Significance
Homeostatic synaptic plasticity has long been considered an anti-Hebbian mechanism that maintains the brain’s network stability, prevents aberrant Hebbian plasticity, and sustains normal cognitive functions. However, evidence supporting this hypothesis is largely lacking. Using region-specific deletions of the retinoic acid receptor RARα, a key mediator of homeostatic synaptic plasticity, we show that experience of an enriched environment (EE) engages homeostatic synaptic plasticity in hippocampal neurons in an RARα-dependent manner. EE experience in mice with deletion of RARα in the hippocampus caused runaway long-term potentiation (LTP), enhanced contextual memory, and reduced cognitive flexibility by hyperactivating mammalian target of rapamycin (mTOR) signaling. Treatment with the mTOR inhibitor rapamycin during an EE experience reversed both runaway LTP and enhanced learning, demonstrating a close functional interaction between Hebbian and homeostatic plasticity.
Keywords: retinoic acid receptor, homeostatic synaptic plasticity, mTOR signaling, enriched environment, Hebbian plasticity
Abstract
Homeostatic synaptic plasticity is a stabilizing mechanism engaged by neural circuits in response to prolonged perturbation of network activity. The non-Hebbian nature of homeostatic synaptic plasticity is thought to contribute to network stability by preventing “runaway” Hebbian plasticity at individual synapses. However, whether blocking homeostatic synaptic plasticity indeed induces runaway Hebbian plasticity in an intact neural circuit has not been explored. Furthermore, how compromised homeostatic synaptic plasticity impacts animal learning remains unclear. Here, we show in mice that the experience of an enriched environment (EE) engaged homeostatic synaptic plasticity in hippocampal circuits, thereby reducing excitatory synaptic transmission. This process required RARα, a nuclear retinoic acid receptor that doubles as a cytoplasmic retinoic acid-induced postsynaptic regulator of protein synthesis. Blocking RARα-dependent homeostatic synaptic plasticity during an EE experience by ablating RARα signaling induced runaway Hebbian plasticity, as evidenced by greatly enhanced long-term potentiation (LTP). As a consequence, RARα deletion in hippocampal circuits during an EE experience resulted in enhanced spatial learning but suppressed learning flexibility. In the absence of RARα, moreover, EE experience superactivated mammalian target of rapamycin (mTOR) signaling, causing a shift in protein translation that enhanced the expression levels of AMPA-type glutamate receptors. Treatment of mice with the mTOR inhibitor rapamycin during an EE experience not only restored normal AMPA-receptor expression levels but also reversed the increases in runaway Hebbian plasticity and learning after hippocampal RARα deletion. Thus, our findings reveal an RARα- and mTOR-dependent mechanism by which homeostatic plasticity controls Hebbian plasticity and learning.
Homeostatic plasticity is a major form of non-Hebbian synaptic plasticity that is thought to maintain the stability of neural networks. Significant progress has recently been made in the characterization and mechanistic appreciation of homeostatic plasticity in vertebrates and invertebrates (1–4). How the development of homeostatic plasticity impacts synapses and their ability to undergo subsequent Hebbian plasticity, as well as how such a synaptic interaction may affect (5–7) an animal’s ability to learn, remains largely unexplored, however (8, 9).
Retinoic acid (RA) is primarily studied as a classical morphogen regulating gene expression during development, but RA additionally acts in adult animals as a nongenomic signaling molecule that controls homeostatic synaptic plasticity (5, 10, 11). A key player mediating synaptic RA signaling is the RA receptor RARα, a nuclear DNA-binding receptor that regulates gene transcription during development (12). In mature neurons, RARα partially translocates out of the nucleus into neuronal dendrites and suppresses dendritic protein synthesis through direct binding to specific target mRNAs (13–15). During homeostatic plasticity, RA synthesis is triggered by chronic suppression of synaptic activity and a reduction in dendritic calcium levels (6, 7, 16). The RA thus synthesized then binds to RARα, changes its conformation, and thereby disinhibits protein synthesis. This process leads to enhanced excitatory synaptic transmission and reduced inhibitory synaptic transmission (5, 11). Conditional deletion of RARα selectively impairs homeostatic synaptic plasticity by blocking the effect of RA without directly affecting Hebbian plasticity (10, 16), making RARα an ideal molecular tool for exploring the functional impact of homeostatic synaptic plasticity on Hebbian long-term plasticity and on animal behavior in vivo.
Our previous studies explored RARα’s function in the context of synaptic RA signaling (action of RA on synaptic function), and found that RARα mediates RA’s action at synapses during homeostatic synaptic plasticity (5, 10, 11). However, as an evolutionarily conserved molecule among vertebrate animals, RARα may have additional functions in the absence of RA (e.g., when synaptic activity is not suppressed). In the present study, we used RARα conditional knockout (cKO) mice to specifically investigate RARα’s role under normal (nonsilent) synaptic transmission, and its relation to homeostatic plasticity. We focused on RARα’s function in the hippocampus, a region of the brain where the behavioral relevance of synaptic function has been well established by many learning and memory studies (17–21). In particular, we explored how an altered activity differentially engages RARα signaling, using a brief enriched environment (EE) experience as a behavioral manipulation of synaptic activity in RARα cKO mice. We found that an EE directly affects excitatory synaptic transmission in hippocampal CA1 neurons. The effect of an EE required normal RARα signaling, as evidenced by the fact that the RARα deletion in CA1 neurons blocks an EE’s effect on basal synaptic transmission. Although conditional genetic deletion of RARα from CA1 neurons did not alter Hebbian long-term potentiation (LTP) and long-term depression (LTD) in home cage control animals, it significantly enhanced LTP and diminished LTD in EE-experienced animals, which correlated with enhanced but less flexible learning in these animals. Moreover, biochemical analysis showed that the activity of extracellular signal-related kinase (ERK) and mammalian target of rapamycin (mTOR) was elevated in EE-experienced neurons after the RARα deletion, revealing a previously undescribed signaling pathway linking RARα to the activity-dependent regulation of protein synthesis. Together, our study establishes RARα as a major player in an activity-dependent metaplasticity mechanism that modulates animal learning through regulation of Hebbian plasticity.
Results
Enhanced LTP and Reduced LTD in CA1-RARα KO Mice with EE Experience.
To engage homeostatic synaptic plasticity in vivo, we chose a short-term experience (10 d) in an EE as a behavioral paradigm (SI Appendix, Fig. S1A) that alters the synaptic excitation/inhibition (E/I) state in the hippocampus. EE experience adds sensory, social, and spatial complexity to the otherwise impoverished home cage environment of a laboratory mouse, and has been shown to improve cognitive functions related to multiple brain regions, including the hippocampus (reviewed in ref. 22). To investigate the potential involvement of RA signaling in EE-induced synaptic changes, we exposed adult RARα cKO mice (10, 23) to an EE (SI Appendix, Fig. S1B), and used injection of Cre-recombinase–expressing adeno-associated viruses (AAVs) to selectively delete RARα expression, thus disrupting RA signaling in the hippocampal CA1 region (SI Appendix, Fig. S1C). Injection of an AAV expressing an inactive truncated form of Cre, a mutant Cre (mCre) was applied as a control. Animals from both wild-type (WT; mCre-infected) and RARα KO (Cre-infected 3 wk before an EE experience) groups show similarly robust activation in many regions of the brain by an EE, including CA1 regions, as indicated by c-Fos immunostaining (SI Appendix, Fig. S1D).
We first investigated whether EE experience in adulthood [postnatal day 60 (P60)–P70] influences Hebbian long-term synaptic plasticity in an RA signaling-dependent manner. Consistent with previous findings (16), we found that deletion of RARα did not alter LTP induced by high-frequency train stimulation at Schaffer collateral (SC)-CA1 synapses in home cage control mice (Fig. 1 A, C, and D). Moreover, EE experience did not significantly change LTP in WT hippocampus (Fig. 1 A, B, and D). Unexpectedly, however, EE experience strongly increased the magnitude of LTP in RARα KO neurons, consistent with “runaway” LTP in the absence of homeostatic plasticity (Fig. 1 A, C, and D). The enhanced LTP in EE-exposed, RARα-deficient neurons resembled classical NMDA receptor (NMDAR)-dependent LTP found at SC-CA1 synapses, as it was completely blocked by the NMDAR antagonist D-2-amino-5-phosphonovalerate (D-APV) in interleaved slices (SI Appendix, Fig. S2A). To explore whether this enhanced LTP could be attributed to increased silent synapse formation in EE-exposed conditions, we examined the excitatory postsynaptic current (EPSC) success rate at −70 mV and +40 mV with a minimal stimulation protocol (24). Home cage- and EE-experienced neurons exhibited a similarly higher success rate at +40 mV than at −70 mV in both WT and RARα KO neurons, suggesting that the enhanced LTP in EE-exposed RARα KO neurons is not due to increased silent synapse formation (SI Appendix, Fig. S2B).
