Significance
In litter-bearing species, females exposed to prenatal testosterone from male littermates exhibit altered traits. In humans, rising twinning rates may be exposing a growing subset of females to similar effects. Data on all twin births in Norway between 1967 and 1978 show that females exposed in utero to a male co-twin have a decreased probability of graduating from high school (15.2%), completing college (3.9%), and being married (11.7%), and have lower fertility (5.8%) and life-cycle earnings (8.6%). These relationships remain unchanged among females whose male co-twin died soon after birth, implicating prenatal testosterone exposure rather than being raised with a male sibling. These findings support the hypothesis that being exposed to a male co-twin in utero can have lasting effects on females.
Keywords: fetal development, steroids, education, earnings, fertility
Abstract
During sensitive periods in utero, gonadal steroids help organize biological sex differences in humans and other mammals. In litter-bearing species, chromosomal females passively exposed to prenatal testosterone from male littermates exhibit altered physical and behavioral traits as adults. The consequences of such effects are less well understood in humans, but recent near-doubling of twinning rates in many countries since 1980, secondary to advanced maternal age and increased reliance on in vitro fertilization, means that an increasing subset of females in many populations may be exposed to prenatal testosterone from their male co-twin. Here we use data on all births in Norway (n = 728,842, including 13,800 twins) between 1967 and 1978 to show that females exposed in utero to a male co-twin have a decreased probability of graduating from high school (15.2%), completing college (3.9%), and being married (11.7%), and have lower fertility (5.8%) and life-cycle earnings (8.6%). These relationships remain unchanged among the subsets of 583 and 239 females whose male co-twin died during the first postnatal year and first 28 days of life, respectively, supporting the interpretation that they are due primarily to prenatal exposure rather than to postnatal socialization effects of being raised with a male sibling. Our findings provide empirical evidence, using objectively measured nation-level data, that human females exposed prenatally to a male co-twin experience long-term changes in marriage, fertility, and human capital. These findings support the hypothesis of in utero testosterone transfer between twins, which is likely affecting a small but growing subset of females worldwide.
Anatomic, physiological, and behavioral differences vary widely among individuals of the same chromosomal sex (1) and begin to be established in utero in response to gene products and gonadal steroids (2). In the human embryo, the testes develop in males and begin producing androgens, notably testosterone, 6 wk after conception. Circulating androgens can directly impact developing brain structures or do so indirectly after local aromatization into estrogens (3). In humans, amniotic testosterone levels are positively related to rates of male-typical play behaviors in both male and female children (4), and chromosomal females with genetic abnormalities that increase prenatal androgen exposure similarly exhibit male-typical behaviors across the lifespan (5). For example, females with congenital adrenal hyperplasia, who are exposed to high levels of androgens starting in utero (6), have been shown to experience an increase in male-typical activities (7), an increase in levels of aggression (8), and changes in processes related to self-socialization of gender-related behavior (9). Although findings vary, some human studies have further found that prenatal testosterone level, as measured in, for instance, amniotic fluid, is inversely related to cognitive test scores in early childhood in females (10).
Although endogenous hormone production varies across individuals of the same chromosomal sex, prenatal exposure to testosterone may be particularly high for the female twin in opposite-sex twin pairs. Studies from such species as rats and guinea pigs have shown that chromosomal female fetuses that are in proximity to male littermates are passively exposed to testosterone and exhibit male-typical morphological (11) and behavioral characteristics (12). Androgen antagonists block these effects (13) while exogenous androgens replicate them (14), implicating a causal role of prenatal testosterone. Although prenatal estrogens are important to female development, in multiple mammalian species, male fetuses exposed in utero to high estrogen levels from female littermates show weak and inconsistent evidence of behavioral and biological changes (12). Work in humans has shown that females, but not males (15), born with a male co-twin exhibit cognitive and behavioral changes consistent with the known effects of prenatal androgen exposure, including such traits as rule-breaking (16), asocial and aggressive behavior (17, 18), sensation-seeking (19), and performance on mental rotation tests (20, 21). On the other hand, data on the effects on fecundity and educational and socioeconomic outcomes have been mixed (22–25).
Although the findings of previous human studies are consistent with prenatal testosterone transfer, they are unable to differentiate the effects of in utero testosterone exposure from the known postnatal effects on female behavior and development of being raised with a male sibling (26). Past studies have shown, for instance, that females raised with male siblings exhibit behaviors more typical of males in preschool (27) and attain higher educational levels (28). Although the latter finding is not universal (29), such socialization effects could contribute to differences in long-term outcomes among opposite-sex female twins, independent of any effects of prenatal testosterone exposure. One exception is a study of historical data from 18th and 19th century Finland reporting that reduced fecundity and marriage rates in females exposed to a male co-twin held among the subsample of females whose male co-twin died during the first 3 mo of life, thus reducing the likelihood that the differences were secondary to postnatal socialization effects (22). While this finding using historical data is consistent with a role of prenatal testosterone exposure as an influence on later-life outcomes in female co-twins, the sample size for this analysis was limited, with outcomes compared between 20 female-male pairs and 10 female-female twin pairs. In addition, no study has used this more conservative design to explore the applicability of prenatal testosterone exposure in contemporary populations, including policy-relevant outcomes beyond reproduction, such as educational attainment and income.
