A striking resection rate and survival benefit of first-line cetuximab-containing chemotherapy were demonstrated in patients with mCRC who were classified into the potentially resectable group.
Keywords: cetuximab, colorectal cancer, prognosis
Abstract
Objective
We aimed to clarify the clinical practice and outcomes of first-line cetuximab-containing chemotherapy in patients with metastatic colorectal cancer. Efficacy and safety were evaluated in each group classified by the European Society for Medical Oncology Guidelines 2012.
Methods
This prospective observational study included patients with previously untreated metastatic colorectal cancer from 158 centers in Japan who started first-line cetuximab-containing chemotherapy from January 2012 to June 2013 and were followed for up to 3 years. The resection rates after chemotherapy were calculated and the overall survival was estimated using the Kaplan–Meier method for Group 1 (G1, potentially resectable), Group 2 (G2, not resectable and tumor-related symptoms) and Group 3 (G3, not resectable and asymptomatic).
Results
Of 578 patients, 562 were classified into G1 (n = 165), G2 (n = 224) or G3 (n = 173). The resection rate of any site was higher in G1 (57.0%) than in G2 (11.2%) and G3 (11.6%). G1, G2 and G3 showed median overall survivals (95% confidence interval) of 45.9 (38.1–not available), 16.7 (14.5–18.8) and 30.6 (23.2–34.8) months, respectively (P < 0.0001). The common tumor-related symptoms in G2 were pain, fatigue and anorexia, from which 31.7, 22.2 and 14.8% of the patients suffered at baseline.
Conclusions
The expected efficacy and safety of first-line cetuximab-containing chemotherapy were demonstrated in patients with metastatic colorectal cancer under clinical practice in Japan.
Registered clinical trial numbers
UMIN000007275
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide and the most frequently occurring cancer in Japan, with 147 200 new cases and 51 600 deaths reported in 2016 (1,2). In a clinical study, ~25% of the patients had distant metastatic disease at the time of diagnosis, and ~50% of the patients developed metastases during the course of the disease, with liver being the most common site (3,4).
First-line treatment of metastatic CRC (mCRC) is determined based on clinical presentation at diagnosis, patient and tumor characteristics, status of metastases, tumor-related symptoms and patient’s preferences. The European Society for Medical Oncology (ESMO) Guidelines of 2012 stratified patients into four subgroups with different treatment intensities, which were defined as follows: Group 0 with resectable metastasis; Group 1 with potentially resectable metastasis after achieving tumor response; Group 2 with non-resectable metastasis, high tumor burden or tumor-related symptoms and Group 3 with unresectable metastasis, initially asymptomatic and less aggressive disease (5). Patients belonging to Groups 1 to 3 receive upfront systemic chemotherapy. Patients of Groups 1 and 2 require intensive upfront chemotherapy to ensure secondary resectability or rapid symptom control, whereas Group 3 patients are treated with a sequential treatment approach (6).
Cetuximab is a human/mouse chimeric monoclonal antibody that targets the epidermal growth factor receptor (EGFR) to inhibit its signaling and shows antitumor effects by binding to EGFR, competing with its ligands (7). Cetuximab, administered alone or in combination with cytotoxic chemotherapy, showed efficacy in the treatment of patients with RAS wild-type CRC (8,9). Cetuximab may yield higher responses with the shrinkage of lesions, potentially leading to long overall survival (OS) in patients with mCRC (10–12).
There have been no reports of treatment results aiming at estimating the hepatic resection rates, overall survival and incidence of tumor-related symptoms in each group by following the ESMO Guidelines 2012. In addition, in Japan, only a few reports have focused on cetuximab combination therapy as the first-line treatment for mCRC (13,14). This prompted us to conduct the present observational study (15,16). The aims of this study were to clarify the clinical practice and outcomes of first-line cetuximab-containing chemotherapy in patients with KRAS wild-type mCRC under practical use in Japan, to collect historical/reference data and to estimate the efficacy of each group classified by the ESMO Guidelines 2012 (5).
Patients and methods
Study population
Cetuximab observational study as first-line therapy (CORAL) is a Japan-based prospective observational study that was conducted at 158 study sites (Table 1). Patients could participate in the study if they met the following criteria: had previously untreated mCRC; had an Eastern Cooperative Oncology Group (ECOG) scale of performance status (PS) of 0–2; were scheduled to receive a first-line chemotherapy regimen containing cetuximab; and provided written informed consent. Patients with multiple primary cancers or previous neoadjuvant chemotherapy for liver metastasis were also included in the study. Patients were considered for this study until death, withdrawal of consent or loss to follow-up. There were no protocol-specified treatments or assessments. All aspects of treatments, including specific chemotherapy agents used alone and/or in combination, dose and schedule, were determined by a physician. The protocol was reviewed by the institutional review board of each participating site.
