greater proportion of patients in the Japanese Phase II study had an ECOG PS of 0 (91.7%) and no prior systemic treatment (50.0%) than patients in the global Phase III study (61.6% and 33.7%, respectively) (14,17). Additionally, the proportion of patients with functioning tumors was 16.7% in the Japanese Phase II study and 29.1% in the global Phase III study (14), and mean body weight was 60.2 kg and 68.0 kg, respectively (Pfizer data on file). Therefore, certain aspects of the patient population may explain the differences observed in efficacy between the Japanese and global studies. Indeed, according to the subgroup analysis of the global Phase III study, some subgroups displayed differences in efficacy—previous systemic regimens, functioning tumor and Ki-67 values (14,17).
AE profiles were similar to those of the Phase III study (14). The most common AE in the current study was diarrhea, which was consistent with the Phase III study, although there were higher proportions of hypertension and hand-foot syndrome AEs in Japanese patients versus the global study population (14). Furthermore, there was a greater incidence of Grade 3–4 hematologic AEs in the current Japanese study population. However, these AEs were manageable with dose reductions, dose interruptions, and/or supportive care in Japanese patients with panNETs.
The occurrence of all-grade AEs and ≥Grade 3 AEs was 100% and 100%, respectively, in the Japanese Phase II study, and 98.8% and 59.0%, respectively, in the global Phase III study (Pfizer data on file); discontinuations due to AEs occurred in 8.3% of patients in the current study and 21.7% of patients in the sunitinib group of the Phase III study (14). Interestingly, mean relative dose intensity was lower in the current study (55.8%) than in the sunitinib group of the Phase III study (91.3%) (14). This difference may be due to the fact that a greater proportion of patients in the Japanese Phase II study had ≥1 dose interruption (91.7%) and ≥1 dose reduction (75.0%) compared with sunitinib-treated patients in the global Phase III study (30% and 31%, respectively) (14). Additionally, AE management through dose interruptions and/or reductions was higher in the current study versus the Phase III study: respectively, 100% and 54.2% of patients had dose interruptions and/or reductions due to AEs (Pfizer data on file). The median duration of treatment (total amount of doses) was 4.6 months in the Phase III study and 10.6 months in the current Phase II study in Japanese patients. Moreover, recent reports suggest that sunitinib-induced AEs could be biomarkers of sunitinib efficacy in renal cell carcinoma and gastrointestinal stromal tumors; thus, it might be important to make efforts to continue sunitinib administration despite AE occurrence (21–24). These data suggest that appropriate AE management through dose reductions and interruptions can prolong the treatment of sunitinib and maximize its efficacy despite lower relative dose intensity. Indeed, a recent study of sunitinib in Japanese hospitals demonstrated that successful AE management with appropriate dose reduction and interruption enabled long-term continuation of sunitinib in Japanese patients with panNETs (25). Furthermore, prolonged treatment period was significantly correlated with decreased relative dose intensity (25).
Some limitations of the current study include the small numbers of patients in this study, i.e. small sample size, and the open-label and non-comparative nature of the study. Additionally, the World Health Organization 2004 classification for panNETs was used, rather than the 2010 classification—the year when the study was initiated; therefore, no information was obtained on Ki-67 values in these Japanese patients with panNETs. Despite these limitations, the results from this study support the efficacy and safety of sunitinib 37.5 mg/day on a CDD schedule in Japanese patients with progressive, advanced/metastatic, well-differentiated, unresectable panNETs. Furthermore, these data support previous studies of sunitinib in patients with panNETs, as well as other indications, in both global and Japanese populations. Finally, appropriate AE management through dose reduction and interruption may prolong treatment with sunitinib and maximize its efficacy.
Acknowledgements
The authors would like to thank Hisanaga Ohashi of Pfizer Japan Inc. for data collection. Medical writing support was provided by Anne Marie McGonigal, PhD, of Engage Scientific Solutions and was funded by Pfizer.
Funding
The study was funded by Pfizer Inc.
Conflict of interest statement
Tetsuhide Ito, Takuji Okusaka, Masayuki Tori and Akira Sawaki do not have any involvement that might raise the question of bias in the work reported or in the conclusions, implications or opinions stated. Satoshi Hashigaki, Nobuyuki Kimura, Kazuo Sato and Emiko Ohki are shareholders and full-time employees of Pfizer.
Contributions
Conception or design of the study-—T.I., E.O., T.O., S.H., N.K.
Acquisition of data-—T.I., E.O., M.T., T.O., S.H., N.K., A.S.
Analysis and interpretation of data-—all authors.
Preparation and final approval of the manuscript-—all authors.