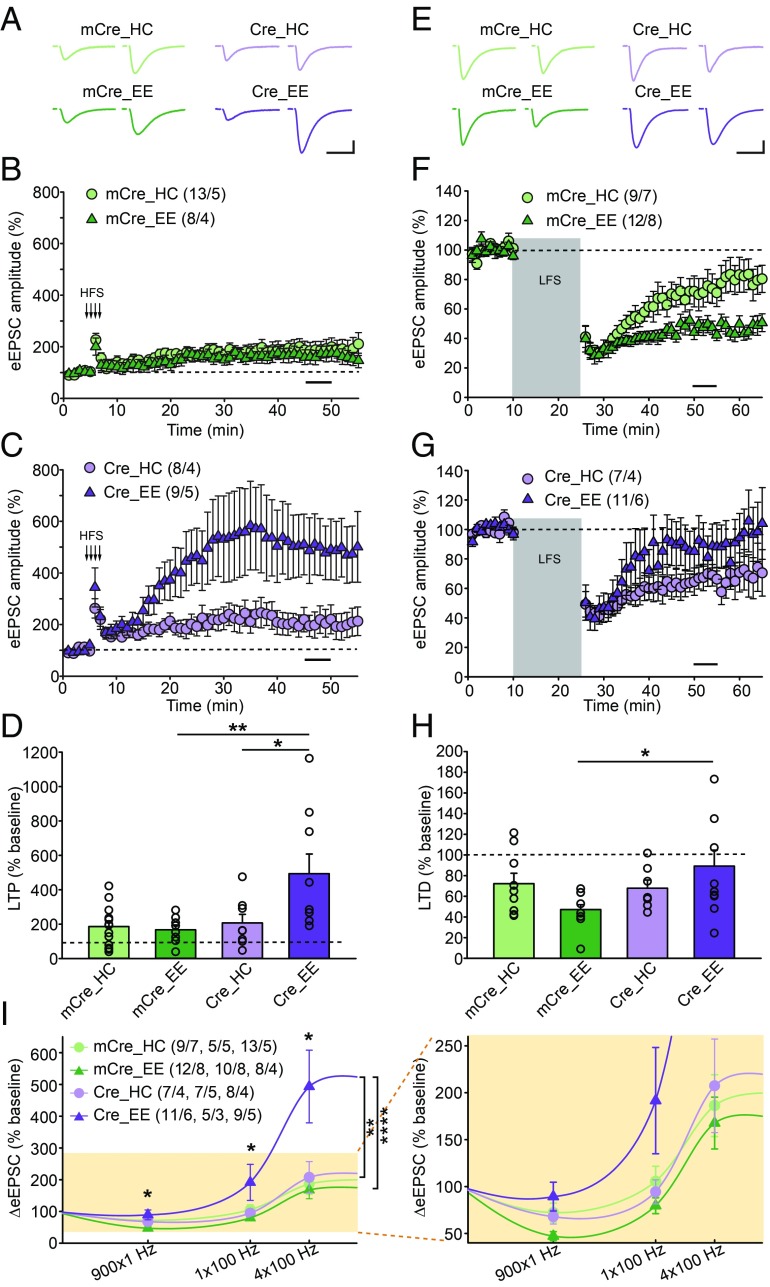
Fig. 1.
Enhanced LTP and reduced LTD in CA1-RARα KO mice with EE experience. (A–D) Quantification of SC-CA1 LTP in WT (mCre) and CA1-RARα KO (Cre) mice exposed to home cage (HC) or EE experience. (A) Representative traces of SC-CA1 evoked EPSCs (eEPSC) before and after LTP induction. (Scale bars: 50 pA, 25 ms.) (B and C) Summary graphs of CA1 LTP in WT or RARα KO neurons with HC or EE exposure. (D) LTP magnitude is measured as average potentiation at 41–45 min (black bar in B and C) after onset of high-frequency train stimulation (HFS) induction (indicated by arrows in B and C) [comparisons by two-way ANOVA: HC/EE × mCre/Cre: F(1,34) = 5.571, P = 0.0241; Tukey post hoc test: *P < 0.05, **P < 0.01]. (E–H) Quantification of SC-CA1 LTD in WT and CA1-RARα KO mice exposed to HC or EE conditions. (E) Representative traces of SC-CA1 eEPSCs. (Scale bars: 50 pA, 25 ms.) (F and G) Summary graphs of CA1 LTD. (H) LTD magnitude is measured as average depression at 40–45 min (black bar in F and G) after onset of low-frequency stimulation (LFS) induction (gray bar in F and G) [comparisons by two-way ANOVA: HC/EE × mCre/Cre: F(1,35) = 4.508, P = 0.0409; Tukey post hoc test: *P < 0.05]. (I) Stimulus strength and CA1 eEPSC response functions derived from HC- and EE-exposed WT and CA1-RARα KO mice. Data points for 900 × 1 Hz represent the average change 40–45 min after the onset of 900 pulses of a 1-Hz stimulus train. Data points for 1 × 100 Hz and 4 × 100 Hz represent the average change 40–45 min after the onset of high-frequency stimulus trains. The asterisk on top of each stimulus pattern indicates significant HC/EE × mCre/Cre interaction (P < 0.05) for that stimulus pattern by two-way ANOVA. For 1 × 100 Hz, comparisons were made by two-way ANOVA [HC/EE × mCre/Cre: F(1,23) = 6.574, P = 0.0173; Tukey post hoc test: *P < 0.05]. For the entire BCM curve, comparisons were made by two-way ANOVA [stimulation pattern × (genotype + experience): F(6,92) = 3.271, P = 0.0058; Tukey post hoc test: mCre_HC vs. mCre_EE, not significant; Cre_HC vs. Cre_EE, **P < 0.01; mCre_EE vs. Cre_EE, ****P < 0.0001]. n/N, number of neurons/number of independent experiments. All graphs represent mean ± SEM.
We next examined LTD. A standard low-frequency stimulation protocol (900 pulses at 1 Hz) induced comparable levels of LTD at SC-CA1 synapses in both WT and RARα KO neurons from mice with home cage experience (Fig. 1 E–H; examined at P70). After EE experience, however, WT neurons exhibited significantly greater LTD than RARα KO neurons (Fig. 1 E–H), indicating that synaptic RA signaling interacts with EE experience in LTD. These results suggest that EE experience shifts the direction of long-term synaptic plasticity in WT mice in a manner dependent on RA signaling.
The altered Hebbian plasticity in the RARα KO neurons with EE experience suggests a potentially shifted modification threshold (θM) in the Bienenstock–Cooper–Munro (BCM) theory of synapse modification (25). Might homeostatic scaling mediated by synaptic RA signaling, in the context of EE exposure, shift the θM of hippocampal Hebbian plasticity? To test this hypothesis directly, we examined the response of EPSCs to a weak tetanic stimulus (one train of a 1-s, 100-Hz stimulus). This weak stimulus train did not induce lasting changes in neurons from WT mice after home cage or EE experience or in neurons from RARα KO neurons after home cage experience, but led to significant LTP in the RARα KO neurons after EE experience (Fig. 1I). Thus, the entire BCM curve shifted significantly in RARα KO neurons after EE experience (Fig. 1I). Taken together, these results indicate that normal RA signaling engaged by an EE experience shifts the θM to constrain the magnitude of LTP while promoting LTD induction.
EE Experience Reduces Basal Excitatory Synaptic Transmission in an RARα-Dependent Manner.