Clarifying the long-term impacts on females of exposure to a male co-twin is becoming increasingly salient. Twinning rates have been increasing globally in developing economies like Brazil (30) and China (31), as well as in high-income countries throughout Europe and North America (32–34). This increase has accompanied a demographic shift toward a later age at first pregnancy and the increased use of assisted reproductive technologies, both of which are associated with an increased likelihood of twinning (35, 36). As a result of these trends, a growing proportion of females are now exposed to a male co-twin in many contemporary societies (36).
To study the potential long-term impacts of being a female opposite-sex co-twin, we used a high-quality longitudinal dataset that includes birth, household composition, schooling, and labor market records for all children born in Norway between 1967 and 1978. From the 728,842 births during that period, we extracted information on 13,717 twins who survived the first year of life, did not present with birth defects, and had available data on outcomes. We then explored links between prenatal exposure to a male co-twin with 30+ years of information on long-term outcomes, including 1,378 individuals (10.0% of twin pairs) whose co-twin died within the first year of life. Using these data, we constructed multivariable linear regression models designed to capture any lasting effects of prenatal exposure of females to a male co-twin on fertility, marriage, and socioeconomic outcomes.
Results
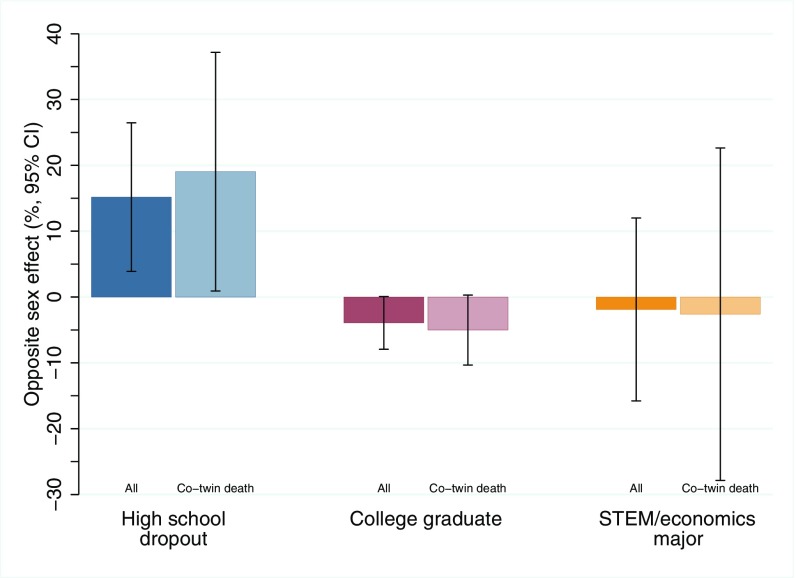
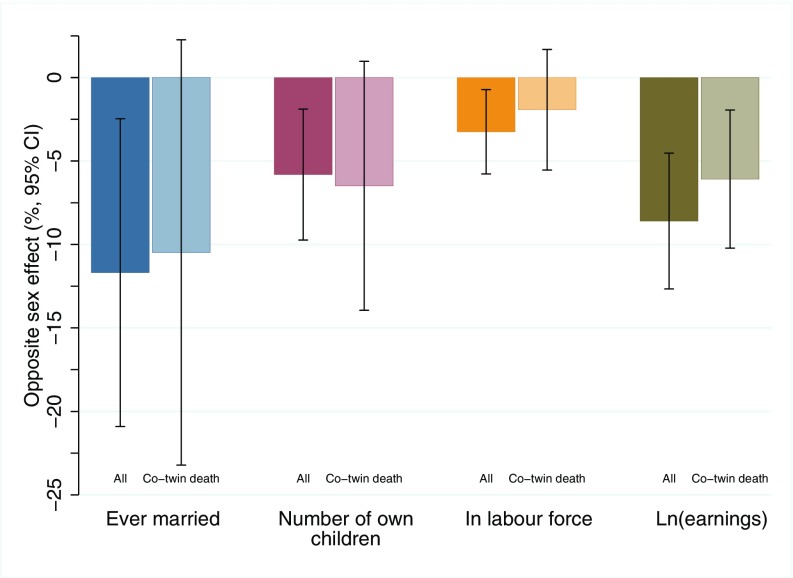
Adjusting for month and year of birth, maternal education and age at the time of birth, and the child’s birth weight, female twins with a male co-twin fared worse in educational attainment and on the labor market and have reduced fecundity and a lower probability of being married compared with female twins with a female co-twin (Figs. 1 and 2 and SI Appendix, Table S1). In the sample of all twins (n = 6,808), female twins with a male co-twin had a 2.8-percentage point (15.2%) higher probability of dropping out of high school and a 1.9-percentage point (3.9%) lower probability of graduating from college; however, conditional on graduating from college, they were no more likely to pursue degrees in traditionally male-dominated science, technology, engineering, and mathematics (STEM)- and economics-oriented fields. In line with findings from 18th and 19th century Finland (22), they also had a 3.8-percentage point (11.7%) lower probability of ever having been married by age 32 y and had 0.09 (5.8%) fewer children on average. When we limited the sample to women who were ever married by age 32 y, the effect on fertility remained similar (4.8%), demonstrating that this effect was not primarily the result of a lower marriage rate. Finally, female twins with a male co-twin were 2.6 percentage points (3.2%) less likely to be in the labor force and had 8.6% lower earnings at age 32 y. The latter gradient is the equivalent of 26% of the gender gap and 27% of the gap between college graduates and high school dropouts in earnings in Norway for the population aged 32–43 y in 2010 observed in our data. Consistent with some past studies, there was minimal evidence of any long-term impacts on males of exposure to a female co-twin (SI Appendix, Table S2).