Table 1.
Baseline characteristics
| Group 1 | Group 2 | Group 3 | Total | |
|---|---|---|---|---|
| N | 165 (100%) | 224 (100%) | 173 (100%) | 562 (100%) |
| Gender | ||||
| Male | 115 (70%) | 138 (62%) | 108 (62%) | 361 (64%) |
| Female | 50 (30%) | 86 (38%) | 65 (38%) | 201 (30%) |
| Age | ||||
| Median (range) | 65 (32–84) | 65 (31–88) | 67 (37–87) | 65 (31–88) |
| ECOG PS | ||||
| 0 | 130 (79%) | 124 (55%) | 139 (80%) | 393 (70%) |
| 1 | 32 (19%) | 82 (37%) | 29 (17%) | 143 (25%) |
| 2 | 3 (2%) | 18 (8%) | 5 (3%) | 26 (5%) |
| KRAS mutation status | ||||
| Wild type | 156 (95%) | 216 (96%) | 166 (96%) | 538 (96%) |
| Mutation type | 5 (3%) | 3 (1%) | 6 (3%) | 14 (2%) |
| Not measured | 2 (1%) | 2 (1%) | 1 (1%) | 5 (1%) |
| Unknown | 2 (1%) | 3 (1%) | 5 (1%) | |
| CEA | ||||
| Median (range) | 13 (0.5–8.3 × 103) | 63 (0.7–9.8 × 104) | 27 (0.4–1.0 × 105) | 29 (0.4–1.0 × 105) |
| LDH | ||||
| < ULN | 99 (60%) | 84 (37%) | 107 (62%) | 290 (52%) |
| ≥ULN | 66 (40%) | 140 (63%) | 66 (38%) | 272 (48%) |
| Primary tumor site | ||||
| Colon | 99 (60%) | 154 (69%) | 117 (67%) | 370 (66%) |
| Rectum | 64 (39%) | 67 (30%) | 55 (32%) | 186 (33%) |
| Other | 2 (1%) | 3 (1%) | 1 (1%) | 6 (1%) |
| Resection of primary tumor | ||||
| Yes | 117 (71%) | 102 (46%) | 149 (86%) | 368 (65%) |
| No | 48 (29%) | 122 (54%) | 24 (14%) | 194 (35%) |
CEA, carcinoembryonic antigen; ECOG PS, Eastern Cooperative Oncology Group scale of performance status.
Classification according to ESMO Guidelines 2012
Patients were classified into three groups at enrollment in the present study. Investigator grouped patients into Group 1, 2 or 3 according to ‘Hierarchy of factors for definition of treatment aim/group’ in ESMO Guidelines 2012. Group 1 was defined as patients with liver or lung metastases which were not completely resectable (R0 or R1), Group 2 as patients who were necessary to receive intermediate intensive treatment, where the treatment aim is palliative rather than curative, most reliable and rapid regression of metastases is important, in particular in case of imminent or present symptoms or tumor associated complications and Group 3 as patients who were not necessary to intensive treatment with maximal shrinkage of metastases.
Clinical outcomes
The measures of the clinical outcomes were based on a physician’s determination and included progression-free survival (PFS) from the date of initiation of first-line treatment to first progressive disease, conversion surgery (with resection margins other than R0) or death. In case of a conversion surgery performed with R0 resection, it is considered a censored case at the time of surgery. Other measures included time to treatment failure (TTF) from the date of initiation of first-line treatment to premature treatment discontinuation, to initiation of other treatments, to disease progression or to death for any reason; OS from the date of initiation of first-line treatment to death; tumor response based on the Response Evaluation Criteria in Solid Tumors (RECIST) criteria without being mandatory for the confirmation of response; time-to-response from the date of initiation of first-line treatment to partial response or complete response; and conversion rate to curative surgery. The transition of tumor-related symptoms was also evaluated for patients of Group 2, in accordance with the ESMO Guidelines 2012. The safety outcomes focused on skin and other specific toxicities (infusion reaction, hypomagnesemia, thrombus and interstitial lung disease) were classified according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), version 3.0 (17,18). Data were collected at baseline and every 2 months until the first 6 months after the initiation of first-line treatment and, thereafter, every 6 months up to 24 months.