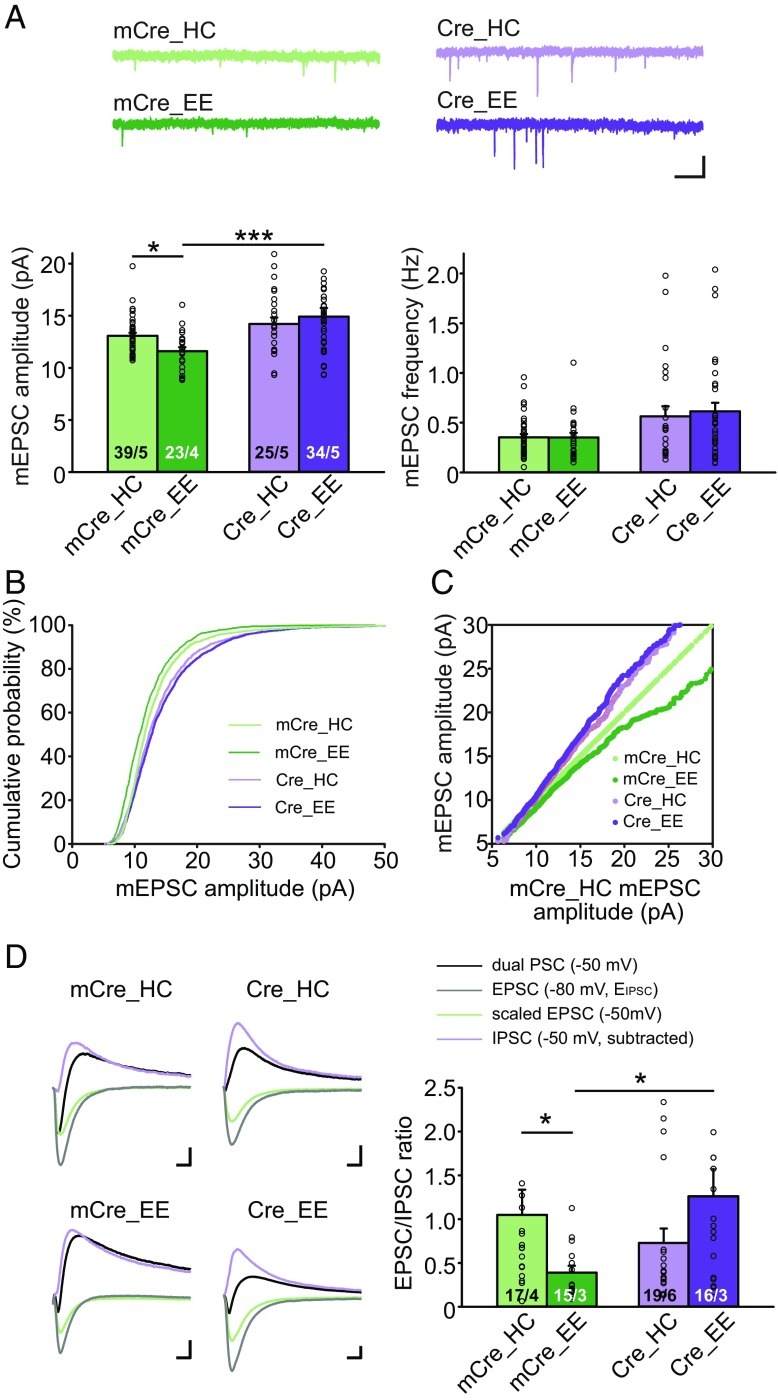
The observation that experiencing an EE alters Hebbian plasticity in an RARα-dependent manner suggests that an EE, as a sensorimotor paradigm, engages RA signaling and homeostatic synaptic plasticity. Given that RA signaling is known to participate in homeostatic plasticity, we asked whether an EE experience indeed alters basal synaptic transmission in mice in an RARα-dependent manner, as would be expected during homeostatic plasticity. In WT neurons, an EE reduced the basal miniature EPSC (mEPSC) amplitude compared with home cage-experienced controls (Fig. 2 A–C). In Cre-infected RARα KO neurons that lack RA signaling, however, an EE no longer had an effect on the mEPSC amplitude (Fig. 2 A–C). The effect of an EE on basal excitatory synaptic transmission was confirmed using evoked synaptic transmission. We measured the EPSC/inhibitory postsynaptic current (IPSC) ratio by monitoring postsynaptic responses to stratum radiatum stimulation, which activates SC axons and local inhibitory interneurons. Dual-component postsynaptic currents (PSCs) were collected at −50 mV. Pure EPSCs were collected at −80 mV (the reversal potential of IPSCs) and scaled based on driving force to estimate EPSCs at −50 mV, which were then subtracted from the dual PSCs to obtain IPSCs at −50 mV. Consistent with the reduced mEPSC amplitude, and unaffected miniature inhibitory synaptic transmission by EE experience (SI Appendix, Fig. S3A), we found a significantly lower E/I ratio in CA1 neurons after EE experience than after home cage experience (Fig. 2D). Again, in RARα KO CA1 neurons, the reduction in E/I ratio by an EE experience was completely blocked (Fig. 2D). Additionally, the paired-pulse ratio, a measure of short-term presynaptic plasticity, was not affected by an EE or by the RARα deletion (SI Appendix, Fig. S3B), corroborating the previously reported postsynaptic action of RA signaling at synapses (5, 16). In summary, all our basal transmission data are consistent with a model positing that in WT CA1 neurons, EE experience induces a homeostatic plasticity response that reduces postsynaptic AMPA receptor (AMPAR) abundance, as evidenced by reduced mEPSC amplitudes, and a reduced E/I ratio. All these changes are blocked by the RARα KO. Thus, impairing RARα signaling blocks EE-induced homeostatic decreases in overall excitatory synaptic strength.
Fig. 2.
EE-induced reduction in basal excitatory synaptic transmission requires intact RARα signaling. (A–C) Quantification of CA1 pyramidal neuron mEPSC recordings from WT and CA1-RARα KO mice exposed to home cage (HC) or EE conditions. (A) Representative traces (Top) and quantification (Bottom) of mEPSC amplitude and frequency (two-tailed Mann–Whitney U test with Bonferroni correction: *P < 0.05, ***P < 0.001). (Scale bars: 10 pA, 1 s.) (B) Cumulative plot of mEPSC amplitudes. (C) Ranked mEPSC amplitude plots. All groups (mCre_HC, mCre_EE, Cre_HC, and Cre_EE) are plotted against the mCre_HC group. (D) Representative traces (Left) and quantification of synaptic E/I ratios (Right) from WT (mCre) and CA1-RARα KO (Cre) mice exposed to HC or EE conditions. Dual PSCs were measured at −50 mV, and pure EPSCs were measured at the reversal potential of IPSCs (−80 mV). Scaled EPSCs at −50 mV were subtracted from dual PSCs to obtain IPSCs at −50 mV, which were used to calculate E/I at −50 mV (two-tailed Mann–Whitney U test with Bonferroni correction: *P < 0.05). (Scale bars: 50 pA, 10 ms.) n/N, number of neurons/number of independent experiments. All graphs represent mean ± SEM.
Enhanced Hippocampus-Dependent Memory but Reduced Learning Flexibility in CA1-RARα KO Mice with EE Experience.
What might be the behavioral effects of the interplay between Hebbian and RA-dependent homeostatic synaptic plasticity? To address this question, we performed a series of behavioral assays that probe different aspects of hippocampus-dependent learning. Specifically, we used classical Pavlovian fear conditioning to examine spatial memories (26–28) and reversal learning in the water T-maze and the Barnes maze to examine behavioral flexibility. Additional behavioral tasks were performed to evaluate animals’ working memory and general anxiety levels.
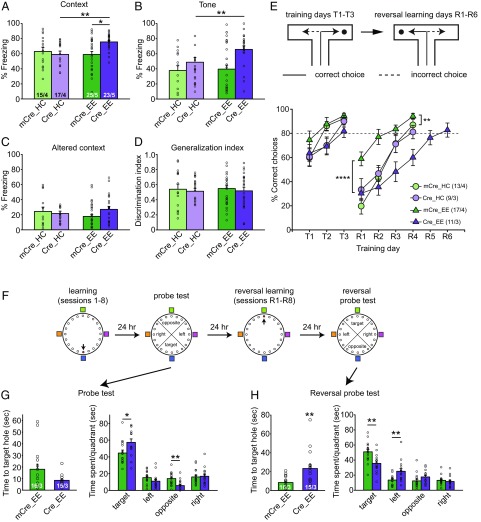
EE experience alone or RARα deletion alone had no obvious effect on fear memory, as indicated by normal contextual or cued (tone) fear memory tested 24 h or 48 h, respectively, after training (Fig. 3 A and B). By contrast, EE experience in CA1-RARα KO mice (i.e., mice with a selective bilateral conditional deletion of RARα in the CA1 region of the hippocampus) significantly enhanced both contextual and cued fear memory (Fig. 3 A and B). Since the hippocampus is thought to be required for both contextual and cued fear memory of delayed fear conditioning (29, 30), the stronger contextual and cued fear memories are likely supported by enhanced LTP in RARα KO EE hippocampus. Measurement of overall unconditioned responses during training (SI Appendix, Fig. S4), freezing levels in an altered context (Fig. 3C), and the fear generalization index (Fig. 3D) showed that the enhanced conditioned response was not due to higher intrinsic freezing levels in EE-experienced CA1-RARα KO mice. The animals also performed similarly in the Y-maze, exhibiting normal working memory (SI Appendix, Fig. S5A). Open-field and elevated plus maze tests also showed that CA1-RARα KO mice exposed to an EE did not have increased nonsocial anxiety (SI Appendix, Fig. S5 B and D), and they did not display altered social anxiety levels (SI Appendix, Fig. S5C), indicating that the higher freezing levels of CA1-RARα KO mice in the memory test were not due to elevated anxiety. Instead, EE experience in CA1-RARα KO mice likely facilitated spatial memory formation.