Fig. 1.
Effect sizes (and 95% CIs) linking in utero exposure to a male co-twin and educational attainment of females. Samples shown in the darker bars are based on all twin births, whereas samples in the lighter bars are based on a subsample of twins in which a co-twin died during the first year of life.
Fig. 2.
Effect sizes (and 95% CIs) linking in utero exposure to a male co-twin and socioeconomic success of females. Samples shown in the darker bars are based on all twin births, whereas samples in lighter bars are based on a subsample of twins in which a co-twin died during the first year of life.
We do not have direct measures of prenatal testosterone exposure in our administrative data (e.g., from amniotic fluid), and models using all twin births cannot differentiate in utero testosterone exposure from the postbirth socialization effects of being raised with a male co-twin. To address this limitation, we present results for the subsample of twin pairs in which the co-twin died early in life (22), thereby comparing females with a deceased male co-twin to females with a deceased female co-twin and dramatically limiting the potential for cross-twin spillover in parenting and socialization. The coefficients in this more stringent subsample (n = 583) are similar to those in the full sample (Figs. 1 and 2 and SI Appendix, Table S1), suggesting that the relationships in the full sample are likely due to prenatal rather than postnatal exposure to a male co-twin. However, as expected, the coefficients in co-twin death sample are at the margin of statistical significance owing to the much smaller sample size of this group (P = 0.84 for STEM/economics major, 0.30 for probability of being in labor force, and between <0.01 and 0.10 for the remaining outcomes).
As a further check, we restricted the analysis to those females with a male co-twin who died within 28 d of birth (SI Appendix, Table S3). In this even smaller sample (n = 239), these coefficients are no longer statistically significant at conventional levels; however, the point estimates are very similar in this subgroup of females who were exposed to a male co-twin only in utero and during the very earliest postnatal period. This stability of estimated effect sizes bolsters our confidence that the findings are predominantly of prenatal origin, with testosterone transfer thus being a likely explanation.
Although prenatal exposure to a male twin likely involves elevated exposure to testosterone, it is also plausible that females with a male co-twin are at a relative nutritional disadvantage compared with females with a female co-twin, owing to the greater prenatal nutritional requirements of males, as reflected in their faster average fetal growth rate and higher birth weight compared with females. Although such an effect should be accounted for in the specifications in which we control for birth weight (SI Appendix, Table S1), we evaluated this possibility more directly by rerunning our models stratified on a median split of the level of between-twin disparity in birth weight. If nutritional disadvantage is an important confounder, then we should observe larger treatment effects in pairs with more disparate birth weights. Contrary to this possibility, the coefficients in these two subsamples are nearly identical (SI Appendix, Table S4), providing strong evidence against a role of nutritional disadvantage in the females of male-female twin pairs.
A potential concern with the deceased co-twin approach is that the sex might affect future fertility or gender composition in the household, which could, through resource competition or socialization effects, have differential postnatal impacts on females with a deceased male versus female co-twin (26). However, we find no evidence of differences in either future fertility or the sex composition of future siblings across these groups. Girls with deceased male co-twins have 1.76 more later-born siblings on average, which is nearly identical to the 1.66 later-born siblings for girls with deceased female co-twins (P = 0.99), and 50.4% of their later-born siblings are male, compared with 49.7% for girls with deceased female co-twins (P = 0.98). Thus, the sex of the deceased co-twin appears to be random with respect to future changes in family composition (SI Appendix, Table S5).
Discussion
We find that females with male co-twins experience adverse educational and labor market outcomes, while also being less likely to marry and have children. These effects are sizable and robust to statistical specifications designed to isolate an effect of prenatal exposure to a male co-twin from postnatal socialization effects, as well as those aimed at ruling out prenatal nutritional pathways. As such, they provide strong support for the applicability of the testosterone-transfer hypothesis to humans, and more generally point to the likely durable impacts of prenatal sex steroids on a range of biological, behavioral, and social outcomes.
