Significance
Despite the role of T cell-mediated events in control of intracellular infections, studies from several laboratories have shown that humoral immunity also plays a critical role. An effective glycoconjugate vaccine to prevent Francisella tularensis infection has many advantages over live-organism vaccines, but protection against pulmonary infection by conjugate vaccines has never been shown. Here, we report a glycoconjugate vaccine that protects against intranasal challenge with F. tularensis. We determined that lipopolysaccharide-specific antibodies induced by a larger sized O antigen exhibit significantly enhanced relative affinity. This observation challenges the paradigm of a direct correlation between the amount of IgG induced by a glycoconjugate and the level of protection conferred, encouraging the development of conjugate vaccines inducing high-affinity antibodies to important pathogens.
Keywords: glycoconjugate vaccine, O antigen, intracellular pathogen, antibody affinity, Francisella
Abstract
Francisella tularensis is the causative agent of tularemia, a category A bioterrorism agent. The lipopolysaccharide (LPS) O antigen (OAg) of F. tularensis has been considered for use in a glycoconjugate vaccine, but conjugate vaccines tested so far have failed to confer protection necessary against aerosolized pulmonary bacterial challenge. When F. tularensis OAg was purified under standard conditions, the antigen had a small molecular size [25 kDa, low molecular weight (LMW)]. Using milder extraction conditions, we found the native OAg had a larger molecular size [80 kDa, high molecular weight (HMW)], and in a mouse model of tularemia, a glycoconjugate vaccine made with the HMW polysaccharide coupled to tetanus toxoid (HMW-TT) conferred better protection against intranasal challenge than a conjugate made with the LMW polysaccharide (LMW-TT). To further investigate the role of OAg size in protection, we created an F. tularensis live vaccine strain (LVS) mutant with a significantly increased OAg size [220 kDa, very high molecular weight (VHMW)] by expressing in F. tularensis a heterologous chain-length regulator gene (wzz) from the related species Francisella novicida. Immunization with VHMW-TT provided markedly increased protection over that obtained with TT glycoconjugates made using smaller OAgs. We found that protective antibodies recognize a length-dependent epitope better expressed on HMW and VHMW antigens, which bind with higher affinity to the organism.
The intracellular pathogen Francisella tularensis causes tularemia, a potentially fatal disease in humans and other mammals (1). Two predominant subspecies are of interest with regard to F. tularensis infection of humans: F. tularensis subspecies tularensis (type A) and F. tularensis subspecies holarctica (type B) (2). While type A strains cause more severe and life-threatening disease, systemic infection with type B strains is the most prevalent form of human tularemia (1). F. tularensis has been classified as a category A bioterrorism agent because it is readily aerosolized and exhibits a high degree of infectivity and lethality in humans. This organism causes disease by diverse routes, including oral, s.c., and pneumonic. The respiratory route is of particular concern because infection with 50 or fewer organisms is associated with mortality rates of 30–60% if untreated (2, 3). An attenuated live vaccine strain (LVS) has been developed by several in vitro passages of a type B clinical isolate and is available for administration to at-risk individuals but has not been licensed because of an incomplete understanding of the basis for its attenuated virulence and associated side effects (4–6). Significant research efforts have been aimed at elucidating Francisella pathogenesis and identifying components for rational vaccine design (7–10). Although F. tularensis is a facultative intracellular pathogen, studies from several laboratories have demonstrated that humoral immunity plays an important role in protection (11–17). The lipopolysaccharide (LPS) of F. tularensis is atypical compared with the LPSs of many other gram-negative pathogens and evades innate immune activation of Toll-like receptor 4, thereby playing a significant role in immune evasion. The O antigen (OAg) has been considered as a potential target for use in a vaccine (13, 18, 19). However, while the OAg-based glycoconjugate vaccines tested so far have been protective against intradermal bacterial infection (13), they have failed to confer protection against intranasal infections with type A and B strains in mice (i.e., against infections acquired by the most challenging and relevant route in potential bioterrorism attacks) (13, 19).