Statistical analysis
The overall response rate (ORR), disease control rate, resection rate, liver resection rate and R0 resection rate were summarized along with the Clopper–Pearson exact confidence interval (CI). The differences in these rates among groups or subgroups were assessed by Fisher’s exact test. We used the Kaplan–Meier method to estimate PFS, OS and relapse-free survival and used the log-rank test to compare the survival distributions of two or more groups. The pairwise test was performed as a post hoc analysis, and the adjusted P value was calculated in accordance with the Holm or Tukey method. All analyses were performed using SAS version 9.3.
Results
Patients’ characteristics
During the period from January 2012 to June 2013, 578 mCRC patients were enrolled in the study from 158 centers in Japan; of those, 562 patients from 152 centers met the inclusion criteria of the study. Out of these 562 patients, KRAS wild-type 538 (96%), KRAS mutation type 14 (2%), not measured 5 (1%) and unknown 5 (1%) were diagnosed with KRAS wild-type and mutation tumors, respectively (Table 1). There were 165 (29%), 224 (40%) and 173 (31%) patients classified into Groups 1, 2 and 3, respectively, in accordance with the ESMO consensus guidelines. Background characteristics are shown in Table 2. The proportions of patients with ECOG PS ≥ 1, high level of LDH and present primary tumor location were higher in Group 2 (45, 63 and 54%) than in Group 1 (21, 40 and 29%) or 3 (20, 38 and 14%). The demographics with regard to gender, age and KRAS status were well balanced among the three groups.
Table 2.
Regimens of cetuximab-containing chemotherapies in each ESMO guideline group
| Regimens | Group 1 | Group 2 | Group 3 | P valuea |
|---|---|---|---|---|
| Total (N = 562) | 165 (100%) | 224 (100%) | 173 (100%) | |
| Oxaliplatin based (65.7%) | 115 (69.7%) | 147 (65.6%) | 107 (61.8%) | 0.1937 |
| FOLFOX+Cmab | 94 (57.0%) | 116 (51.8%) | 63 (36.4%) | 0.0006 |
| SOX+Cmab | 12 (6.4%) | 21 (9.2%) | 29 (16.7%) | 0.0103 |
| Cape+Cmab | 9 (8.7%) | 10 (5.2%) | 15 (8.6%) | 0.2887 |
| Irinotecan based (30.1%) | 44 (26.7%) | 64 (28.4%) | 61 (35.6%) | 0.1437 |
| FOLFIRI+Cmab | 33 (20.3%) | 54 (24.0%) | 36 (21.3%) | 0.6512 |
| IRIS+Cmab | 10 (5.8%) | 6 (2.6%) | 19 (10.9%) | 0.0026 |
| IRI+Cmab | 1 (0.6%) | 3 (1.3%) | 6 (3.4%) | 0.1352 |
| IFL+Cmab | 0 | 1 (0.4%) | 0 | |
| Cmab monotherapy (3.6%) | 6 (3.5%) | 10 (4.4%) | 4 (2.9%) | 0.6271 |
| Others+Cmab Combinations | 0 | 3 (1.3%) | 1 (0.6%) |
Cape, capecitabine; Cmab, Cetuximab; ESMO, European Society for Medical Oncology; SOX, TS-1 and oxaliplatin.
aBy two-tailed Fisher’s exact test.
Treatment regimen
The cetuximab-containing regimens used in each group of patients are listed in Table 2. Oxaliplatin-based regimens seemed to be preferable over irinotecan-based chemotherapy in Japan; 65.7% of the patients received oxaliplatin-based chemotherapy combined with cetuximab, while 30.1% of the patients received irinotecan-based cetuximab-containing chemotherapy. Cetuximab monotherapy was used in 3.6% of patients in the study. The proportions of patients treated with FOLFOX (5-FU, leucovorin and oxaliplatin) + cetuximab were 57.0 and 51.8% in Groups 1 and 2, respectively. This was higher than the rate of 36.4% in Group 3 (P < 0.001). The percentage of patients who received SOX (TS-1 and oxaliplatin) + cetuximab and IRIS (irinotecan + TS-1) + cetuximab were 16.7 and 10.9% in Group 3, which showed a tendency to be higher than those in Group 1 (6.4 and 5.8%, respectively) and Group 2 (9.2 and 2.6%, respectively).