Causes of these relationships plausibly include the direct effects of high prenatal testosterone exposure in chromosomal females. For instance, umbilical testosterone has been shown to predict traits like lower cognitive test scores (10) and difficulties with social communication (37). Although we do not have prenatal measures of testosterone concentration, which due to ethical constraints may never be available in humans, past research reveals evidence for intrauterine transfer of hormones in multiple pregnancies. It has been shown that testosterone transfers across fetal membranes through diffusion in amniotic fluid among rats (38). Another potential pathway involves increased testosterone exposure of female twins secondary to higher concentrations of maternal testosterone induced by their male co-twin. Such a mother-child transfer has been observed in rabbits (39) and Rhesus monkeys (40), and human mothers bearing male fetuses have been shown to have elevated circulating testosterone (41, 42).
Behavioral changes downstream of prenatal testosterone exposure, such as any effect of disruptive behavior on schooling or educational attainment, are likely candidate pathways linking male co-twin exposure to later earnings. Consistent with this interpretation, conditioning on educational attainment in the earnings equation reduced the coefficient on opposite-sex from −0.086 (SE, 0.021) to −0.042 (SE, 0.014), suggesting that roughly 50% of intensive margin labor market effects could be explained by educational outcomes that are upstream of earnings. We are aware of only one similar previous study, conducted in The Netherlands, that used an opposite-sex twin comparison and reported no or only small negative effects on female earnings (24). The results of that cross-sectional study were sensitive to specification of the sibling comparison group, suggesting that twins and singletons may vary in relation to other unmeasured factors, such as gendered socialization or prenatal development. In contrast, using longitudinal panel data, we find that the negative long-term differences in Norwegian females with a male co-twin are consistent across a range of long-term outcomes measured at age 32 y and are also largely unchanged in more conservative subsamples in which the potential for postnatal socialization effects were minimized.
Our finding of reduced fertility among females exposed to male co-twins is in agreement with some, but not all, previous human studies. The study by Lummaa et al (22) using historical Finnish data documented significant decrements in fertility among females with male co-twins that related to a combination of a reduced likelihood of marriage along with reduced fecundity. In contrast, a more recent study of 913 opposite-sex twin pairs failed to find evidence of reduced fertility in females exposed to male co-twins in the United States, The Netherlands, and Australia (25). The reason for the disparate findings among studies is unclear, but a negative impact of prenatal testosterone on female fecundity is supported by experimental work in animal models. In mice, females positioned in utero near male littermates have relatively masculinized morphological, endocrine, and behavioral phenotypes, as well as a tendency for longer estrus cycles and altered fertility. In sheep, females exposed to excess testosterone in utero have been shown to have altered negative feedback inhibition of hypothalamic-pituitary-gonadal function (43), and in multiple species, lower reproductive rates have been reported in females from male-biased litters (12, 44).
In addition to any direct in utero effects on female reproductive biology, prenatal testosterone also might have indirect effects on fertility by altering behaviors or social relationships, as suggested by our finding that females with a male co-twin are less likely to enter marriage. For instance, prenatal testosterone has been shown to predict antisocial and aggressive behaviors (18). However, it is notable that the negative effect of male co-twin exposure on female fertility in our sample was similar in the subsample of married women, demonstrating that the effect is largely independent of any effect on marriage success. Although we are not able to rule out other behavioral or social pathways not captured in our data, our findings are consistent with a small but nonetheless significant negative impact on human female fecundity among females exposed in utero to a male co-twin.
Although females exposed to a male co-twin exhibited a range of long-term differences compared with females exposed to a female co-twin, we found that males exposed in utero to a female co-twin had similar long-term outcomes as males exposed in utero to a male co-twin. This finding is consistent with some previous studies in humans and animal models and points to likely nonlinearities in the effect of prenatal testosterone. Females have much lower baseline testosterone levels than males, and thus passive exposure to testosterone from a male co-twin represents a relatively larger increase in exposure to the hormone (45). Although little is known about the magnitude of this increase, studies have reported marked differences in the concentration of amniotic fluid testosterone in males and females during early gestation (46, 47), and female non-twins have variable testosterone levels that predict a wide range of postnatal behaviors (4). Studies of other mammals have demonstrated increases in female fetal testosterone of 25%–50% in rats and mice exposed to two male siblings (48, 49) and of roughly 50% in gerbil females exposed to one male sibling in utero (50). Our average differences are based on a sample of twin pregnancies, which, although providing a natural experiment, are inherently different from singleton pregnancies. Therefore, it is possible that effects could be different if prenatal testosterone levels were experimentally manipulated or the natural variability in testosterone exposure was considered in singleton females.
The deleterious educational and labor market effects of having a male co-twin are of policy interest, as they now affect a larger fraction of the female population in many nations than once was the case. In Norway, 0.6% of newborn girls were exposed to a male co-twin in 2010, compared with 0.3% in 1971. Although similar statistics generally are not available outside of Scandinavia, based on National Center for Health Statistics vital statistics data, we estimate that in the United States, the percentage of all newborn girls with male co-twins rose from 0.6% in 1971 to 1.1% in 2010. While of increasing importance globally (36, 51), our present findings may have particular relevance for subgroups with especially high increases in twinning rates, such as college-educated families.