Acid hydrolysis of LPS has been the preferred method for breaking the ketosidic bond between the immunodominant OAg and the endotoxic lipid A moiety (20). We discovered that F. tularensis OAg was considerably reduced in size when subjected to the conditions previously used for its cleavage from LPS (13, 19). By modifying the hydrolytic conditions, we were able to extract the OAg without affecting its native molecular size. The native OAg was incorporated into a glycoconjugate that resulted in greater protection than a conjugate produced with a reduced-size polysaccharide. To further increase the size of the OAg, we generated an F. tularensis mutant with an increased number of OAg repeating units by genetic modification of the chain-length regulator gene wzz. Surprisingly, a conjugate vaccine produced with the genetically induced, very-high-molecular-size OAg provided markedly increased protection, despite inducing IgG levels similar to those elicited by the native OAg conjugate and even lower than those induced by the reduced-size OAg conjugate. The more efficacious IgG induced by this very-high-molecular-size OAg vaccine had a higher relative affinity for the organism than the antibodies induced by the reduced-size and native-size OAg conjugates. These results revealed the importance of conformational OAg epitopes in the protection generated by the vaccine.
Results
F. tularensis OAg Is Susceptible to Acid Hydrolysis.
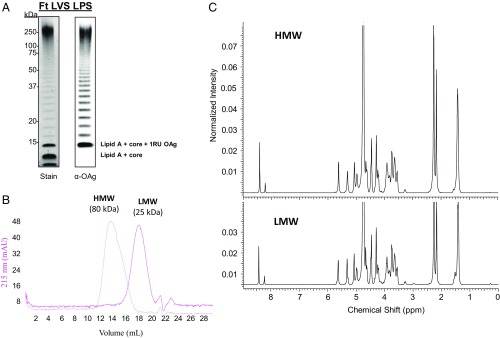
LPS was extracted and purified from F. tularensis LVS and was characterized by silver staining and immunoblot analysis with an OAg-specific monoclonal antibody (mAb) (Fig. 1A). Both blots showed the characteristic bacterial LPS stepladder patterns (Fig. 1A). OAg was extracted from F. tularensis LPS (13, 19) with 6% (vol/vol) acetic acid at 100 °C for 2 h, and the average molecular size of the purified sugar was determined by size-exclusion chromatography (SEC) with dextran standards (Fig. 1B). Surprisingly, the average measured size of OAg was only ∼25 kDa, in sharp contrast to the profile seen for the intact LPS by silver staining and western blot. To evaluate the sensitivity of F. tularensis LVS OAg to acid hydrolysis, we tested different hydrolytic conditions (Table 1). The size of the OAg obtained was highly dependent on the conditions used for acid hydrolysis. Use of 6% (vol/vol) acetic acid at 90 °C reduced the sugar size from 80 kDa after 1 h of hydrolysis to 11 kDa after 5 h of reaction. When we decreased the acetic acid concentration for hydrolysis to 2% (vol/vol), there was significantly less change in molecular size (Table 1). To confirm that OAg is cleaved by acid hydrolysis, we directly hydrolyzed the extracted high-molecular-weight (HMW) OAg, using 6% (vol/vol) acetic acid at 90 °C; the molecular size decreased substantially over time (SI Appendix, Fig. S1). Two hydrolytic conditions were selected for further analysis: a lower molecular size [low-molecular-weight (LMW) OAg, 25 kDa, condition C] and a higher molecular size [high-molecular-weight (HMW) OAg, 80 kDa, condition F] (Fig. 1B and Table 1). The 1H NMR spectra of HMW and LMW OAgs were identical and comparable to previously reported structures (21, 22); therefore, the depolymerization caused by hydrolysis did not affect the structure of the repeating unit (Fig. 1C).
Fig. 1.
F. tularensis OAg is susceptible to acid hydrolysis, but its structural identity is conserved. (A) Silver staining and immunoblot analysis of LPS from F. tularensis (Ft) LVS using an OAg-specific mAb (mAb2034). RU, repeating unit. (B) SEC profiles of HMW and LMW OAgs extracted from F. tularensis LVS. A profile run on a Superose 6 10/300 GL column at 0.5 mL⋅min−1 with 1× PBS (pH 7.4) is shown. The average molecular weight was calculated with a dextran calibration curve. mAU, milli-absorbance unit. (C) 1H NMR spectrum of HMW and LMW OAgs extracted from F. tularensis LVS.