Efficacy
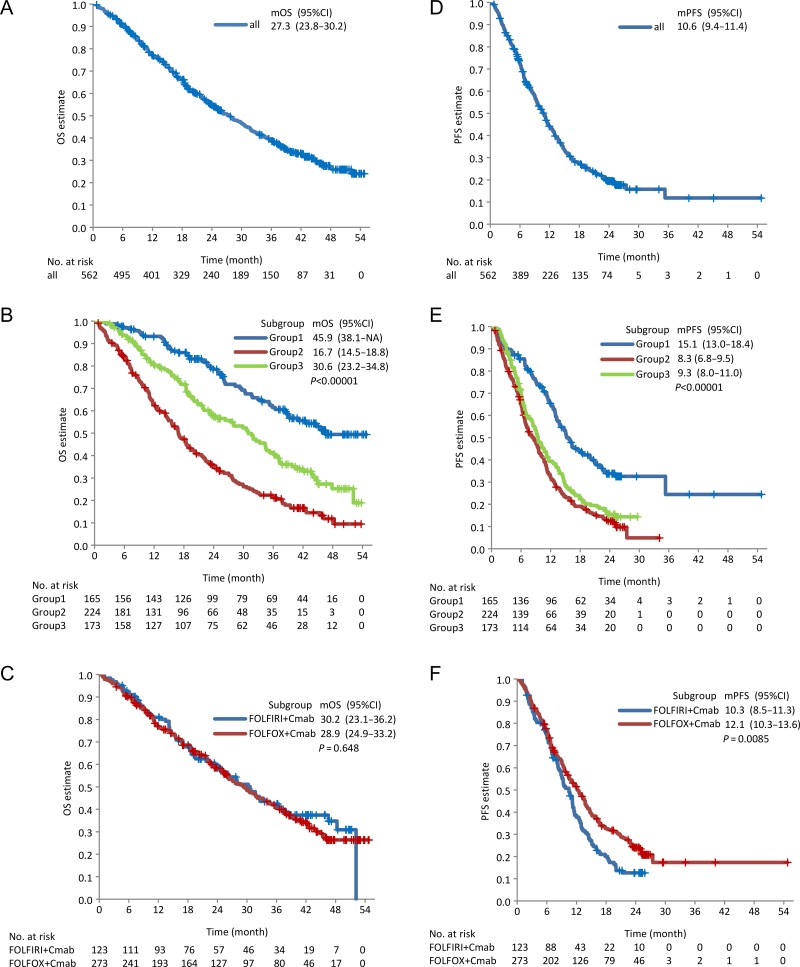
The median OS (mOS) and median PFS (mPFS) were recorded as 27.3 (95% CI: 23.8–30.2) and 10.6 (9.4–11.4) months, respectively, in all eligible patients (Fig. 1A and D). In terms of regimens, the PFS was longer in patients treated with FOLFOX + cetuximab than in those treated with FOLFIRI + cetuximab (P = 0.0085). The mPFS was 12.1 (10.3–13.6) and 10.3 (8.5–11.3) months in patients treated with FOLFOX + cetuximab and FOLFIRI + cetuximab, respectively (Fig. 1C and F). Groups 1, 2 and 3 showed the mOS of 45.9 (38.1–NA), 16.7 (14.5–18.8) and 30.6 (23.2–34.8) months, respectively (P < 0.0001). Pairwise test P values were < 0.0001 for Group 1 vs. Group 2 or 3, and the P values were < 0.0001 for Group 2 vs. Group 3 (Fig. 1B). The mPFSs of Groups 1, 2 and 3 were found to be 15.1 (13.0–18.4), 8.3 (6.8–9.5) and 9.3 (8.0–11.0) months, respectively (P < 0.0001). The P value for the pairwise test for Group 1 vs. Group 2 or 3 in comparison to Group 2 vs. Group 3 (Fig. 1E) was found to be < 0.0001. ORR was 56.9% in all evaluable patients (n = 531). In Groups 1, 2 and 3, the ORRs were 63.5, 55.6 and 59.1%, respectively (Table 3) (P = 0.3200). However, the complete response rate differed among them (P = 0.0356), with 5.1% in Group 1, 1.9% in Group 2 and 6.9% in Group 3. The medians of maximum tumor shrinkage (depth of response) were almost the same between groups (Table 3).
Figure 1.
Kaplan–Meier estimates for OS and PFS. OS and PFS in all patients (A and D) in Groups 1, 2 and 3 (B and E) and those in patients treated with FOLFOX + cetuximab and FOLFIRI + cetuximab (C and F, respectively). Two-sided P values were evaluated by the log-rank test. The median OS or PFS is shown in each figure with 95% CI. mOS, median OS; mPFS, median PFS; OS, overall survival; PFS, progression-free survival; CI, confidence interval.
Table 3.