Fig. 3.
Enhanced hippocampus-dependent memory but reduced learning flexibility in CA1-RARα KO mice with EE experience. (A–D) Behavioral quantification of contextual and cued fear conditioning. Contextual fear memory (A) and cued fear memory (B) were measured 24 h and 48 h after training, respectively (two-tailed Mann–Whitney U test with Bonferroni correction: *P < 0.05, **P < 0.01). (C) Fear memory generalization in an altered context was measured 48 h after training. (D) Discrimination index = [Fz (Training Context) − Fz (Altered Context)]/[Fz (Training Context) + Fz (Altered Context)]. Fz, percent freezing. (E) Performance of WT and CA1-RARα KO mice in the water T-maze. After reaching the 80% learning criteria (T1–T3), the platform was moved to the opposite arm to start reversal training (R1–R6) the next day. Training was terminated after 4 d of reversal training, with the exception of the case of the Cre_EE group, where 2 additional days of training were necessary for the animals to reach learning criteria [statistical analysis was performed using two-way repeated-measures ANOVA: T1–T3: group factor, F(3,46) = 2.012, P = 0.1253; time factor, F(2,92) = 30.67, P < 0.0001; interaction, F(6,92) = 1.389, P = 0.2276; R1–R4: group factor, F(3,46) = 9.66, P < 0.0001; time factor, F(3,138) = 72.78, P < 0.0001; interaction, F(9,138) = 3.44, P = 0.0008; Tukey post hoc test: mCre_EE vs. mCre_HC, **P < 0.01; mCre_EE vs. Cre_EE, ****P < 0.0001]. (F) Schematics of training and testing paradigm for the Barnes maze. (G) Performance of WT and CA1-RARα KO mice during the first probe test, measured as time to the target hole and time spent in each quadrant (two-tailed Mann–Whitney U test: *P < 0.05, **P < 0.01). (H) Performance of WT and CA1-RARα KO mice during the second probe test after reversal learning, measured as time to the target hole and time spent in each quadrant (two-tailed Mann–Whitney U test: **P < 0.01). n/N, number of mice/number of independent litters. All graphs represent mean ± SEM.
In contrast to hippocampal LTP, which is thought to support memory formation, hippocampal NMDAR-dependent LTD is thought to support behavioral flexibility through the weakening of previously encoded memory traces when new information is learned (31–33). Using a water T-maze paradigm, we showed that all naive mice groups exhibited similar learning curves to find the hidden platform, reaching a criterion of 80% correct trials in each session after 3 d of learning (Fig. 3E). However, in the reversal learning test, where the hidden platform was moved to the opposite arm, EE-experienced WT mice, which exhibited enhanced hippocampal LTD compared with EE-experienced CA1-RARα KO mice, learned to switch significantly faster than the WT mice with home cage experience and the CA1-RARα KO mice (Fig. 3E). We observed similar results with the Barnes maze, another hippocampus-dependent spatial memory task (Fig. 3F). While the Barnes maze learning curve was similar between WT and CA1 RARα KO mice after EE experience (SI Appendix, Fig. S6), CA1-RARα KO mice spent significantly more time in the target quadrant and less time in the opposite quadrant during the probe test performed 24 h after initial training (Fig. 3G), supporting stronger spatial memory formation in the KO animals. However, the reversal probe test, performed 24 h after the last reversal learning sessions (change of target hole location), showed the opposite results. Not only did the CA1-RARα KO mice spend significantly less time in the target quadrant and more time in the wrong quadrant, but it also took them a much longer time than WT mice to locate the new target hole (Fig. 3H). Thus, with multiple hippocampus-dependent learning tasks, we demonstrate that under a more natural EE (our EE condition), conditional deletion of RARα from the hippocampal CA1 region of adult mice enhances learning but reduces behavioral flexibility, which is consistent with the enhanced LTP and decreased LTD observed under these conditions.
EE Experience Enhances LTP in RARα KO Mice by Stimulating AMPAR Protein Synthesis.
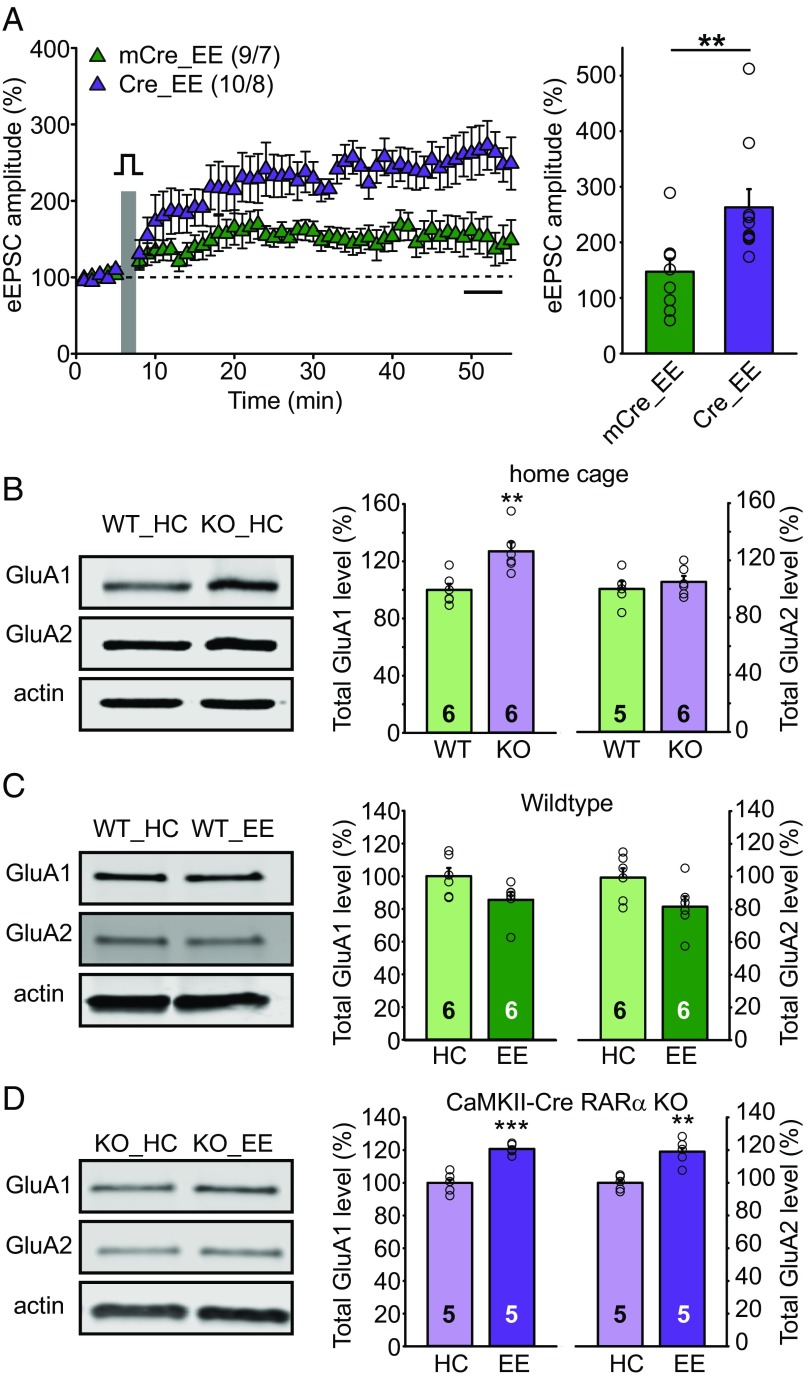
What may be the underlying mechanism that drives changes in synaptic plasticity after ablation of RA-dependent homeostatic plasticity in EE-exposed CA1-RARα KO mice? To address this question, we focused on the enhanced NMDAR-dependent LTP in EE CA1-RARα KO mice because of its robustness. We first tested whether another induction protocol of LTP, calcium influx via L-type calcium channels induced by postsynaptic depolarization in the presence of the NMDAR antagonist D-APV (referred to as voltage-pulse LTP) (34, 35), also produced enhanced LTP in EE-experienced CA1-RARα KO mice. Strikingly, voltage-pulse LTP was also significantly enhanced in EE-experienced CA1-RARα KO mice (Fig. 4A), suggesting that the CA1-RARα KO enhances LTP expression downstream of LTP induction.
