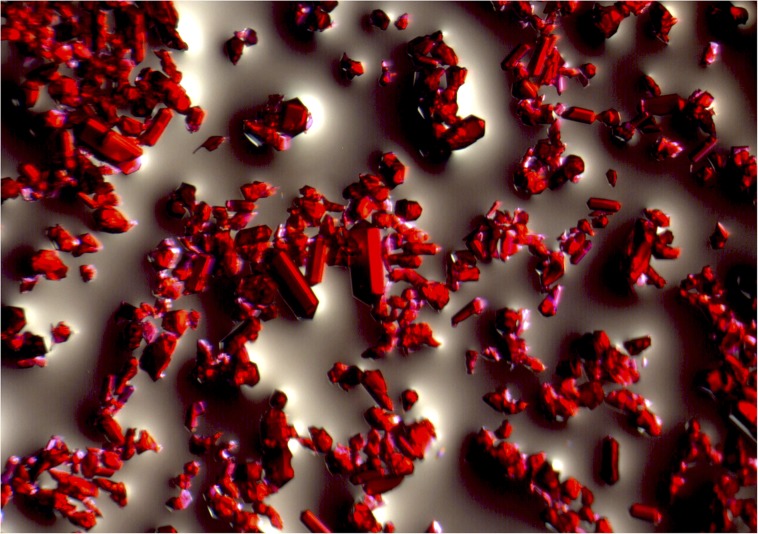
The grandness of metal–organic frameworks (MOFs) lies hidden beneath an unremarkable exterior. To the naked eye, MOFs typically look like little more than a fine powder, not all that different from flour. But place them under an ordinary optical microscope to reveal their crystalline structure, and you see what looks like a forest of gems. Place them under a scanning electron microscope, and you might see not only crystals but numerous pores.
These red crystals (magnification 10x), a type of MOF called cobalt zeolitic imidazolate framework-8 (Co ZIF-8), have several desirable properties, including the ability to capture and store copious amounts of carbon dioxide. Image credit: Omar Yaghi.
It’s those pores that make MOFs so special. The amazing number of cavities affords MOFs a huge internal surface area. Unfolding just 7 grams of the holeyest MOF would create an area on par with the 77,000-square-meter floor space of Buckingham Palace.
For 2 decades, MOFs have been lauded for their ability to host atoms and molecules within those copious pores. The first commercial products to use MOFs, launched in 2016, offer a way to store gases more efficiently than conventional containers. And other MOF applications are in the pipeline, including gas purification, catalysis, and even the ability to suck water out of desert air.
Now MOFs are making headway in medical applications. In July 2018, RiMO Therapeutics, a University of Chicago spinoff, announced that it had injected what it believes to be the first patient with a MOF-based therapy as part of a phase I clinical trial to treat advanced tumor growth (1, 2). Many researchers are developing such medi-MOFs, which could carry concentrated doses of pharmaceuticals inside patients and then gradually release their cargo. Others are using MOFs to enhance medical devices or even integrating biological molecules into the MOF’s structure to create what they call bio-MOFs.
“The biological and medical applications of MOFs are developing quickly,” says chemist Omar Yaghi at University of California, Berkeley, who has pioneered MOF research. “They are ideally suited for having a well-defined molecular interface between the synthetic and the living worlds.”
Storage Solutions
The structure of a MOF resembles the 3D scaffolds made from a ball-and-stick magnetic construction toy. Inside the MOF, metal-based clusters act as nodes that are held together by carbon-based linker molecules, creating large pores connected by channels that crisscross the material.
The first robust MOFs were unveiled in 1999: MOF-5 emerged from Yaghi’s lab, and HKUST-1 was built by chemist Ian Williams at Hong Kong University of Science and Technology in Kowloon (3, 4). MOF-5 had an internal surface area of 2,900 square meters per gram of material, breaking all previous porosity records in other materials, says Yaghi. Steady improvements since then have produced MOFs with internal surface areas more than 10,000 square meters per gram (5). They are much more porous than zeolites, a class of inorganic materials with open crystal structures that are commonly used as catalysts, water softeners, and even cat litter. “MOFs are more porous because their pores have no walls—they are scaffolds,” Yaghi says.