ORR, RR, DpR and TTF in each ESMO guideline group
| Group 1 | Group 2 | Group 3 | P valuea | |
|---|---|---|---|---|
| Response rate | ||||
| Number of patients analyzed (N = 531) | 156 | 216 | 159 | |
| Complete response | 8 (5.1%) | 4 (1.9%) | 11 (6.9%) | 0.03555 |
| Partial response | 91 (58.3%) | 116 (53.7%) | 83 (52.2%) | 0.5188 |
| Stable disease | 33 (21.1%) | 51 (23.6%) | 33 (20.8%) | 0.7807 |
| Progressive disease | 13 (8.3%) | 29 (13.4%) | 15 (9.4%) | 0.2462 |
| Not evaluable | 6 (3.8%) | 13 (6.0%) | 12 (7.5%) | |
| Unknown | 5 (3.2%) | 3 (1.4%) | 5 (3.1%) | |
| ORR (56.9%) | 99 (63.5%) | 120 (55.6%) | 94 (59.1%) | 0.3169 |
| Disease control rate (80.9%) | 132 (84.6%) | 171 (79.2%) | 127 (79.9%) | 0.3744 |
| Depth of response | ||||
| Number of patients analyzed (N = 473) | 145 | 189 | 139 | |
| Median | 43.0% | 43.3% | 39.6% | <0.6511 |
| Interquartile range | (20.6, 58.8) | (11.9, 57.9) | (15.0, 58.8) | |
| Range | (0, 100%) | (0, 100%) | (0, 100%) | |
| Group 1 | Group 2 | Group 3 | P valuea | ||
| Resection rate | |||||
| Number of patients analyzed (N = 562) | 165 | 224 | 173 | ||
| Resection | 94 (57.0%) | 25 (11.2%) | 20 (11.6%) | <0.00001 | |
| Liver resection | 66 (40.0%) | 10 (4.5%) | 11 (6.4%) | <0.00001 | |
| R0 resection | 68 (41.2%) | 9 (4.0%) | 7 (4.0%) | <0.00001 | |
| Time to treatment failure | |||||
| Number of patients analyzed (N = 562) | 165 | 224 | 173 | ||
| Median (day) | 126 | 157.5 | 175 | <0.019 | |
| Interquartile range | (64.0, 250.0) | (72.5, 269.0) | (93.0, 322.0) | ||
| Range (day) | (0, 1204) | (0, 1022) | (0, 771) | ||
ORR, overall response rate; RR, resection rate; DpR, depth of response; TTF, time to treatment failure.
aOverall P value for the three groups was evaluated by Fisher’s exact test. When it was <0.05, post hoc pairwise comparisons by Fisher’s exact test were conducted, where multiplicity was adjusted by Holm’s method.
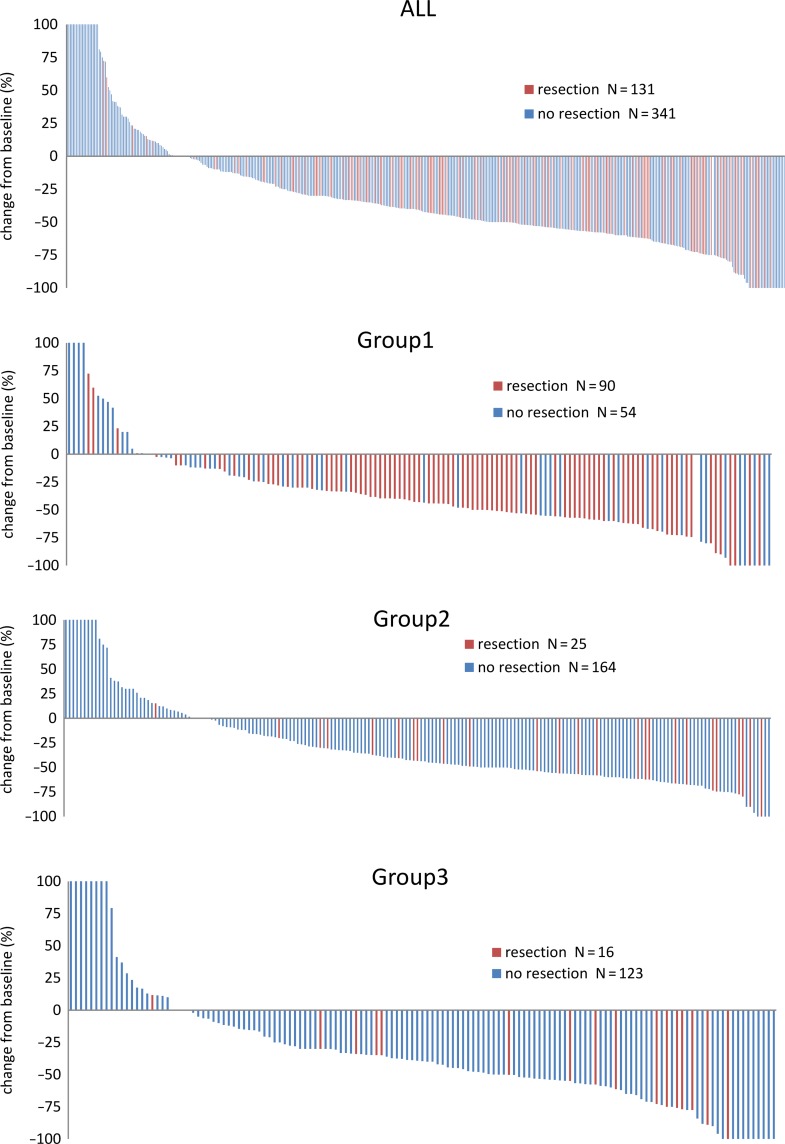
The resection rate was higher in Group 1 than in Groups 2 and 3 (P < 0.0001). Specifically, the resection, the hepatic resection and the R0 resection rates were found to be 57.0% (49.0–64.6%), 40.0% (32.5–47.9%) and 41.2% (33.6–49.1%) in Group 1 compared with 11.2% (7.4–16.0%), 4.5% (2.2–8.1%), and 4.0% (1.9–7.5%) in Group 2 and 11.6% (7.2–17.3%), 6.4% (3.2–11.1%) and 4.0% (1.6–8.2%) in Group 3 (Table 3). Among patients whose metastases were resected, the rates of R0 resection (Group 1, 2 and 3) were 72, 36 and 35%. Waterfall plots for each group are shown in Fig. 2.