We emphasize that we do not advocate against delayed reproduction or the use of in vitro fertilization, which are complex decisions made by individuals balancing a range of biological, social, and individual factors. While the effects that we document are important at the population level, they represent an unlikely outcome of any single fertility decision. We are also limited to administrative records, and it may be that females gestating with male co-twins excel in other domains of life that we are unable to measure in our data. Moreover, the specific pattern of long-term societal impacts may change with time and across societies as gender norms and other cultural mores change. These caveats aside, our results suggest that in utero testosterone transfer could present a hidden impact of practices that increase multiple zygote implantation, and provide long-term perspectives concerning the risks and returns of these fertility decisions.
Materials and Methods
This study has been approved by Statistics Norway and Norwegian Data Authorities. Our data are from the Norwegian Registry Data (52), a linked administrative dataset that covers the entire population of Norway. The dataset, maintained by Statistics Norway, is a compilation of different administrative registers, including the Central Population Register, Medical Birth Register, Education Register, and Tax and Earnings Register. These administrative registers can be linked together using unique personal identifiers. Linkage is deterministic rather than probabilistic, and the linkage rate is 100% if an individual exists in both datasets (e.g., foreign-born individuals would not be present in the Medical Birth Register). Since our analysis requires birth records, we limited the sample to individuals born in Norway.
The Medical Birth Register provides information about children and mothers at the time of birth: month and year of birth, plurality, birth weight, sex, and maternal age. The Education Register provides information about maternal years of education as well as children’s educational outcomes: high school dropout, college graduate, and STEM/economics major. The Tax and Earnings Register provides information on labor market outcomes: labor force participation and earnings. The Central Population Register provides demographic outcomes for children (ever married, number of own children) and information on family composition that allows children to be linked to their parents.
We included all twins from twin pairs born in Norway between 1967 and 1978 who met the following criteria: (i) at least one twin survived the first year of life and did not present with birth defects and (ii) at least one twin was observed at age 32 y, when long-run outcomes were assessed. We imposed a panel structure on the data and discarded any observations for which we observed only education information but not earnings information, due to, for example, emigration or early adulthood mortality. Our results were substantively unchanged in repeated cross-sections in which we allowed sample sizes to differ for each outcome. These restrictions yielded a sample of 13,717 observations (out of 13,800 possible twin births surviving to age 1 y without birth defects) with 36.2% of female-female pairs, 37.0% of male-male pairs, and 26.7% of female-male pairs. Corresponding proportions in the total population of twin births between 1967 and 2010 in Norway are 32.4% for female-female pairs, 33.9% for male-male pairs, and 33.7% for female-male pairs. The decline in the fraction of opposite-sex twin pairs in our estimation sample is due to a greater likelihood of birth defects and congenital malformations for this set of pregnancies. Furthermore, approximately 4.1% of infants from multifetal pregnancies do not reach 1 y of age or are born with serious congenital malformations (compared with 1.3% of those from singleton pregnancies). We necessarily excluded these pregnancies from the analysis.
We used linear regression models to study the effects of testosterone transfer. The primary analysis sample was restricted to female same-sex and female-male opposite-sex twin pairs, and we included only females from these pairs in our estimation sample. The empirical sample used in the main analysis included 6,808 observations from 4,533 twin pairs; however, when analyzing college major choice, we conditioned on college completion, which reduced the sample to 3,376 observations from 2,568 twin pairs. We considered seven long-run outcomes measured at the individual level, as outlined in the dataset description.
The theory stipulates that testosterone transfer occurs in female-male pairs, and thus females from these pairs constituted our treatment group, while females from female-female pairs composed a control group. Analytically, we regressed one of the outcomes on the indicator for opposite sex (β) and the set of control variables (λ). Control variables included year of birth indicator variables, month of birth indicator variables, maternal age and years of education at the time of birth, as well as child’s birth weight to control for initial birth endowments. Birth endowments may be important because females are smaller at birth on average; however, in our data, females with a male co-twin were actually larger than females with a female co-twin. We estimated this opposite-sex favorable difference to be 58.1 g (SE, 20.5 g). Because birth weight is positively related to later human capital and similar outcomes (53, 54), including most likely behavioral changes, this pattern of results leads to an overestimation of the effects without controlling for birth weight. Nonetheless, we present the less conservative results without controlling for birth endowments in SI Appendix, Table S6. It could also be the case that females exposed to larger male fetuses receive higher in utero testosterone transfers. We tested this hypothesis by interacting an indicator for opposite-sex twin pair with an indicator for above-median birth weight of the male fetus, and found that this interaction was neither consistently signed nor statistically significant at conventional levels (SI Appendix, Table S7). Furthermore, coefficients for the opposite-sex indicator in these expanded models remained very similar to those in our main results.