One of the main attractions of MOFs is that chemists have an almost unlimited toolbox of clusters and linkers they can use to build new MOFs, which allows them to fine-tune their properties for specific functions. At least 70,000 MOFs are now known, according to Yaghi. He has explored MOFs for storing hydrogen and methane as transportation fuels and capturing carbon dioxide from the exhaust gases of power plants, for example. These applications take advantage of interactions between gas molecules and the surfaces of the MOF’s pores, which cram the gas molecules closer together. The upshot is that a MOF-based gas storage container can hold much more gas than an empty vessel of the same size.
Yaghi hasn’t commercialized this work yet, but two other MOF-based products for gas storage have reached the market (6). The ION-X gas cylinder was developed by NuMat Technologies, a company spun off from Omar Farha’s chemistry lab at Northwestern University in Evanston, IL. The cylinder uses a MOF to safely store toxic gases used in the electronics industry. Meanwhile, MOF Technologies in Belfast, United Kingdom, has launched Trupick, a sachet containing a MOF with the gas 1-methylcyclopropene (1-MCP) in its pores. 1-MCP inhibits ripening in fruit and is commonly released into warehouses to prolong the shelf life of fresh food.
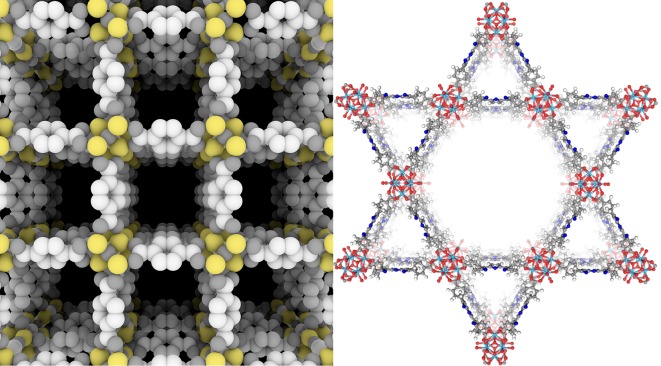
This MOF model (Left) illustrates the materials’ highly porous, crystalline composition. Researchers have started to adapt MOFs such as this one (Right) to biological applications. The structure of 5,10,15,20-tetra(p-benzoato)porphyrin-Hf (TBP-Hf) was designed to host an anticancer immunotherapy agent. Image credit: ScienceSource/USTC Institute of Advanced Technology & Tsinghua University Press (Left) and Wenbin Lin (Right).
Controlled Release
The ability of MOFs to store and release molecules isn’t restricted to gases—it also works with medicines. Gérard Férey and Patricia Horcajada at the Versailles Lavoisier Institute in Paris unveiled the first successful laboratory demonstration of this in 2006, using MOFs that could slowly release ibuprofen over a couple days (7). Controlled drug release can allow patients to take less frequent doses, and MOFs have potential here for two reasons: They can hold a lot of drug molecules inside their pores, and their structures can be tailored to control how quickly the drugs are released.
Fast forward a dozen years, and MOFs are moving from the laboratory to the clinic. Chemist Wenbin Lin at the University of Chicago developed a treatment called RiMO-301 that is being trialed by his company RiMO Therapeutics as a radiation enhancer (8). Lin’s team will inject the MOF into patients’ tumors to increase the destructive power of therapeutic X-rays, boosting their efficiency while reducing overall radiation doses and the related side effects. Other types of radiation enhancers have been explored in recent years but have not yet been approved for clinical use.