Table 1.
Impact of LPS hydrolytic conditions on OAg size
| Condition | AcOH%, vol/vol | Time, h | OAg size, kDa |
| A | 6 | 1 | 70 |
| B | 2 | 35 | |
| C | 3 | 25 | |
| D | 5 | 11 | |
| E | 2 | 0.5 | 80 |
| F | 1 | 80 | |
| G | 2 | 55 |
Only the Larger OAg Produces a Glycoconjugate Vaccine Providing Full Protection Against Intranasal Challenge.
Having observed the sensitivity of F. tularensis OAg to acid hydrolysis, we hypothesized that the failure of previous conjugate vaccines to protect against intranasal challenge may have been related to the size of OAg incorporated into the vaccines. It has been shown that larger polysaccharides are more immunogenic than smaller polysaccharides of the same repeating unit structure (23–25). We evaluated the relationship of OAg size to the immunogenicity of F. tularensis glycoconjugate vaccines. HMW (80 kDa) and LMW (25 kDa) OAgs were covalently coupled to the tetanus toxoid (TT) carrier protein. The HMW-TT and LMW-TT glycoconjugates were synthesized and purified as previously reported (13, 19) (SI Appendix, Fig. S2). Conjugates were characterized (Table 2) and subsequently tested in a mouse immunization and challenge study. As a positive control, we vaccinated mice with the live-attenuated F. tularensis mutant ∆kdhAB, which we had previously shown to be highly immunogenic and protective (26). Four weeks after the last vaccine dose (Fig. 2A), sera were collected and OAg-specific IgG antibody titers were determined by ELISA. LMW-TT generated higher levels of anti-LPS IgG than HMW-TT. The relative IgG titers induced by the different vaccines were confirmed by use of UV-killed F. tularensis LVS as an ELISA coating agent. Like the LPS-coated plates, the organism-coated plates revealed that the LMW-TT conjugate induced higher IgG levels than the HMW-TT conjugate (SI Appendix, Fig. S3). All groups of mice were subsequently challenged intranasally with 5 × 103 cfu (50-fold the intranasal LD50) of F. tularensis LVS. Surprisingly, although the LMW-TT conjugate induced higher antibody titers (Fig. 2B), only the HMW-TT conjugate was protective (Fig. 2C).
Table 2.
OAg-TT conjugates generated with different OAg sizes
| Conjugate | OAg size, kDa | Sugar/protein, wt/wt | Kd conjugate (FPLC-SEC) |
| LMW-TT | 25 | 3.84 | 0.32 |
| HMW-TT | 80 | 1.13 | 0.25 |
| VHMW-TT | 220 | 2.25 | 0.16 |
Fig. 2.
HMW OAg produces a glycoconjugate vaccine conferring protection against intranasal challenge with F. tularensis LVS. (A) Immunization schedule. BALB/c mice received three immunizing doses (2 wk apart) and were challenged intranasally after a 4-wk rest period with F. tularensis LVS (50-fold the intranasal LD50). Animals were monitored daily for 25 d after challenge. (B) Anti-OAg IgG ELISA units to the F. tularensis (Ft) LPS coating agent measured at day 49. Dots represent individual animals. Horizontal bars represent mean ± SD values. i.n., intranasally. (C) Survival of mice challenged intranasally (IN) with F. tularensis after immunization with HMW-TT, LMW-TT, or F. tularensis ∆kdhAB or administration of adjuvant alone. There were six mice per group. **P ≤ 0.01.
OAg Molecular Size Is Increased by Genetic Manipulation of LPS Biosynthesis.