Figure 2.
Waterfall plot. Tumor shrinkage from baseline is plotted in each group.
Tumor-related symptoms in Group 2
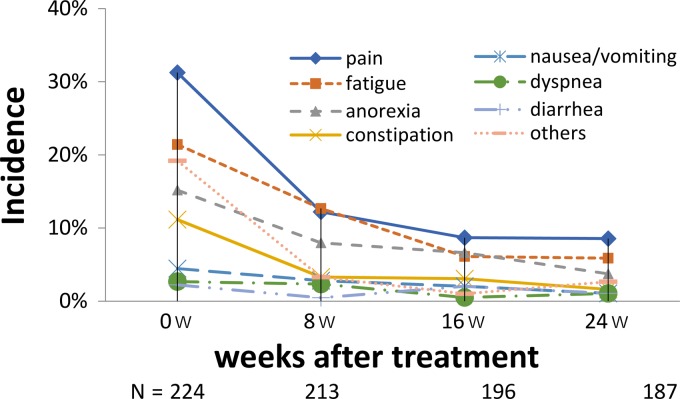
The changes in tumor-related symptoms (CTCAE Grade 1≤) in Group 2 after initiation of the first-line treatment are summarized in Fig. 3. The common tumor-related symptoms were pain, fatigue and anorexia, from which 31.7, 22.2 and 14.8% of the patients suffered at baseline, but the proportions decreased to 8.6, 5.9 and 3.7%, respectively, at 24 weeks after the treatment in a time-dependent manner with a decreasing trend (P < 0.0001).
Figure 3.
Time course of tumor-related symptoms and cetuximab-related adverse reactions (CTCAE Grade 1≤) in Group 2. Percentage of patients with tumor-related symptoms at each time point. Decreasing trend was evaluated by Chi-squared test for the proportions of each symptom and P value is shown at the right edge of the last time point in the figure.
Skin toxicities and hypomagnesemia
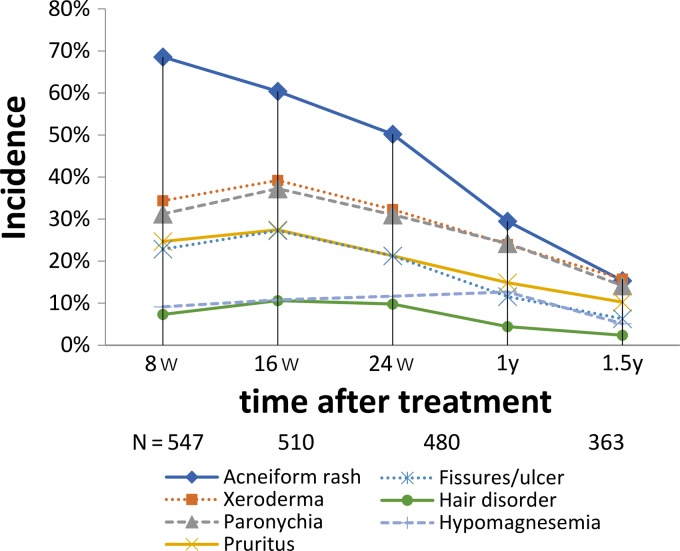
Skin reactions (CTCAE Grade 1≤) were frequently observed in the study and were considered to be cetuximab-related adverse events, including acneiform rash, xeroderma, paronychia, pruritus, fissures/skin ulcer and hair disorder. Acneiform rash was observed with the highest incidence of 68.6% of the patients at 8 weeks after cetuximab treatment was started, and the incidence gradually decreased to 50.2% at 24 weeks and to 18.2% at 1.5 years (Fig. 4). Other skin reactions developed gradually with a peak at 16 weeks, later than that of acneiform rash, and then decreased in frequency (Fig. 4). The incidence of hypomagnesemia increased gradually to 12.7% with a peak at 1 year after treatment and then decreased to 5.1% at 1.5 years (Fig. 4).