In the main regressions, the estimate on opposite sex (β) compared the mean outcomes between females exposed to a male and females exposed to a female. Figs. 1 and 2 plot effect sizes, that is, the point estimate divided by mean of an outcome in the estimation sample; this does not apply to earnings, which are expressed in logs rather than levels. In the larger sample, we adjusted SEs for within-family correlations by clustering them at the mother level and used heteroskedasticity-robust errors when regressions included only a single twin from a pair. We clustered at the mother rather than twin pair level because in a few cases (<1% of our larger sample) we included multiple twin pregnancies from the same mother, and our approach accounts for potential correlation within family and across births. Even though we observed a near-census of individuals, SE estimation was still warranted because a small fraction of children could die or migrate internationally between the birth and outcome measurements. Moreover, there is always a concern about coding errors, even in administrative data, which introduce measurement error. Finally, we performed similar analyses for males, with the sample restricted to female-male and male-male twin pairs (SI Appendix, Table S2).
We augmented the primary analysis with two additional sets of results addressing the possibility of postnatal socialization. Here we replicated the main analysis on two samples aimed at minimizing the potential for postnatal socialization effects: (i) the group in which both twins were born alive but one of them died within the first year of life; and (ii) the more restrictive sample in which both twins were born alive but one of them died within the first 28 d of life. Sample sizes were 583 when analyzing death within the first year and 239 when analyzing death within the first month (476 and 202, respectively, when analyzing college major choice). Irrespective of the sample, in these analyses we required death to occur in both female-female and female-male pairs; however, in the former, a female dies, and in the latter, a male dies. We also analyzed fertility in SI Appendix, Table S5, were we regressed the number of children born to a mother after the twin pregnancy or the percentage of males among these children on the opposite-sex indicator.
When analyzing subsequent fertility and sex composition with respect to sex of the deceased twin, we restricted the sample to these 583 observations from families in which a co-twin died within the first year of life. Here we counted the number of subsequent live births to a mother (by 2010) after the twin birth. We then applied a t test and reported P values for the difference between two groups: deceased female and deceased male co-twin. We performed similar procedures when computing the sex ratio, but counted the number of subsequent female and male births separately and constructed a variable, the number of females over number of males.
To approximately quantify the population affected in the United States, we first computed the percentage of twin births in all births reported in publicly available National Center for Health Statistics (NCHS) Vital Statistics Data for birth cohorts 1971 and 2010. These values were 1.8% and 3.4% for 1971 and 2010, respectively. However, NCHS data only indicate whether the birth is a twin and do not allow linking children into twin pairs. In a separate dataset restricted to the universe of births in Florida between 1992 and 2002 (53), twin identities are known, and we computed that among all twin births, 33.8% were male-male pairs, 33.8% were female-female pairs, and 32.4% were female-male pairs. Based on these percentages, we multiplied 1.8 and 3.4 × 0.324 to obtain approximate percentages of newborn girls with a male co-twin in the United States as 0.6% in 1971 and 1.1% in 2010. For this extrapolation to be correct, we need to assume that the sex composition of twin pairs born in Florida between 1992 and 2010 is a good approximation for the entire United States and over time between the 1970s and 2010s.
Supplementary Material
Acknowledgments
We thank Sarah Richardson and an anonymous reviewer for comments.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1812786116/-/DCSupplemental.
References
- 1.Joel D, et al. Sex beyond the genitalia: The human brain mosaic. Proc Natl Acad Sci USA. 2015;112:15468–15473. doi: 10.1073/pnas.1509654112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auyeung B, et al. Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychol Sci. 2009;20:144–148. doi: 10.1111/j.1467-9280.2009.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hines M. Sex-related variation in human behavior and the brain. Trends Cogn Sci. 2010;14:448–456. doi: 10.1016/j.tics.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000;21:245–291. doi: 10.1210/edrv.21.3.0398. [DOI] [PubMed] [Google Scholar]
- 7.Berenbaum SA. Effects of early androgens on sex-typed activities and interests in adolescents with congenital adrenal hyperplasia. Horm Behav. 1999;35:102–110. doi: 10.1006/hbeh.1998.1503. [DOI] [PubMed] [Google Scholar]