Fig. 4.
EE experience enhances LTP in CA1-RARα KO mice by stimulating AMPAR protein synthesis. (A) SC-CA1 LTP induced by the voltage pulse protocol in EE-experienced WT and CA1-RARα KO mice (two-tailed Mann–Whitney U test: **P < 0.01). n/N, number of neurons/number of animals. (B) Immunoblot analysis of total GluA1 and GluA2 expression in home cage (HC) WT and CaMKII-Cre RARα KO CA1 (two-tailed Student’s t test: GluA1: t = 3.572, df = 10, **P < 0.01; GluA2: t = 0.7464, df = 9, P > 0.4). (C) Immunoblot analysis of total GluA1 and GluA2 expression in HC- and EE-experienced WT CA1 (two-tailed Student’s t test: GluA1: t = 2.079, df = 10, P = 0.064; GluA2: t = 2.096, df = 10, P = 0.063). (D) Immunoblot analysis of total GluA1 and GluA2 expression in HC- and EE-experienced CaMKII-Cre RARα KO CA1 (two-tailed Student’s t test: GluA1: t = 6.539, df = 8, ***P < 0.001; GluA2: t = 4.681, df = 8, **P < 0.01). All graphs represent mean ± SEM.
It is generally accepted that postsynaptic LTP is mediated by an increase in postsynaptic AMPAR abundance (36). Thus, we examined the effect of RARα deletion on the expression levels of the AMPAR subunits GluA1 and GluA2 in CA1 neurons. For this purpose, we dissected and compared CA1 tissues from littermate RARα cKO mice that lacked (RARαfl/fl, no Cre) or contained a transgene expressing Cre-recombinase under control of the CaMKIIα promoter (CaMKII-Cre::RARαfl/fl). We chose this approach instead of bilateral injection of Cre-expressing AAVs for our biochemical analysis because it allowed us to obtain tissues that are more genetically homogeneous. Compared with WT home cage controls, RARα KO home cage CA1 neurons contained significantly higher levels of GluA1, but not of GluA2 (Fig. 4B), which is consistent with our previous findings that RARα regulates GluA1, but not GluA2, synthesis (5, 37). EE experience did not alter expression levels of GluA1 or GluA2 in the CA1 region of WT mice (Fig. 4C), but dramatically elevated the levels of both GluA1 and GluA2 in CaMKII-Cre RARα KO mice (Fig. 4D). By contrast, expression levels of excitatory postsynaptic scaffold proteins, such as PSD95 and SAP97, remained unperturbed (SI Appendix, Fig. S7). Thus, the RARα deletion enhanced GluA1, but not GluA2, levels under impoverished home cage conditions, and further increased both GluA1 and GluA2 levels under more natural EE conditions, suggesting that synaptic RA signaling operating on RARα normally serves as a break on GluA1 and GluA2 synthesis and correlating with the altered synaptic plasticity observed under these conditions.
Overactive mTOR Signaling Underlies Enhanced AMPAR Expression, LTP, and Fear Memory in EE-Experienced RARα KO Mice.
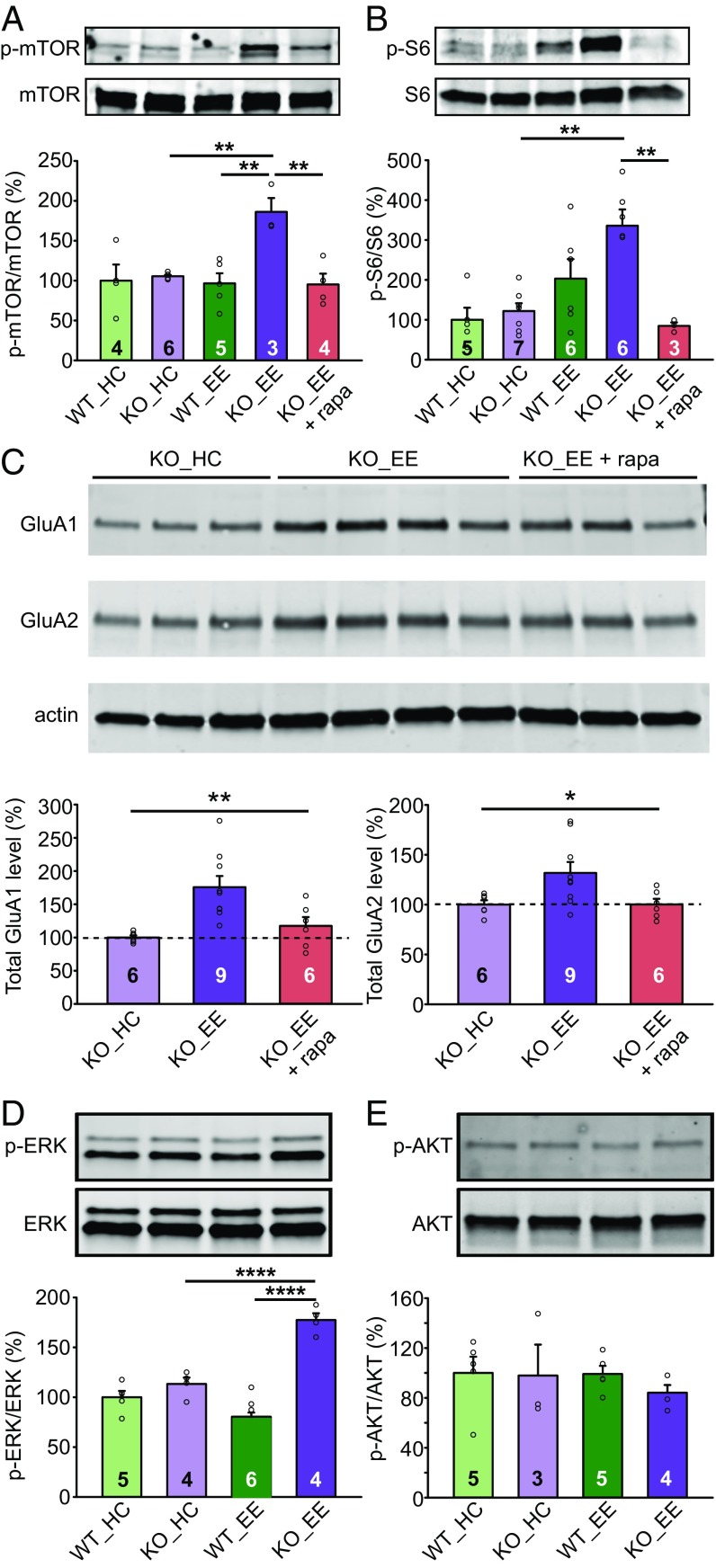
We next asked whether the observed concomitant increases in GluA1 and GluA2 expression in CaMKII-Cre::RARαfl/fl mice are due to RARα’s regulation of protein synthesis pathways. For this purpose, we examined mTOR complex 1 (mTORC1) activation in CA1 region samples. Strikingly, samples from CaMKII-Cre::RARαfl/fl mice, but not WT mice, with EE experience exhibited significantly higher levels of mTOR activation (mTOR S2448 phosphorylation normalized to total mTOR levels; Fig. 5A), as well as stronger activation of the best-characterized downstream mTOR effector, the p70 ribosomal S6 protein kinase, quantified via phosphorylation of the ribosomal protein S6 (Fig. 5B). Treating mice with rapamycin (5-mg/kg daily injection) during the entire EE experience blocked the effect of the EE experience on mTOR activation and S6 phosphorylation in CA1 tissue of CaMKII-Cre::RARαfl/fl mice (Fig. 5 A and B) and restored GluA1 and GluA2 expression levels back to normal (Fig. 5C). These results suggest that in the absence of synaptic RA signaling, EE experience results in increased activation of mTOR that then causes increased GluA1 and GluA2 expression.
Fig. 5.