After observing that OAg size is a critical parameter in glycoconjugate vaccine immunogenicity against F. tularensis, we sought to increase the polysaccharide chain length by genetically engineering F. tularensis LVS. In most gram-negative bacteria, the assembly of OAg depends on an inner-membrane OAg polymerase, Wzy (27–29). Wzy activity is often associated with another membrane protein, Wzz, a polysaccharide copolymerase that regulates the number of repeating units in OAg (27, 28). To determine whether the molecular size of OAg is conserved in Francisella species, we purified and characterized LPS from Francisella novicida U112, a closely related subspecies with a distinct OAg composition (30, 31). LPS staining analysis revealed a banding pattern distinct from that of F. tularensis (Fig. 3A). F. novicida OAg had a higher molecular weight than F. tularensis OAg. Distinct clusters of OAg formation suggested OAg chain-length regulation by wzz-like gene(s) in the Francisella genome. Bioinformatics analysis identified two wzz gene candidates in both F. tularensis and F. novicida. Initially, we generated an F. tularensis ∆wzz1 mutant and found it unable to regulate OAg size (Fig. 3B). We then transformed the ∆wzz1 mutant with a plasmid carrying the wzz2 gene from F. novicida, which is responsible for generating the higher molecular-weight OAg. The resultant F. tularensis ∆wzz1/wzz2Fn had an OAg of significantly larger molecular size than the LVS strain (Fig. 3B). The optimized hydrolytic condition (condition F, Table 1) was used to extract the LPS from the F. tularensis ∆wzz1/wzz2Fn mutant strain, and an OAg population with an average molecular size of 220 kDa was obtained [very high molecular weight (VHMW); Fig. 3C]. The 1H NMR spectrum of VHMW OAg was identical to those of LMW and HMW OAg and similar to previously reported structures (21, 22). Therefore, genetic manipulation did not affect the structure of the single repeating unit (SI Appendix, Fig. S4).
Fig. 3.
Heterologous expression of Wzz2 from F. novicida to produce OAg of larger molecular size in F. tularensis. (A) Silver staining analysis comparing LPS from F. tularensis (Ft) LVS with LPS from F. novicida U112. (B) Generation of an Ft LVS mutant with an increased OAg size by heterologous expression of chain-length regulator gene wzz2 from the related subspecies F. novicida. (C) SEC profile of VHMW in comparison to HMW and LMW OAgs. This profile was run on a Superose 6 10/300 GL column at 0.5 mL⋅min−1 with 1× PBS (pH 7.4). The average molecular weight was calculated with a dextran calibration curve.
Conjugation of VHMW OAg Results in a Glycoconjugate Vaccine Providing Full Protection Against High-Dose Intranasal Challenge.
To evaluate the importance of OAg size in glycoconjugate vaccines against F. tularensis, we performed a second immunization study, coupling TT to each of the three sizes of OAg: the native size (HMW-TT), the reduced size obtained by acid hydrolysis (LMW-TT), and the increased size obtained by modification of the chain-length regulator gene wzz (VHMW-TT). Glycoconjugates were synthesized as previously reported (13, 19) (SI Appendix, Fig. S2). After purification, conjugates were characterized (Table 2) and tested in vivo. As a positive control, mice were vaccinated with the live-attenuated F. tularensis mutant ∆kdhAB (26). Four weeks after the last vaccine dose, sera were collected and OAg-specific IgG antibody titers were determined by ELISA on LPS-coated plates (Fig. 4A). LMW-TT generated higher levels of anti-OAg IgG than either HMW-TT or VHMW-TT. To evaluate the impact of OAg size on conjugate vaccine-induced protective immunity, we increased the bacterial challenge dose over that used in the earlier study shown in Fig. 2. All groups were challenged intranasally with 1 × 104 cfu (∼100 LD50) of F. tularensis LVS (Fig. 4B). Again, the LMW-TT conjugate induced higher antibody titers than either the HMW-TT or VHMW-TT conjugate; however, of great interest, only the VHMW-TT conjugate was protective.
Fig. 4.
VHMW OAg produces a glycoconjugate vaccine conferring protection against intranasal challenge with a high dose of F. tularensis LVS. (A) Anti-OAg IgG ELISA units to the F. tularensis (Ft) LPS coating agent measured at day 49. Dots represent individual animals. Horizontal bars represent mean ± SD values. i.n., intranasal. (B) Survival of mice intranasally (IN) challenged with Ft (100-fold the IN LD50) after immunization with VHMW-TT, HMW-TT, LMW-TT, or Ft ∆kdhAB or administration of adjuvant alone. There were eight mice per group. **P ≤ 0.01. ns, not significant.