- 8.Pasterski V, et al. Increased aggression and activity level in 3- to 11-year-old girls with congenital adrenal hyperplasia (CAH) Horm Behav. 2007;52:368–374. doi: 10.1016/j.yhbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hines M, et al. Prenatal androgen exposure alters girls’ response to information indicating gender-appropriate behaviour. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150125. doi: 10.1098/rstb.2015.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finegan JAK, Niccols GA, Sitarenios G. Relations between prenatal testosterone levels and cognitive abilities at 4 years. Dev Psychol. 1992;28:1075–1089. [Google Scholar]
- 11.Vandenbergh JG, Huggett CL. The anogenital distance index, a predictor of the intrauterine position effects on reproduction in female house mice. Lab Anim Sci. 1995;45:567–573. [PubMed] [Google Scholar]
- 12.Ryan BC, Vandenbergh JG. Intrauterine position effects. Neurosci Biobehav Rev. 2002;26:665–678. doi: 10.1016/s0149-7634(02)00038-6. [DOI] [PubMed] [Google Scholar]
- 13.Clemens LG, Gladue BA, Coniglio LP. Prenatal endogenous androgenic influences on masculine sexual behavior and genital morphology in male and female rats. Horm Behav. 1978;10:40–53. doi: 10.1016/0018-506x(78)90023-5. [DOI] [PubMed] [Google Scholar]
- 14.Hrabovszky Z, Hutson JM. Androgen imprinting of the brain in animal models and humans with intersex disorders: Review and recommendations. J Urol. 2002;168:2142–2148. doi: 10.1016/S0022-5347(05)64338-8. [DOI] [PubMed] [Google Scholar]
- 15.Tapp AL, Maybery MT, Whitehouse AJ. Evaluating the twin testosterone transfer hypothesis: A review of the empirical evidence. Horm Behav. 2011;60:713–722. doi: 10.1016/j.yhbeh.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Loehlin JC, Martin NG. Dimensions of psychological masculinity-femininity in adult twins from opposite-sex and same-sex pairs. Behav Genet. 2000;30:19–28. doi: 10.1023/a:1002082325784. [DOI] [PubMed] [Google Scholar]
- 17.Cohen-Bendahan CC, Buitelaar JK, van Goozen SH, Orlebeke JF, Cohen-Kettenis PT. Is there an effect of prenatal testosterone on aggression and other behavioral traits? A study comparing same-sex and opposite-sex twin girls. Horm Behav. 2005;47:230–237. doi: 10.1016/j.yhbeh.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Crespi BJ. Oxytocin, testosterone, and human social cognition. Biol Rev Camb Philos Soc. 2016;91:390–408. doi: 10.1111/brv.12175. [DOI] [PubMed] [Google Scholar]
- 19.Slutske WS, Bascom EN, Meier MH, Medland SE, Martin NG. Sensation seeking in females from opposite- versus same-sex twin pairs: Hormone transfer or sibling imitation? Behav Genet. 2011;41:533–542. doi: 10.1007/s10519-010-9416-3. [DOI] [PubMed] [Google Scholar]
- 20.Vuoksimaa E, et al. Having a male co-twin masculinizes mental rotation performance in females. Psychol Sci. 2010;21:1069–1071. doi: 10.1177/0956797610376075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heil M, Kavšek M, Rolke B, Beste C, Jansen P. Mental rotation in female fraternal twins: Evidence for intra-uterine hormone transfer? Biol Psychol. 2011;86:90–93. doi: 10.1016/j.biopsycho.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Lummaa V, Pettay JE, Russell AF. Male twins reduce fitness of female co-twins in humans. Proc Natl Acad Sci USA. 2007;104:10915–10920. doi: 10.1073/pnas.0605875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahrenfeldt L, Petersen I, Johnson W, Christensen K. Academic performance of opposite-sex and same-sex twins in adolescence: A Danish national cohort study. Horm Behav. 2015;69:123–131. doi: 10.1016/j.yhbeh.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gielen AC, Holmes J, Myers C. Prenatal testosterone and the earnings of men and women. J Hum Resour. 2016;51:30–61. [Google Scholar]
- 25.Medland SE, et al. Males do not reduce the fitness of their female co-twins in contemporary samples. Twin Res Hum Genet. 2008;11:481–487. doi: 10.1375/twin.11.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raley S, Bianchi S. Sons, daughters, and family processes: Does gender of children matter? Annu Rev Sociol. 2006;32:401–421. [Google Scholar]
- 27.Rust J, Golombok S, Hines M, Johnston K, Golding J. ALSPAC Study Team The role of brothers and sisters in the gender development of preschool children. J Exp Child Psychol. 2000;77:292–303. doi: 10.1006/jecp.2000.2596. [DOI] [PubMed] [Google Scholar]
- 28.Butcher K, Case A. The effect of sibling sex composition on women’s education and earnings. Q J Econ. 1994;109:531–563. [Google Scholar]
- 29.Kaestner R. Are brothers really better? Sibling sex composition and educational achievement revisited. J Hum Resour. 1997;32:250–284. [Google Scholar]
- 30.Homrich da Silva C, et al. The rise of multiple births in Brazil. Acta Paediatr. 2008;97:1019–1023. doi: 10.1111/j.1651-2227.2008.00791.x. [DOI] [PubMed] [Google Scholar]
- 31.Lu X, et al. Epidemiology of twin births in southeast China: 1993-2005. Twin Res Hum Genet. 2013;16:608–613. doi: 10.1017/thg.2013.7. [DOI] [PubMed] [Google Scholar]