Overactive mTOR signaling enhances AMPAR expression in EE-experienced CA1-RARα KO mice. (A) Immunoblot analysis of mTOR and phospho-mTOR in home cage (HC)- and EE-exposed WT and CaMKII-Cre RARα KO CA1 [one-way ANOVA with Tukey’s multiple comparison test: F(4,17) = 6.857, P = 0.0018, **P < 0.01]. (B) Immunoblot analysis of ribosomal protein S6 and phospho-S6 in CA1 [one-way ANOVA with Tukey’s multiple comparison test: F(4,22) = 8.143, P = 0.0003, **P < 0.01]. (C) Rapamycin treatment during EE experience restores AMPAR levels back to normal in the CA1 of RARα cKO mice. Immunoblot analysis of total GluA1 and GluA2 expression in HC- and EE-experienced RARα KO CA1 with and without rapamycin [one-way ANOVA with Tukey’s multiple comparison test: GluA1: F(2,18) = 8.433, P = 0.0026; GluA2: F(2,18) = 4.558, P = 0.025, *P < 0.05, **P < 0.01]. (D) Immunoblot analysis of MAP kinase ERK and phospho-ERK in CA1 [two-way ANOVA test: HC/EE × WT/KO: F(1,15) = 36.51, P < 0.0001; Tukey post hoc test: ****P < 0.0001]. (E) Immunoblot analysis of AKT and phospho-AKT in CA1 [two-way ANOVA test: HC/EE × WT/KO: F(1,13) = 0.2659, P > 0.5]. All graphs represent mean ± SEM.
Multiple cellular inputs can activate mTORC1 to promote growth and energy storage (38). Downstream signaling from these inputs mainly converges on ERK and on AKT (also known as protein kinase B), both of which inhibit the activity of the tuberous sclerosis complex, which is a suppressor of mTORC1. We found that phosphorylation of ERK, but not of AKT, is selectively elevated in the EE-exposed CA1 region from CaMKII-Cre::RARafl/fl mice (Fig. 5 D and E). Thus, in the absence of RARα, environmental stimulation leads to enhanced activation of mTORC1 via the ERK pathway.
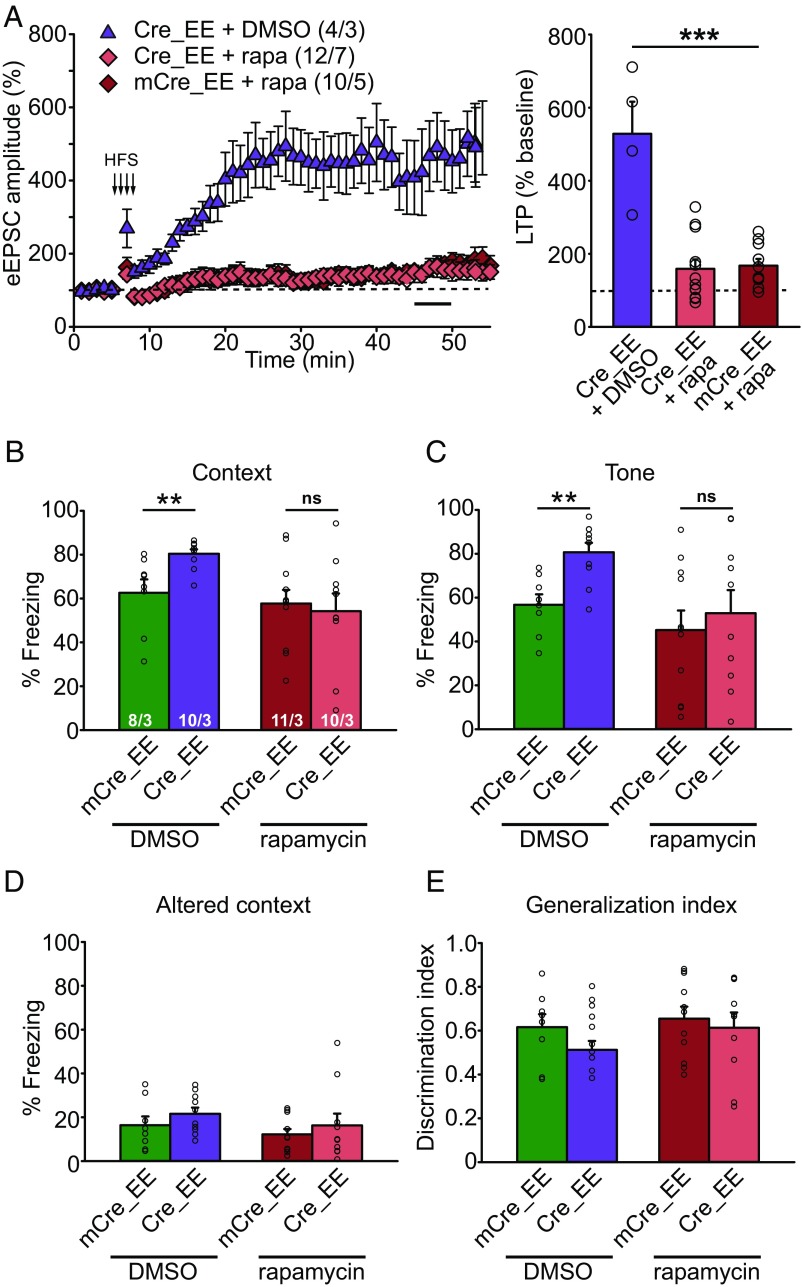
To test the causality between overactive mTOR signaling and enhanced LTP and learning in CA1-specific RARα KO EE animals, we administered daily injections of 5 mg/kg of rapamycin during 10 d of EE experience, which was sufficient to block mTOR activation (Fig. 5 A and B), and tested LTP and fear conditioning learning 24 h after the last injection. Whereas slices from CA1-RARα KO mice with EE experience and DMSO injection exhibited greatly enhanced LTP as expected, rapamycin injection reversed the greater LTP back to normal (Fig. 6A). Additionally, enhanced fear memory in the CA1-RARα KO EE animals was blocked by rapamycin treatment without affecting fear generalization (Fig. 6 B–E). Together, we conclude that activation of mTOR signaling contributes to enhanced LTP and learning in the RARα KO EE animals.
Fig. 6.
Rapamycin treatment in CA1-RARα KO mice during EE experience reverses LTP and fear memory to normal levels. (A) SC-CA1 LTP in EE-experienced WT and CA1-RARα KO mice treated with rapamycin (rapa) during an EE experience [one-way ANOVA with Tukey’s multiple comparison test: F(2,23) = 24.80, P < 0.0001, ***P < 0.001]. n/N, number of neurons/number of animals. (B–E) Behavioral quantification of contextual and cued fear conditioning in DMSO- or rapamycin-treated WT and CA1-RARα KO mice with EE experience. n/N, number of mice/number of independent litters. (B) Quantification of contextual memory (two-tailed Mann–Whitney U test with Bonferroni correction: **P < 0.01). ns, not significant. (C) Quantification of cued memory (two-tailed Mann–Whitney U test with Bonferroni correction: **P < 0.01). (D) Quantification of freezing in an altered context. (E) Quantification of memory generalization. All graphs represent mean ± SEM.
Discussion
As one of the best-studied developmental morphogens, RA is known to modulate gene transcription through nuclear receptors during embryogenesis (12). The discovery of a nongenomic, postdevelopmental action of RA in homeostatic plasticity in mature brain expands the functional repertoire of RA and its receptors (39). In particular, RARα, which is highly expressed in adult brain, has been found to repress synthesis of specific proteins, most prominently AMPARs, via binding to their mRNAs (14, 15). Deletion of RARα impairs synaptic RA signaling and homeostatic synaptic plasticity, but does not affect Hebbian plasticity directly (5, 16, 24). Previous work had focused on RA signaling via RARα in the context of synaptic inactivity-induced homeostatic synaptic plasticity. However, RARα’s function on its own in the absence of RA (i.e., when synaptic activity is not chronically suppressed) has never been explored in an intact circuit. In this study, we examined the in vivo impact of the RARα deletion on basal synaptic transmission, on hippocampal plasticity, and on learning and memory. A key behavioral element introduced in this study is an EE experience that better resembles a normal experience of mice than standard and relatively impoverished home cage conditions. An EE enhances behaviorally relevant sensory input and provides structured cortical and hippocampal circuit activations. Most previous EE experiments were performed in young animals (3–4 wk old) for prolonged periods of time (1–3 mo). The prolonged EE experience paradigm produced mixed effects on basal synaptic transmission (22) and has been reported to enhance learning and social interactions and to improve cognitive performances (40), likely due to developmental effects in young animals on synaptic plasticity and potential engagement of multiple forms of synaptic plasticity. For the purpose of this study, we thus chose a short-term (10 d) EE experience in young adult mice to minimize developmental effects induced by an EE, thereby allowing a more focused investigation of how RARα, operating in a relatively stable and functionally mature circuit, impacts synaptic plasticity in an activity-dependent manner.