Figure 4.
Change in incidence of skin toxicities and hypomagnesemia (CTCAE Grade 1≤), which are known as cetuximab-related adverse reactions.
Discussion
This observational study showed the efficacy and safety of cetuximab-containing regimens as the first-line treatment for patients with mCRC in Japan and the difference of efficacies between patients who were classified into three groups according to the ESMO Guidelines 2012. To our knowledge, this is the first report comparing the efficacies of cetuximab-containing regimens among these three groups.
Among the patients enrolled in the present study, 70% of patients were classified into Groups 1 and 2. This might be because cetuximab-containing regiments may enhance the depth of response and early tumor shrinkage and are tended to be chosen in patients classified into Groups 1 and 2 in expectation of metastasectomy and reduction of tumor-related symptoms, respectively. The reasons for the higher proportion of patients with ECOG PS ≥ 1 and high level of LDH in Group 2 and without primary tumor in Group 3 may be that patients in Group 2 had tumor-related symptoms and patients in Group 3 had time to undergo resection of the primary tumor due to shrinkage of metastatic tumors. High proportion of patients with poor PS and high level of LDH led to shorter OS in Group 2 than in Group 1 or 3.
The present study showed that PFS and OS differed among the three groups while ORR was similar among them, showing that the efficacy of cetuximab-containing regimens was similar among the three groups. In Group 1, the higher resection rate likely led to longer PFS and OS. PFS of Group 2 was similar to that of Group 3, while OS was shorter than that of Group 3, suggesting that the baseline tumor burden in Group 2 was higher than that in Group 3. Although the present study was observational and a single-arm study, the higher resection rate in Group 1, the reduced rate of tumor-related symptoms in Group 2, and the similar OS to those in previous reports on Japanese trials suggested that cetuximab-containing regimens can achieve goals of chemotherapy in each group, showing that the classification in the ESMO Guidelines 2012 is useful in actual clinical practice (15,16). In addition, a waterfall plot showed that receiving cetuximab-containing regimens resulted in good tumor shrinkage in each group, suggesting that patients whose goal is cytoreduction in the ESMO Guidelines 2016, which is nearly equivalent to Groups 1 and 2 in the ESMO Guidelines 2012, should receive treatment regimens containing anti-EGFR antibodies.
Previous studies did not compare the efficacy of FOLFOX + cetuximab with that of FOLFIRI + cetuximab. However, in the present study, the OS of patients treated with FOLFOX + cetuximab was similar to that of those treated with FOLFIRI + cetuximab. Although the PFS of patients treated with FOLFOX + cetuximab was longer than that of those treated with FOLFIRI + cetuximab, the fact that more patients were classified into Group 1 among those treated with FOLFOX + cetuximab than among those treated with FOLFIRI + cetuximab may have led to a longer PFS.
There were several limitations in the present study. First, it was an observational study; therefore no analysis was performed on proportions of KRAS mutation status or ECOG PS, and definitive conclusions cannot be drawn from the obtained results. Second, when this trial was planned, expanded RAS testing and BRAF testing have not been approved in Japan, yet. Additionally, it was not reported that primary tumor location can be predictive factor for the efficacy of anti-EGFR antibodies including cetuximab. Therefore, our results did not include RAS status, BRAF status and sidedness. Third, the present study featured several cetuximab-containing regimens, including non-standard regimens such as SOX or IRIS + cetuximab. This may have affected the results on the efficacy and safety. However, because a large number of patients were enrolled in the present study, and the efficacy and safety of cetuximab-containing regimens were similar to those in the FIRE-3 study, these findings seem to be reliable and might provide guidance for physicians in clinical practice.
Conclusions
This observational study suggests that cetuximab-containing regimens as the first-line treatment are effective and tolerable in Japanese patients with mCRC, and that the classification of the ESMO Guidelines 2012 is applicable to Japanese patients.
Acknowledgements
The authors would like to thank the study patients, study investigators and clinical research teams.
Funding
This study was supported by the Public Health Research Foundation, Comprehensive Support Project for Oncology Research in Japan.