The Size of the OAg Affects Relative Affinity for LPS-Specific Antibodies.
We further characterized the antibodies induced by immunization with the different glycoconjugates by measuring polysaccharide-specific IgM titers (SI Appendix, Fig. S5A) as well as IgG subclass responses (SI Appendix, Fig. S5B). The IgM titers induced by the three conjugates were comparable. The IgG1 subclass was induced by all three vaccines, but IgG3 was induced only by LMW-TT. We hypothesized that the highly functional IgG antibodies induced by VHMW-TT were recognizing a chain-length–dependent epitope in the glycan that is less well presented in the HMW-TT conjugate and poorly presented in the LMW-TT glycoconjugate. To test this hypothesis, we measured the ability of antibodies generated by the different vaccines to bind to the VHMW OAg (Fig. 5A). Interestingly, we observed improved recognition of VHMW OAg by VHMW-TT–induced antibodies (Fig. 4A vs. Fig. 5A). We further investigated whether the highly protective IgG antibodies induced by VHMW-TT possess a higher relative affinity for binding to saccharides of different chain length. Using an F. tularensis OAg ELISA inhibition assay (32, 33), we measured the relative binding affinities of F. tularensis OAg-specific antibodies to UV-killed bacteria or LPS (Fig. 5B and SI Appendix, Fig. S6A). These results demonstrate that antibodies directed to the OAg recognize a chain-length–dependent (i.e., conformational) epitope best expressed on the VHMW OAg. Importantly, similar results were obtained when inhibition was compared with use of the corresponding conjugate vaccines bearing different-sized OAg as inhibitors (SI Appendix, Fig. S6B).
Fig. 5.
Size of the OAg affects relative affinity to LPS-specific antibodies. (A) Anti-OAg IgG ELISA units to the F. tularensis ∆wzz1/wzz2Fn LPS coating agent measured at day 49. Dots represent individual animals. Horizontal bars represent mean ± SD values. (B) F. tularensis (Ft) OAg competitive ELISA. Recognition of Ft OAg-specific polyclonal antibody by UV-killed Ft-coated ELISA plates in the presence of LMW (orange), HMW (green), or VHMW (red) OAg as a soluble competitor is shown. The inhibition percentage is calculated in relation to the ELISA signal (A405) with no competition. Data points represent competition percentage values at indicated inhibitor concentrations. Each data point is the mean of duplicate determinations from a representative experiment. Horizontal bars represent mean ± SD values. *P ≤ 0.05.
Discussion
F. tularensis is a facultative intracellular pathogen that causes the potentially fatal disease tularemia in humans and animals. The clinical manifestations of the disease depend on the biotype of the organism, the size of the inoculum, and the port of entry (34). While cutaneous tularemia (the most common form of the disease, resulting from the bite of an infected tick, deerfly, or mosquito) is rarely fatal, pneumonic tularemia (the most potent form of the disease, resulting from inhalation of F. tularensis via the aerosol route) can result in up to 60% mortality if not treated with antibiotics (35). The aerosol route of infection, which can result in fatal pulmonary tularemia, is a likely choice for use in bioterrorism.
Currently, no vaccine is approved for the prevention of tularemia (36). Live-attenuated prototype vaccines have provided the most promising results thus far, revealing the key role of T cell-mediated events in the control of F. tularensis, an intracellular pathogen (14, 37–42). F. tularensis LVS is a type B, live-attenuated investigational vaccine strain that has been used by the US Army for decades to protect laboratory workers. LVS has also been used as a model of murine tularemia in most of the preclinical studies conducted so far. Concerns over safety and reversion represent significant impediments to the licensure of an attenuated vaccine.