RiMO-301 is built from hafnium oxide clusters and ruthenium-containing organic linkers. The MOF absorbs X-ray energy and uses it to generate two different reactive oxygen species, which attack the mitochondria in nearby cells to help kill the tumor. In mouse studies, Lin’s team reported a significant reduction in the size of colorectal tumors using their MOF. Localizing the MOF within the tumor means that the therapy can use very low X-ray doses, producing no side effects. “RiMO-301 did not show any toxicity in rats and dogs in studies,” Lin explains.
The pores in RiMO-301 are empty, but Lin has filled a similar MOF with an anticancer immunotherapy agent to create a treatment that attacks tumors with a one-two punch (9, 10). Tests in mice showed that this MOF not only lowered the X-ray dose needed but also helped destroy nearby tumor cells not exposed to X-rays. Lin says he hopes to advance this MOF into clinical trials soon.
Meanwhile, Horcajada, now at the IMDEA Energy Institute in Madrid, has been working on an orally administered MOF called MIL-127 that could treat aspirin overdose. It works by absorbing aspirin in a victim’s stomach before the drug can enter the bloodstream. The concept has been proven in rats, where the MOF reduced the amount of aspirin absorbed more than 40-fold (11).
MOFs could also make an impact as functionalized coatings for medical devices (12). Russell Morris’s chemistry group at the University of St Andrews in the United Kingdom is developing coatings using MOFs that carry nitric oxide (NO). NO acts, for example, as a signaling molecule for the formation of new blood vessels and as a natural defense against bacteria. Morris’s group is embedding NO-filled MOFs in bandages to promote wound healing (13) and coating stents and catheters with the material so that NO can prevent blood clots and reduce infection risk. His spin-off company MOFgen is currently raising funds to begin registering NO-filled MOFs for use in medical devices.
Biomolecular Building Blocks
Toxicity is a major consideration when designing a MOF for use in biotech, though selecting the right components should mitigate concerns. “Using biomolecules as MOF constituents can potentially lower the toxicity of the degradation products and improve biocompatibility,” explains chemist Nathaniel Rosi at the University of Pittsburgh, noting that MOFs will degrade in vivo for some applications. Rosi has used a component of DNA called adenine as a MOF linker (14, 15), and others are exploring linkers made of amino acids and peptides. At the University of California, San Diego, Akif Tezcan has used proteins in lieu of the traditional metal-containing clusters (16, 17). Tezcan’s goal is to use the MOF’s structure to help proteins construct themselves without outside guidance. “We took the synthetic modularity of MOFs and exploited that to control protein self-assembly,” he says.
MOFs are also being explored as a way to encapsulate—and therefore protect—delicate biomolecules. Some enzymes, for example, can catalyze industrial chemical reactions and offer huge practical advantages over the more standard inorganic catalysts. But those enzymes, despite their potential utility, often aren’t stable enough. MOF protection could potentially enable industry to utilize many more enzymes in a wider range of chemical processes.
Encapsulation could also carry biologic drugs through the harsh environment of the stomach before the MOF releases its payload into the bloodstream. “The ability to make MOFs that are stable under certain conditions, but unstable under other conditions to allow the release of the enzymes, is much easier with MOFs than other [porous] materials,” Northwestern’s Farha explains. His lab recently reported encapsulating the hormone insulin in a MOF, with the end goal of developing an oral diabetes drug (18), but he concedes that readying the treatment for therapeutic use will require a lot more research.
Much of the work on biological applications of MOFs is still in its early stages, so chemists are increasingly looking to biologists and biomedical engineers to help guide their efforts. “I believe they might play an important role in moving the field forward,” notes Lin.
Perhaps the largest challenge that MOFs will face in their charge onto the biotech scene is finding acceptance in a naturally cautious market. “But for those applications where capacity is important,” predicts Morris, “then MOFs should be the first class of material on the team sheet.”
References
- 1.RiMO Therapeutics Inc 2018 First patient enrolled in phase 1 study of RiMO-301. Available at www.rimorx.com/2018/07/press-release-july-13-2018/. Accessed December 21, 2018.