Conflict of interest statement
Dr Muro has received personal fees from Chugai, Eli Lilly, Takeda, Ono, Taiho and Bayer Yakuhin. Dr Masuishi has received personal fees from Merck Serono, and Takeda. Dr Denda has received personal fees from Sanofi, Taiho and Yakult Honsha. Prof. Yamanaka has received personal fees from Takeda, Taiho, Boehringer Ingelheim Japan and Chugai. Prof. Ohashi has received personal fees from Sanofi, Eisai, Chugai, Taiho, Shionogi, Kowa, Public Health Research Foundation and Daiichi Sankyo. Dr Sugihara has received personal fees from Chugai, Sanofi, Bristol-Myers Squibb, Eli Lilly and Merck Serono. All remaining authors have declared no conflicts of interest.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. . Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Information Service, Japan National Cancer Center [Internet]. Cancer Statistics in Japan ‘16. [cited 2018 July 5]. Available from: https://ganjoho.jp/en/professional/statistics/brochure/2016_en.html.
- 3. Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: metastases to a single organ. World J Gastroenterol 2015;21:11767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doi T, Boku N, Kato K, et al. . Phase I/II study of capecitabine plus oxaliplatin (XELOX) plus bevacizumab as first-line therapy in Japanese patients with metastatic colorectal cancer. Jpn J Clin Oncol 2010;40:913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmoll HJ, Van Cutsem E, Stein A, et al. . ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol 2012;23:2479–516. [DOI] [PubMed] [Google Scholar]
- 6. Van Cutsem E, Cervantes A, Adam R, et al. . ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–422. [DOI] [PubMed] [Google Scholar]
- 7. Galizia G, Lieto E, De Vita F, et al. . Cetuximab, a chimeric human mouse anti-epidermal growth factor receptor monoclonal antibody, in the treatment of human colorectal cancer. Oncogene 2007;26:3654–60. [DOI] [PubMed] [Google Scholar]
- 8. Van Cutsem E, Köhne CH, Láng I, et al. . Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011–9. [DOI] [PubMed] [Google Scholar]
- 9. Bokemeyer C, Bondarenko I, Hartmann JT, et al. . Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol 2011;22:1535–46. [DOI] [PubMed] [Google Scholar]
- 10. Folprecht G, Gruenberger T, Bechstein WO, et al. . Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol 2010;11:38–47. [DOI] [PubMed] [Google Scholar]
- 11. Ye LC, Liu TS, Ren L, et al. . Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wildtype unresectable colorectal liver-limited metastases. J Clin Oncol 2013;31:1931–8. [DOI] [PubMed] [Google Scholar]
- 12. Wilke H, Glynne-Jones R, Thaler J, et al. . Cetuximab plus irinotecan in heavily pretreated metastatic colorectal cancer progressing on irinotecan: MABEL study. J Clin Oncol 2008;26:5335–43. [DOI] [PubMed] [Google Scholar]
- 13. Uehara K, Ishiguro S, Hiramatsu K, et al. . Conversion chemotherapy using cetuximab plus FOLFIRI followed by bevacizumab plus mFOLFOX6 in patients with unresectable liver metastases from colorectal cancer. Jpn J Clin Oncol 2011;41:1229–32. [DOI] [PubMed] [Google Scholar]
- 14. Uemura M, Kim HM, Hata T, et al. . First-line cetuximab-based chemotherapies for patients with advanced or metastatic KRAS wild-type colorectal cancer. Mol Clin Oncol 2016;5:375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsuji A, Sunakawa Y, Ichikawa W, et al. . Early tumor shrinkage and depth of response as predictors of favorable treatment outcomes in patients with metastatic colorectal cancer treated with FOLFOX plus cetuximab (JACCRO CC-05). Target Oncol 2016;11:799–806. [DOI] [PubMed] [Google Scholar]
- 16. Hazama S, Maeda H, Iwamoto S, et al. . A phase II study of XELOX and cetuximab as first-line therapy in patients with KRAS wild type metastatic colorectal cancer (FLEET2 study). Clin Colorectal Cancer 2016;15:329–36. [DOI] [PubMed] [Google Scholar]
- 17. Lacouture ME, Mitchell EP, Piperdi B, et al. . Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:1351–7. [DOI] [PubMed] [Google Scholar]
- 18. Horie Y, Yamazaki K, Funakoshi T, et al. . Predictability of antitumor efficacy of cetuximab plus irinotecan based on skin rash severity according to observation period in patients with metastatic colorectal cancer following failure of fluorouracil, irinotecan and oxaliplatin. Mol Clin Oncol 2015;3:1029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]