The role of humoral immunity, especially in the early phase of intradermal and i.p. infections with type B F. tularensis, has been documented by multiple studies (11–17). The ubiquitous bacterial LPS is the immunodominant antibody target (43), and its importance in the generation of a protective humoral response has been extensively investigated (7, 11, 17–19, 44). More recent observations showed that passive immunization with antibodies to LPS can provide protection against pulmonary tularemia caused by LVS. Passive i.p. transfer of immune serum confers complete protection against intranasal challenge with an otherwise lethal dose of LVS, even when the transfer takes place 24–48 h after the start of infection (45). Immune serum generated by vaccination of mice with inactivated LVS rather than live LVS also affords protection against lethal intranasal LVS challenge (46). In addition, a number of Francisella OAg-specific mAbs have been shown to be protective in the mouse model of F. tularensis LVS infection (47). While the role of antibodies in protection against intracellular pathogens has been controversial, the evidence that LVS can exist in an extracellular form implies that antibodies may be able to access and clear the bacteria. It is generally thought that F. tularensis spreads from the lungs via the hematogenous route to systemic organs such as the liver and spleen, possibly extracellularly (45, 48). As a matter of fact, very few bacteria are recovered from the blood, livers, and spleens of immune serum-treated mice after infection with LVS (45). Recently, other studies have shown that antibodies are active in clearing infections with intracellular pathogens, such as Salmonella and Ehrlichia (49), influenza virus (50), and Legionella pneumophila and Mycobacterium bovis bacillus Calmette–Guérin (51).
Glycoconjugate vaccines directed against encapsulated bacteria have resulted in an enormous decrease in the incidence of serious bacterial infections. These vaccines elicit T cell-dependent immunogenicity against the saccharide (52, 53). With the involvement of T cells, immunological memory is induced, and affinity maturation and isotype switching from IgM to IgG occur. F. tularensis OAg-based glycoconjugate (13, 19), bioconjugate (54, 55), and synthetically derived core-based glycoconjugate (56) vaccines have been tested; however, so far, no protection has been reported against the intranasal route of infection.
Herein, we report the successful prevention of pneumonic LVS tularemia by two conjugate vaccines. A key difference between our approach and previous attempts (19) is the attention paid to the molecular size of the OAg. The size of the OAg was considerably reduced during its purification from LPS after standard acid hydrolytic liberation of lipid A from the OAg. We implemented milder hydrolysis that retains the native polysaccharide size. While we did not perform structural investigation studies to elucidate the site of glycosidic bond breaking during hydrolysis, a previous publication (21) reported the generation of tetrasaccharides with breaking between Qui4NFm and QuiNAc, and partial loss of the formamido bond after partial hydrolysis with 0.1 M hydrochloride. Conjugation of native OAg to TT yielded a vaccine that confers protection against intranasal challenge with live bacteria, while the hydrolyzed LMW OAg conjugate did not confer protection. We hypothesized a direct correlation between OAg size and protection, and in a second study, we tested a larger molecular-size polysaccharide conjugated to TT. In that study, we increased the bacterial challenge dose and compared the efficacy of the VHMW OAg conjugate with that of the HMW and LMW OAg conjugates. Only immunization with the VHMW OAg conjugate induced protection.
To produce the VHMW OAg, we investigated the Wzz family of proteins, which consist of membrane‐associated OAg chain-length regulators controlling the modal number of OAg repeats polymerized by the Wzy protein (57). We found that the OAgs of both F. tularensis and F. novicida are synthesized through the Wzx‐/Wzy‐dependent pathway; however, in contrast to F. tularensis, which possesses only a single functional wzz, F. novicida encodes two different Wzz proteins, each producing a unique OAg modal length. We named the second protein Wzz2 and found that it is responsible for the production of a very large OAg. The complementation of an F. tularensis wzz1 mutant with the plasmid-encoded wzz2 gene from F. novicida led to the synthesis by F. tularensis of an OAg incorporating the number of repeat units governed by the heterologous F. novicida Wzz2 protein. The presence of distinct wzz genes controlling OAg size in gram-negative species has been reported before (58, 59), as has the use of such a strategy to increase the production of an OAg population of a defined size (60), but these genes have not been used to manipulate the molecular size of the polysaccharide, and hence the immunogenicity of a vaccine.