- 32.Astolfi P, Ulizzi L, Zonta LA. Changes in twinning rate: Italy 1950-1996. Hum Reprod. 2003;18:207–211. doi: 10.1093/humrep/deg036. [DOI] [PubMed] [Google Scholar]
- 33.Luke B, Martin JA. The rise in multiple births in the United States: Who, what, when, where, and why. Clin Obstet Gynecol. 2004;47:118–133. doi: 10.1097/00003081-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Hardin J, Carmichael SL, Selvin S, Shaw GM. Trends in the probability of twins and males in California, 1983-2003. Twin Res Hum Genet. 2009;12:93–102. doi: 10.1375/twin.12.1.93. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds MA, Schieve LA, Martin JA, Jeng G, Macaluso M. Trends in multiple births conceived using assisted reproductive technology, United States, 1997-2000. Pediatrics. 2003;111:1159–1162. [PubMed] [Google Scholar]
- 36.Pison G, Monden C, Smits J. Twinning rates in developed countries: Trends and explanations. Popul Dev Rev. 2015;41:629–649. [Google Scholar]
- 37.Whitehouse AJ, et al. Fetal androgen exposure and pragmatic language ability of girls in middle childhood: Implications for the extreme male-brain theory of autism. Psychoneuroendocrinology. 2010;35:1259–1264. doi: 10.1016/j.psyneuen.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Even MD, Dhar MG, vom Saal FS. Transport of steroids between fetuses via amniotic fluid in relation to the intrauterine position phenomenon in rats. J Reprod Fertil. 1992;96:709–716. doi: 10.1530/jrf.0.0960709. [DOI] [PubMed] [Google Scholar]
- 39.Bánszegi O, Altbäcker V, Dúcs A, Bilkó A. Testosterone treatment of pregnant rabbits affects sexual development of their daughters. Physiol Behav. 2010;101:422–427. doi: 10.1016/j.physbeh.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 40.Phoenix CH. Prenatal testosterone in the non-human primate and its consequences for behavior. In: Friedman RC, Richart RM, Vande Wiele RL, editors. Sex Differences in Behavior. Wiley; New York: 1974. pp. 19–32. [Google Scholar]
- 41.Meulenberg PM, Hofman JA. Maternal testosterone and fetal sex. J Steroid Biochem Mol Biol. 1991;39:51–54. doi: 10.1016/0960-0760(91)90012-t. [DOI] [PubMed] [Google Scholar]
- 42.Simmons D, France JT, Keelan JA, Song L, Knox BS. Sex differences in umbilical cord serum levels of inhibin, testosterone, oestradiol, dehydroepiandrosterone sulphate, and sex hormone-binding globulin in human term neonates. Biol Neonate. 1994;65:287–294. doi: 10.1159/000244074. [DOI] [PubMed] [Google Scholar]
- 43.Veiga-Lopez A, Astapova OI, Aizenberg EF, Lee JS, Padmanabhan V. Developmental programming: Contribution of prenatal androgen and estrogen to estradiol feedback systems and periovulatory hormonal dynamics in sheep. Biol Reprod. 2009;80:718–725. doi: 10.1095/biolreprod.108.074781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drickamer LC, Arthur RD, Rosenthal TL. Conception failure in swine: Importance of the sex ratio of a female’s birth litter and tests of other factors. J Anim Sci. 1997;75:2192–2196. doi: 10.2527/1997.7582192x. [DOI] [PubMed] [Google Scholar]
- 45.Miller EM. Prenatal sex hormone transfer: A reason to study opposite-sex twins. Pers Individ Dif. 1994;17:511–529. [Google Scholar]
- 46.Warne GL, Faiman C, Reyes FI, Winter JS. Studies on human sexual development, V: Concentrations of testosterone, 17-hydroxyprogesterone and progesterone in human amniotic fluid throughout gestation. J Clin Endocrinol Metab. 1977;44:934–938. doi: 10.1210/jcem-44-5-934. [DOI] [PubMed] [Google Scholar]
- 47.Carson DJ, et al. Amniotic fluid steroid levels: Fetuses with adrenal hyperplasia, 46,XXY fetuses, and normal fetuses. Am J Dis Child. 1982;136:218–222. [PubMed] [Google Scholar]
- 48.vom Saal FS, Bronson FH. Sexual characteristics of adult female mice are correlated with their blood testosterone levels during prenatal development. Science. 1980;208:597–599. doi: 10.1126/science.7367881. [DOI] [PubMed] [Google Scholar]
- 49.Houtsmuller EJ, de Jong FH, Rowland DL, Slob AK. Plasma testosterone in fetal rats and their mothers on day 19 of gestation. Physiol Behav. 1995;57:495–499. doi: 10.1016/0031-9384(94)00291-c. [DOI] [PubMed] [Google Scholar]
- 50.Clark MM, Crews D, Galef BG., Jr Concentrations of sex steroid hormones in pregnant and fetal Mongolian gerbils. Physiol Behav. 1991;49:239–243. doi: 10.1016/0031-9384(91)90038-p. [DOI] [PubMed] [Google Scholar]
- 51.Smits J, Monden C. Twinning across the developing world. PLoS One. 2011;6:e25239. doi: 10.1371/journal.pone.0025239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Figlio D, Karbownik K, Salvanes K. Education research and administrative data. In: Hanushek E, Machin S, Woessmann L, editors. Handbook of the Economics of Education. Elsevier; Amsterdam: 2016. pp. 75–138. [Google Scholar]
- 53.Figlio D, Guryan J, Karbownik K, Roth J. The effects of poor neonatal health on children’s cognitive development. Am Econ Rev. 2014;104:3921–3955. doi: 10.1257/aer.104.12.3921. [DOI] [PubMed] [Google Scholar]
- 54.Black S, Devereux P, Salvanes K. From the cradle to the labor market? The effect of birth weight on adult outcomes. Q J Econ. 2007;122:409–439. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.