Retinoic acid (RA) was originally identified as a morphogen, a signaling molecule that is produced by a specific region but diffuses away, thereby producing a concentration gradient. The morphogen’s nonuniform distribution governs embryo patterning during development. In its role as a morphogen, RA was shown to bind to the RA receptor (RAR) to directly modulate transcription in the nucleus. Recently, though, a noncanonical role for the RARα subtype was identified at neuronal synapses. At the synapse, RARα promotes local translation of AMPA receptors in response to persistently low neuronal activity (1). Increasing AMPA receptors in this way strengthens synapses in a form of homeostatic synaptic plasticity (HSP). HSP shifts the overall synaptic strengths across the neuron while maintaining relative synaptic weights. HSP is bidirectional; synapse may “scale up” in response to decreased neuronal activity or “scale down” in response to increased neuronal activity. HSP is thought to play a vital role in holding synaptic activity within the physiological range and preventing information loss due to a “maxed-out” signal. The mechanistic basis of HSP is poorly understood, particularly in relation to synaptic downscaling.
In PNAS, Hsu et al. (2) discover that RARα, through its effects on the ERK-mammalian target of rapamycin complex 1 (mTORC1) pathway, also plays a pivotal role in synaptic downscaling. In an exhaustive series of experiments, the authors show that loss of RARα in the CA1 region of the dorsal hippocampus has no effect on baseline electrophysiological or behavioral properties of mice. However, after a manipulation that elicits HSP [environmental enrichment (EE)], Hebbian long-term potentiation (LTP) is enhanced while long-term depression (LTD) is repressed (a sign of “runaway plasticity”). This imbalance results in improved performance in spatial memory tests but reduced capacity for reversal learning. Based on these findings, the authors conclude that RARα signaling normally dampens synapses in response to EE, thereby defining a molecular mechanism for HSP. It has long been known that synaptic plasticity induced according to Hebbian-type rules is important for memory, but that there must be additional mechanisms to prevent runaway dynamics. Many theories have included homeostatic plasticity in their models to solve this problem. However, examining the molecular basis of homeostatic plasticity has proved difficult, especially in vivo. The current manuscript (2) represents a broadly important finding regarding the mechanisms of homeostatic plasticity and their importance in memory.
Many Functions of RARα: Transcription, Translation, and Beyond
Historically, it was thought that RARs function solely in the nucleus; RAR is bound to DNA, and binding of RA changes the conformation of RAR to alter the transcription of nearby genes. Initial studies in the brain found RAR at the synapse, a location inconsistent with its canonical role in the nucleus. Synaptically localized RARα was implicated in the homeostatic synaptic upscaling observed in response to prolonged synaptic silencing. This homeostatic role of RARα was independent of its previously identified function in DNA binding (1). Instead, RARα directly activates the translation of specific subtypes of AMPA receptors to scale up the neuron. Therefore, in addition to its well-known function as a transcriptional modulator, RARα took on another role as a translational regulator.
Hsu et al. (2) discover an additional role for RARα in synaptic downscaling and, moreover, define a molecular mechanism that allows RARα to mediate both synaptic upscaling and downscaling (Fig. 1). Homeostatic downscaling is thought to counterbalance Hebbian potentiation of synapses by keeping the activity of the neuron within its physiological range. In this elegant study, the authors use EE as a means to ramp up synaptic activity in mice. They find that EE, via RARα, decreases AMPA receptor levels (synaptic downscaling) by regulating the ERK-mTORC1 pathway (2). Interfering with this pathway has profound effects on memory. Together, their findings show a function of RAR whereby RARα mediates AMPA translation via an intermediate pathway rather than having a direct role on translational regulation or the canonical transcriptional role of RARα.
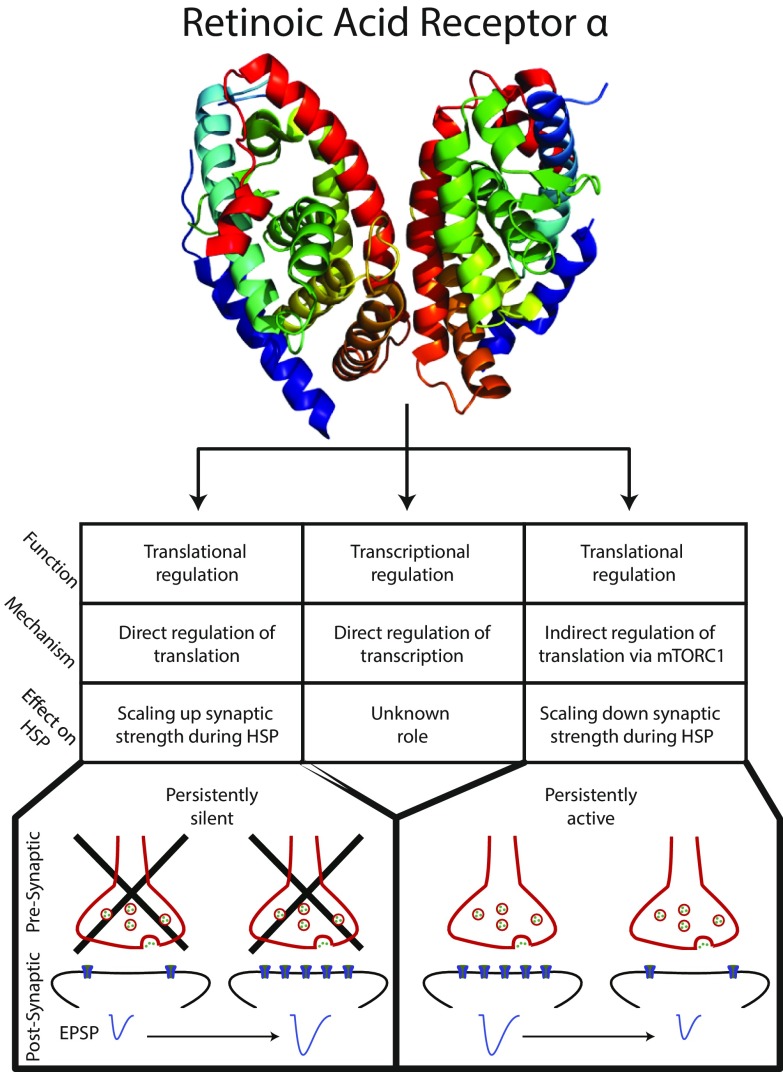
Fig. 1.