- 2.ClinicalTrials.gov 2018 Phase I study of RiMO-301 with radiation in advanced tumors. Available at clinicaltrials.gov/ct2/show/NCT03444714. Accessed December 21, 2018.
- 3.Li H, et al. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature. 1999;402:276–279. [Google Scholar]
- 4.Chui SSY, Lo SM, Charmant JP, Orpen AG, Williams ID. A chemically functionalizable nanoporous material. Science. 1999;283:1148–1150. doi: 10.1126/science.283.5405.1148. [DOI] [PubMed] [Google Scholar]
- 5.Farha OK, et al. Metal-organic framework materials with ultrahigh surface areas: Is the sky the limit? J Am Chem Soc. 2012;134:15016–15021. doi: 10.1021/ja3055639. [DOI] [PubMed] [Google Scholar]
- 6.Frameworks for commercial success. Nat Chem. 2016;8:987. doi: 10.1038/nchem.2661. [DOI] [PubMed] [Google Scholar]
- 7.Horcajada P, et al. Metal-organic frameworks as efficient materials for drug delivery. Angew Chem Int Ed Engl. 2006;45:5974–5978. doi: 10.1002/anie.200601878. [DOI] [PubMed] [Google Scholar]
- 8.Ni K, et al. Nanoscale metal-organic frameworks for mitochondria-targeted radiotherapy-radiodynamic therapy. Nat Commun. 2018;9:4321. doi: 10.1038/s41467-018-06655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu K, et al. Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nat Biomed Eng. 2018;2:600–610. doi: 10.1038/s41551-018-0203-4. [DOI] [PubMed] [Google Scholar]
- 10.Ni K, et al. Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nat Commun. 2018;9:2351. doi: 10.1038/s41467-018-04703-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rojas S, et al. Metal–organic frameworks as efficient oral detoxifying agents. J Am Chem Soc. 2018;140:9581–9586. doi: 10.1021/jacs.8b04435. [DOI] [PubMed] [Google Scholar]
- 12.Gregg ST, et al. Functionalised solids delivering bioactive nitric oxide gas for therapeutic applications. Mater Today Commun. 2017;12:95–105. [Google Scholar]
- 13.Xiao B, et al. High-capacity hydrogen and nitric oxide adsorption and storage in a metal-organic framework. J Am Chem Soc. 2007;129:1203–1209. doi: 10.1021/ja066098k. [DOI] [PubMed] [Google Scholar]
- 14.An J, Geib SJ, Rosi NL. Cation-triggered drug release from a porous zinc-adeninate metal-organic framework. J Am Chem Soc. 2009;131:8376–8377. doi: 10.1021/ja902972w. [DOI] [PubMed] [Google Scholar]
- 15.Li T, et al. Systematic modulation and enhancement of CO2:N2 selectivity and water stability in an isoreticular series of bio-MOF-11 analogues. Chem Sci. 2013;4:1746–1755. [Google Scholar]
- 16.Sontz PA, Bailey JB, Ahn S, Tezcan FA. A metal organic framework with spherical protein nodes: Rational chemical design of 3D protein crystals. J Am Chem Soc. 2015;137:11598–11601. doi: 10.1021/jacs.5b07463. [DOI] [PubMed] [Google Scholar]
- 17.Bailey JB, Zhang L, Chiong JA, Ahn S, Tezcan FA. Synthetic modularity of protein–metal–organic frameworks. J Am Chem Soc. 2017;139:8160–8166. doi: 10.1021/jacs.7b01202. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Li P, Modica JA, Drout RJ, Farha OK. Acid-resistant mesoporous metal–organic framework toward oral insulin delivery: Protein encapsulation, protection, and release. J Am Chem Soc. 2018;140:5678–5681. doi: 10.1021/jacs.8b02089. [DOI] [PubMed] [Google Scholar]