Results from our study suggest four major conclusions. First, RARα plays a significant role in downward homeostatic synaptic plasticity. Consistent with its previously reported action on translational repression of GluA1, RARα deletion led to elevated basal levels of GluA1 protein and blocked downward homeostatic plasticity in mature neurons. Thus, unlike its action in upward homeostatic plasticity, which is to trigger local mRNA translation upon RA synthesis during chronically suppressed synaptic activity, RARα is required in downward homeostatic plasticity as a permissive signal by keeping basal AMPAR expression levels in check when synaptic activity is elevated. Second, RARα-mediated homeostatic plasticity prevents runaway Hebbian plasticity. This is evidenced by greatly enhanced LTP after EE experience in RARα-deficient, but not WT, neurons. Moreover, EE experience enhanced LTD in an RARα-dependent manner, suggesting that RARα may also play essential roles in constraining LTD. Note that deletion of RARα does not directly alter Hebbian plasticity in the absence of chronic activity manipulations, but shifts the BCM curve when the affected circuit is challenged with altered activity. Thus, RARα-dependent homeostatic plasticity may be operating as a form of metaplasticity mechanism. Third, we demonstrated that shifting Hebbian rules have behavioral consequences. EE experience not only impacts Hebbian plasticity but also strongly influences the strength and flexibility of learning, both in an RARα-dependent manner. Fourth, we uncovered a previously undescribed connection between RARα and mTOR signaling. Deletion of RARα permitted hyperactive ERK/mTOR activation by EE experience, which leads to greatly enhanced AMPAR synthesis and runaway LTP (Fig. 7). Importantly, blocking hyperactive mTOR signaling with daily administration of rapamycin in hippocampal RARα KO mice during an EE experience restored both LTP and learning levels back to normal.
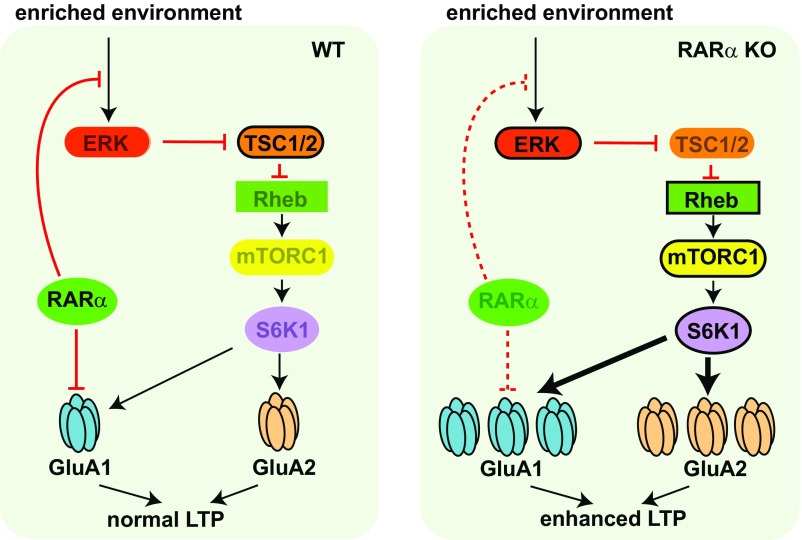
Fig. 7.
Schematic model depicting RARα’s involvement in regulation of intracellular signaling, protein synthesis, and synaptic plasticity in postsynaptic neurons. In addition to its known function in suppressing GluA1 protein synthesis, RARα, through an unknown mechanism, clamps the ERK/mTOR activation pathway in response to intracellular/extracellular inputs triggered by environmental enrichment, thus stabilizing LTP. In the absence of RARα, GluA1 protein synthesis is derepressed. Moreover, the ERK/mTOR pathway is overactivated by environmental enrichment, leading to enhanced GluA1 and GluA2 synthesis and greater LTP. Rheb, Ras homolog enriched in brain.
Perhaps the most unexpected finding in our study is the fact that normal RARα expression is required for stable synaptic function when synaptic activity is enhanced by EE experience. Largely influenced by our finding that RARα acts as an mRNA-binding protein that suppresses translation of target mRNAs and that RA disinhibits protein synthesis upon binding to RARα (15), our previous conceptual framework concerning RARα’s function in mature neurons had been centered on this RA-regulated inhibition of protein synthesis. Results from the current study, however, suggest that RARα’s function in controlling protein synthesis also has a significant biological role during active synaptic transmission (thus, in the absence of RA); that is, RARα broadly regulates protein synthesis. Importantly, RARα not only regulates the expression levels of proteins synthesized from its target mRNAs (e.g., GluA1) but also contributes to clamping overall mTOR-dependent protein synthesis by acting on ERK via a previously unidentified signaling pathway. Thus, while RARα participates in homeostatic upscaling through RA-dependent translational derepression, its action in homeostatic downscaling may be independent of RA. Instead, RARα may mediate homeostatic downscaling through direct regulation of an mTOR activation pathway that then limits the pool of AMPARs available for synaptic insertion during LTP induction. Another prime example demonstrating the significance of maintaining normal basal protein synthesis levels is the function of Fragile X mental retardation protein (FMRP), whose primary role is identified as an mRNA-binding protein that suppresses mRNA translation (41–43). In the case of Fragile X syndrome (FXS), where FMRP expression is absent, dysregulated protein synthesis leads to multifaceted pathophysiology, including altered synaptic plasticity (44). Interestingly, in Fmr1 KO mice and in human FMR1 mutant neurons, synaptic RA signaling is completely lost (11, 37, 45), raising the intriguing possibility that compromised RARα function contributes to some of the synaptic and behavioral phenotypes in FXS. Further investigation is required to dissect the molecular mechanism of functional interactions between RARα and FMRP.
Proposed in the 1940s by Donald Hebb, Hebbian plasticity is probably the most well-known theory for associative learning, basically postulating that learning involves input-specific, activity-induced, long-term changes in synaptic strength (i.e., synaptic plasticity) (46). The BCM model introduced the concept of a modification threshold, θM, which is thought to dictate the direction of the synaptic strength change (strengthening or weakening) in response to neuronal activity (25). Physiological evidence has since validated the BCM model by showing that the activity history of a neuron determines its current biochemical state and its ability to undergo future synaptic plasticity (47, 48), a phenomenon now referred to as metaplasticity, the plasticity of synaptic plasticity (49). Homeostatic plasticity, conversely, is a distinct mechanism that stabilizes neural networks through negative feedback-based modifications. Functionally, Hebbian and homeostatic plasticity are thought to achieve distinct purposes (associative learning versus network stability). However, the induction and expression of these two forms of plasticity often involve modulation of the same biological parameters. Thus, it may not be surprising that Hebbian and homeostatic plasticity robustly interact at the biochemical, molecular, and structural levels, and that homeostatic plasticity strongly impacts Hebbian plasticity within the same network (8, 9, 16, 24). Given its purpose of stabilizing neural systems, we suggest that homeostatic plasticity may serve as a form of Hebbian metaplasticity. Results from our present study reveal the in vivo interactions between RARα-dependent homeostatic plasticity and Hebbian plasticity, suggest the RARα/mTOR signaling pathway as a crucial molecular mechanism supporting such interactions, and demonstrate the robust influence of synaptic RARα signaling on Hebbian rules for synaptic long-term plasticity and behavioral learning. Thus, our study establishes that RARα-mediated synaptic regulation is not only a major determinant of synaptic strength in vivo, but that such regulation is also behaviorally relevant in a defined circuit, namely, the hippocampal networks mediating contextual learning.
Experimental Procedures
RARα floxed mice (C57BL/6 background) were a generous gift from Pierre Chambon and Norbert Ghyselinck, Institute of Genetics, Molecular and Cellular Biology, Strasbourg, France. Breeding colonies are maintained in the animal facility at Stanford Medical School following standard procedures approved by the Stanford University Administrative Panel on Laboratory Animal Care. The homozygous RARα floxed mice were bilaterally stereotaxically injected with AAVs expressing Cre recombinase or a truncated and inactive version of Cre (mCre). Mice were group-housed with littermates and maintained under a 12/12-h daylight cycle. Animal experiments were conducted following protocols approved by the APLAC at Stanford University.
Full experimental procedures and associated references are available in SI Appendix.