The many roles of RARα. (Top) Representation of RARα from ref. 3. (Middle) Three described functions of RARα: direct translational regulation (Left), direct transcriptional regulation (Center), and indirect translational regulation (Right). The transcriptional function of RARα has no known role in homeostatic synaptic plasticity (see Effect on HSP); however, both translational functions of RARα play important roles in HSP. The direct translational role of RARα (Bottom, Left) promotes the translation of AMPA receptors, which are inserted into the postsynaptic membrane after prolonged synaptic silencing, scaling up the synaptic response [illustrated by an increase in the evoked postsynaptic potential (EPSP)]. This is in direct opposition to the indirect translational role of RARα (Bottom, Right), which reduces translation of AMPA receptors by affecting the ERK-mTORC1 pathway, scaling down the synaptic response after persistent activity.
Role of RARα in HSP
HSP is a vital process within neurons that normalizes synaptic responses to keep them within the physiological working range of a neuron while maintaining the relative strength between synapses of a neuron. HSP is thought to be a necessary counterpoint to Hebbian plasticity that, if left unchecked, could lead to a cycle of constant synaptic potentiation. This runaway plasticity would lead to the loss of information, because such constant potentiation would reach a ceiling (or a floor, in the case of depression), preventing any further potentiation. HSP comes in two “flavors,” synaptic upscaling and synaptic downscaling.
Previously, this research group showed an elegant mechanism of action for RARα in synaptic upscaling (1). To induce synaptic upscaling in HSP, neurons were artificially silenced with drugs. The lack of synaptic activity results in a prolonged drop of calcium concentrations in the postsynaptic compartment, triggering the production of RA, which binds to synaptically localized RARα. Activation of RARα directly increases translation of calcium-permeable AMPA receptors (AMPA receptors lacking the GluA2 subunit), thus scaling up the synaptic weights and acting as a negative feedback loop to prevent further upscaling. That is, as more calcium enters the synapse through these receptors, it limits the production of RA (1).
With the role of RARα in synaptic upscaling defined in their previous work, Hsu et al. (2) explore the role of RARα in synaptic downscaling. First, the authors exposed mice to EE, a treatment that produced sustained increases in synaptic activity, to trigger HSP. When RARα was knocked out in the CA1 region of the dorsal hippocampus, mice in the EE condition had greater LTP and impaired LTD. These results suggest that the absence of RARα results in deficits in synaptic downscaling, thereby allowing LTP mechanisms to dominate. Behaviorally, mice lacking RARα in the hippocampus showed a marked improvement in the acquisition of the Morris water maze memory task (among other tasks). In this spatial memory task, a mouse is trained to find a hidden platform in a pool of opaque water by using spatial cues in the room. Consistent with the improved LTP, mice lacking RARα found the hidden platform faster than wild-type control mice. The Morris water maze can also be modified in a reversal task in which, after training, the platform is moved to a new location. Reversal learning differs from normal learning, in that the mice are not asked to learn a new task but are asked to modify an already learned task, a process termed behavioral flexibility. Mice lacking RARα showed profound impairments in reversal learning, spending a significant amount of time searching for the platform in the old, rather than the new, location. This finding is intriguing because impairing HSP by removing RARα had the curious effect of making these mice perform better when learning a task but fail when modifying the already learned task. This observation is in line with the idea that these mice can no longer downscale their synapses, and the LTP caused by the “first solution to the water maze” cannot be changed to make room for the second.
Together, these findings highlight RARα as a major molecular player in memory. Not only is RARα a member of a select group of known molecular players important in HSP, but RARα is one of the only proteins shown to have a role in both the synaptic upscaling and downscaling flavors of HSP. Additionally, this work represents one of the few studies to successfully study HSP in vivo and underline the profound behavioral phenotypes due to impairment in HSP. RARα and potentially RA have arrived as potent regulators of both HSP and memory formation.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research, the National Sciences and Engineering Research Council, and the Canadian Institute for Advanced Research (S.A.J.).
Footnotes
The authors declare no conflict of interest.
See companion article on page 7113.
References
- 1.Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu Y-T, Li J, Wu D, Südhof TC, Chen L. Synaptic retinoic acid receptor signaling mediates mTOR-dependent metaplasticity that controls hippocampal learning. Proc Natl Acad Sci USA. 2019;116:7113–7122. doi: 10.1073/pnas.1820690116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourguet W, et al. Crystal structure of a heterodimeric complex of RAR and RXR ligand-binding domains. Mol Cell. 2000;5:289–298. doi: 10.1016/s1097-2765(00)80424-4. [DOI] [PubMed] [Google Scholar]