Data Sharing Statement
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the USA and/or EU or (2) in programs that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
- 1. Klöppel G, Couvelard A, Hruban RH, Klimstra DS, Komminoth P, Osamura RY. Neoplasms of the neuroendocrine pancreas In: Lloyd RV, Osamura RY, Klöppel G, et al., editors. WHO Classification of Tumours Vol 10, 4th Edn Lyon, France: IARC Publications, 2017; 209–40. Available at: http://publications.iarc.fr/Book-And-Report-Series/Who-Iarc-Classification-Of-Tumours/Who-Classification-Of-Tumours-Of-Endocrine-Organs-2017 (1 March 2018, date last accessed). [Google Scholar]
- 2. Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterol 2008;135:1469–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ito T, Igarashi H, Nakamura K, et al. . Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol 2015;50:58–64. [DOI] [PubMed] [Google Scholar]
- 4. Ito T, Lee L, Hijioka M, et al. . The up-to-date review of epidemiological pancreatic neuroendocrine tumors in Japan. J Hepatobiliary Pancreat Sci 2015;22:574–7. [DOI] [PubMed] [Google Scholar]
- 5. Oberg K, Kvols L, Caplin M, et al. . Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol 2004;15:966–73. [DOI] [PubMed] [Google Scholar]
- 6. McCollum AD, Kulke MH, Ryan DP, et al. . Lack of efficacy of streptozocin and doxorubicin in patients with advanced pancreatic endocrine tumors. Am J Clin Oncol 2004;27:485–8. [DOI] [PubMed] [Google Scholar]
- 7. Saltz L, Trochanowski B, Buckley M, et al. . Octreotide as an antineoplastic agent in the treatment of functional and nonfunctional neuroendocrine tumors. Cancer 1993;72:244–8. [DOI] [PubMed] [Google Scholar]
- 8. Sun W, Lipsitz S, Catalano P, Mailliard JA, Haller DG. Eastern Cooperative Oncology Group . Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol 2005;23:4897–904. [DOI] [PubMed] [Google Scholar]
- 9. Ito T, Okusaka T, Ikeda M, et al. . Everolimus for advanced pancreatic neuroendocrine tumours: a subgroup analysis evaluating Japanese patients in the RADIANT-3 trial. Jpn J Clin Oncol 2012;42:903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ito T, Honma Y, Hijioka S, et al. . Phase II study of lanreotide autogel in Japanese patients with unresectable or metastatic well-differentiated neuroendocrine tumors. Invest New Drug 2017;35:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 2005;8:299–309. [DOI] [PubMed] [Google Scholar]
- 12. Fjällskog ML, Hessman O, Eriksson B, Janson ET. Upregulated expression of PDGF receptor beta in endocrine pancreatic tumors and metastases compared to normal endocrine pancreas. Acta Oncol 2007;46:741–6. [DOI] [PubMed] [Google Scholar]
- 13. Kulke MH, Lenz HJ, Meropol NJ, et al. . Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol 2008;26:3403–10. [DOI] [PubMed] [Google Scholar]
- 14. Raymond E, Dahan L, Raoul JL, et al. . Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501–13. [DOI] [PubMed] [Google Scholar]
- 15. Pellat A, Dreyer C, Couffignal C, et al. . Clinical and biomarker evaluations of sunitinib in patients with grade 3 digestive neuroendocrine neoplasms. Neuroendocrinology 2018;107:24–31. [DOI] [PubMed] [Google Scholar]
- 16. Rinzivillo M, Fazio N, Pusceddu S, et al. . Sunitinib in patients with pre-treated pancreatic neuroendocrine tumors: a real-world study. Pancreatology 2018;18:198–203. [DOI] [PubMed] [Google Scholar]
- 17. Ito T, Okusaka T, Nishida T, et al. . Phase II study of sunitinib in Japanese patients with unresectable or metastatic, well-differentiated pancreatic neuroendocrine tumor. Invest New Drug 2013;31:1265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Igarashi H, Okusaka T, Ito T, et al. . Phase II study of sunitinib (SU) in Japanese patients with well-differentiated pancreatic neuroendocrine tumor (NET). Ann Oncol 2013;24:ix82–ix3. [Google Scholar]
- 19. Mendel DB, Cherrington JM, Laird AD. CCR 20th anniversary commentary: determining a pharmacokinetic/pharmacodynamic relationship for sunitinib—a look back. Clin Cancer Res 2015;21:2415–7. [DOI] [PubMed] [Google Scholar]
- 20. Faivre S, Niccoli P, Castellano D, et al. . Sunitinib in pancreatic neuroendocrine tumors: updated progression-free survival and final overall survival from a phase III randomized study. Ann Oncol 2017;28:339–43. [DOI] [PubMed] [Google Scholar]
- 21. Poprach A, Pavlik T, Melichar B, et al. . Skin toxicity and efficacy of sunitinib and sorafenib in metastatic renal cell carcinoma: a national registry-based study. Ann Oncol 2012;23:3137–43. [DOI] [PubMed] [Google Scholar]
- 22. Donskov F, Michaelson MD, Puzanov I, et al. . Sunitinib-associated hypertension and neutropenia as efficacy biomarkers in metastatic renal cell carcinoma patients. Br J Cancer 2015;113:1571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bono P, Rautiola J, Utriainen T, Joensuu H. Hypertension as predictor of sunitinib treatment outcome in metastatic renal cell carcinoma. Acta Oncol 2011;50:569–73. [DOI] [PubMed] [Google Scholar]
- 24. Komatsu Y, Ohki E, Ueno N, et al. . Safety, efficacy and prognostic analyses of sunitinib in the post-marketing surveillance study of Japanese patients with gastrointestinal stromal tumor. Jpn J Clin Oncol 2015;45:1016–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee L, Ito T, Igarashi H, et al. . Dose and schedule modification are required for long-term continuation of sunitinib in Japanese patients with advanced pancreatic neuroendocrine tumors. Cancer Chemother Pharmacol 2018;81:163–9. [DOI] [PubMed] [Google Scholar]