Supplementary Material
Acknowledgments
We thank members of the L.C. laboratory for comments and help during the course of the study. The work was supported by NIH Grants MH086403 (to L.C.), MH091193 (to L.C.), HD084215 (to L.C.), and MH086403 (to T.C.S.) and by a postdoctoral fellowship from the Stanford Maternal and Child Health Research Institute (to J.L.).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 6528.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1820690116/-/DCSupplemental.
References
- 1.Marder E, Prinz AA. Current compensation in neuronal homeostasis. Neuron. 2003;37:2–4. doi: 10.1016/s0896-6273(02)01173-x. [DOI] [PubMed] [Google Scholar]
- 2.Turrigiano G. Homeostatic synaptic plasticity: Local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol. 2012;4:a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis GW, Müller M. Homeostatic control of presynaptic neurotransmitter release. Annu Rev Physiol. 2015;77:251–270. doi: 10.1146/annurev-physiol-021014-071740. [DOI] [PubMed] [Google Scholar]
- 4.Vitureira N, Letellier M, Goda Y. Homeostatic synaptic plasticity: From single synapses to neural circuits. Curr Opin Neurobiol. 2012;22:516–521. doi: 10.1016/j.conb.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang HL, Zhang Z, Hintze M, Chen L. Decrease in calcium concentration triggers neuronal retinoic acid synthesis during homeostatic synaptic plasticity. J Neurosci. 2011;31:17764–17771. doi: 10.1523/JNEUROSCI.3964-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arendt KL, et al. Calcineurin mediates homeostatic synaptic plasticity by regulating retinoic acid synthesis. Proc Natl Acad Sci USA. 2015;112:E5744–E5752. doi: 10.1073/pnas.1510239112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keck T, et al. Integrating Hebbian and homeostatic plasticity: The current state of the field and future research directions. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160158. doi: 10.1098/rstb.2016.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yee AX, Hsu YT, Chen L. A metaplasticity view of the interaction between homeostatic and Hebbian plasticity. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160155. doi: 10.1098/rstb.2016.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarti F, Schroeder J, Aoto J, Chen L. Conditional RARα knockout mice reveal acute requirement for retinoic acid and RARα in homeostatic plasticity. Front Mol Neurosci. 2012;5:16. doi: 10.3389/fnmol.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarti F, Zhang Z, Schroeder J, Chen L. Rapid suppression of inhibitory synaptic transmission by retinoic acid. J Neurosci. 2013;33:11440–11450. doi: 10.1523/JNEUROSCI.1710-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 13.Huang H, et al. Changes in the expression and subcellular localization of RARalpha in the rat hippocampus during postnatal development. Brain Res. 2008;1227:26–33. doi: 10.1016/j.brainres.2008.06.073. [DOI] [PubMed] [Google Scholar]
- 14.Maghsoodi B, et al. Retinoic acid regulates RARalpha-mediated control of translation in dendritic RNA granules during homeostatic synaptic plasticity. Proc Natl Acad Sci USA. 2008;105:16015–16020. doi: 10.1073/pnas.0804801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poon MM, Chen L. Retinoic acid-gated sequence-specific translational control by RARalpha. Proc Natl Acad Sci USA. 2008;105:20303–20308. doi: 10.1073/pnas.0807740105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arendt KL, et al. Retinoic acid and LTP recruit postsynaptic AMPA receptors using distinct SNARE-dependent mechanisms. Neuron. 2015;86:442–456. doi: 10.1016/j.neuron.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 18.Mayford M, Siegelbaum SA, Kandel ER. Synapses and memory storage. Cold Spring Harb Perspect Biol. 2012;4:a005751. doi: 10.1101/cshperspect.a005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 20.Mitsushima D, Ishihara K, Sano A, Kessels HW, Takahashi T. Contextual learning requires synaptic AMPA receptor delivery in the hippocampus. Proc Natl Acad Sci USA. 2011;108:12503–12508. doi: 10.1073/pnas.1104558108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eichenbaum H. On the integration of space, time, and memory. Neuron. 2017;95:1007–1018. doi: 10.1016/j.neuron.2017.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckert MJ, Abraham WC. Effects of environmental enrichment exposure on synaptic transmission and plasticity in the hippocampus. Curr Top Behav Neurosci. 2013;15:165–187. doi: 10.1007/7854_2012_215. [DOI] [PubMed] [Google Scholar]
- 23.Chapellier B, et al. A conditional floxed (loxP-flanked) allele for the retinoic acid receptor alpha (RARalpha) gene. Genesis. 2002;32:87–90. doi: 10.1002/gene.10071. [DOI] [PubMed] [Google Scholar]
- 24.Arendt KL, Sarti F, Chen L. Chronic inactivation of a neural circuit enhances LTP by inducing silent synapse formation. J Neurosci. 2013;33:2087–2096. doi: 10.1523/JNEUROSCI.3880-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. Eur J Pharmacol. 2003;463:217–223. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- 27.Josselyn SA, Köhler S, Frankland PW. Finding the engram. Nat Rev Neurosci. 2015;16:521–534. doi: 10.1038/nrn4000. [DOI] [PubMed] [Google Scholar]
- 28.Tonegawa S, Liu X, Ramirez S, Redondo R. Memory engram cells have come of age. Neuron. 2015;87:918–931. doi: 10.1016/j.neuron.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Sutherland RJ, O’Brien J, Lehmann H. Absence of systems consolidation of fear memories after dorsal, ventral, or complete hippocampal damage. Hippocampus. 2008;18:710–718. doi: 10.1002/hipo.20431. [DOI] [PubMed] [Google Scholar]
- 30.Quinn JJ, Wied HM, Ma QD, Tinsley MR, Fanselow MS. Dorsal hippocampus involvement in delay fear conditioning depends upon the strength of the tone-footshock association. Hippocampus. 2008;18:640–654. doi: 10.1002/hipo.20424. [DOI] [PubMed] [Google Scholar]
- 31.Nicholls RE, et al. Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron. 2008;58:104–117. doi: 10.1016/j.neuron.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 32.Mills F, et al. Cognitive flexibility and long-term depression (LTD) are impaired following β-catenin stabilization in vivo. Proc Natl Acad Sci USA. 2014;111:8631–8636. doi: 10.1073/pnas.1404670111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JI, et al. PI3Kγ is required for NMDA receptor-dependent long-term depression and behavioral flexibility. Nat Neurosci. 2011;14:1447–1454. doi: 10.1038/nn.2937. [DOI] [PubMed] [Google Scholar]
- 34.Wyllie DJ, Manabe T, Nicoll RA. A rise in postsynaptic Ca2+ potentiates miniature excitatory postsynaptic currents and AMPA responses in hippocampal neurons. Neuron. 1994;12:127–138. doi: 10.1016/0896-6273(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 35.Kato HK, Watabe AM, Manabe T. Non-Hebbian synaptic plasticity induced by repetitive postsynaptic action potentials. J Neurosci. 2009;29:11153–11160. doi: 10.1523/JNEUROSCI.5881-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: The last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soden ME, Chen L. Fragile X protein FMRP is required for homeostatic plasticity and regulation of synaptic strength by retinoic acid. J Neurosci. 2010;30:16910–16921. doi: 10.1523/JNEUROSCI.3660-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen L, Lau AG, Sarti F. Synaptic retinoic acid signaling and homeostatic synaptic plasticity. Neuropharmacology. 2014;78:3–12. doi: 10.1016/j.neuropharm.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 41.Bassell GJ, Warren ST. Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet. 2001;10:329–338. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- 43.Li Z, et al. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 2001;29:2276–2283. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfeiffer BE, Huber KM. The state of synapses in fragile X syndrome. Neuroscientist. 2009;15:549–567. doi: 10.1177/1073858409333075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, et al. The fragile X mutation impairs homeostatic plasticity in human neurons by blocking synaptic retinoic acid signaling. Sci Transl Med. 2018;10:eaar4338. doi: 10.1126/scitranslmed.aar4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hebb DO. The Organization of Behavior: A Neuropsychological Theory. Wiley; New York: 1949. [Google Scholar]
- 47.Christie BR, Abraham WC. Priming of associative long-term depression in the dentate gyrus by theta frequency synaptic activity. Neuron. 1992;9:79–84. doi: 10.1016/0896-6273(92)90222-y. [DOI] [PubMed] [Google Scholar]
- 48.Huang YY, Colino A, Selig DK, Malenka RC. The influence of prior synaptic activity on the induction of long-term potentiation. Science. 1992;255:730–733. doi: 10.1126/science.1346729. [DOI] [PubMed] [Google Scholar]
- 49.Abraham WC, Bear MF. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.