Although protective, the large-sized OAg conjugate induced a much lower IgG titer than the smaller sized, nonprotective OAg glycoconjugate. This observation challenges the paradigm of a direct correlation between the amount of IgG induced by a glycoconjugate and protection. When we characterized the serological response elicited by immunization with different glycoconjugates, we found no differences in IgM levels, and all of the vaccines induced IgG1 antibodies. The LMW-TT conjugate also induced IgG3 production, as well as inducing the highest IgG1 levels of the three vaccines. We hypothesized that the highly functional IgG antibodies induced by the VHMW-TT conjugate possess greater affinity and/or avidity than the antibodies generated by the HMW-TT and LMW-TT conjugates. The relative affinity of antibody binding to saccharides of different chain lengths was measured in an F. tularensis OAg ELISA inhibition assay, where we measured the recognition of polyclonal serum and OAg-specific mAb by UV-killed bacteria and LPS-coated plates in the presence of LMW, HMW, or VHMW OAg as a soluble competitor. The outcome suggested that antibodies induced by immunization recognize a chain-length–dependent (e.g., conformational) epitope better expressed on the larger molecular-size antigen. Importantly, the same result was obtained when we used the differently sized conjugate vaccines as inhibitors, showing that the random technology used for the conjugation of the polysaccharides to the protein did not impair their ability to recognize antibodies. The ELISA methodologies to measure the antibodies against the polysaccharide can be highly dependent on the type of polysaccharide used to coat the plates or in solution. However, while we cannot completely exclude ELISA artifacts, we have tested two different coating antigens (LPS and UV-killed organism) and obtained similar trends in IgG antibody responses [LPS (Fig. 2A) vs. UV-killed organism (SI Appendix, Fig. S3)]. Also, using a competitive ELISA to measure the relative affinity of IgG to OAgs of different size, we saw similar trends with plates coated with either LPS (SI Appendix, Fig. S6A) or UV-killed organism (Fig. 5B). In the competitive ELISA, it is of interest that we observed the same trend when using either a monoclonal (OAg-specific mAb 2034, SI Appendix, Fig. S6A) or polyclonal (α-F. tularensis LVS rabbit polyclonal serum, Fig. 5B) source of IgG. However, the data reported here do not completely exclude the possibility of immune mechanisms (i.e., cellular) in addition to IgG contributing to protection. We think this is less likely since glycoconjugates are humoral vaccines, and it has been shown with many other organisms that they provide protection by inducing antigen-specific IgG.
In conclusion, these results support the notion that the use of large-molecular-size polysaccharide in the context of F. tularensis glycoconjugate vaccines provides superior protection against bacterial challenge. This study is an example of the use of genetic manipulation of OAg size to improve the immunogenicity of a glycoconjugate vaccine. A direct correlation between the sugar size and the immunogenicity of random conjugate vaccines has been observed previously (23, 24, 33), but a relationship of polysaccharide size to the relative affinity of antibody responses has not been reported. The design of antigen able to call into action B cells producing high-affinity antibodies has been a “holy grail” in the vaccine field. It has been predicted that high-affinity antibodies may be the future in the treatment of emerging infectious diseases (61). Further studies will evaluate the impact of this vaccination strategy against the more challenging F. tularensis type A and will characterize the mechanisms involved in B-cell recognition of glycoconjugates bearing differently sized polysaccharides.
Materials and Methods
Mice.
Six-week-old female BALB/c mice were purchased from Taconic Biosciences. All mouse experiments were approved by the Harvard Medical Area Standing Committee on Animals (Animal Protocol IS00000636).
Bacterial Strains and Growth Conditions.
F. tularensis LVS was provided by Karen Elkins (US Food and Drug Administration, Washington, DC), while F. novicida U112 was purchased from BEI Resources. All strains were grown at 37 °C in tryptic soy broth (Sigma) supplemented with ferric pyrophosphate (0.025%) and l-cysteine (0.1%) or on cysteine heart agar (Difco) supplemented with 1% hemoglobin solution (BD Biosciences). When appropriate, hygromycin B (200 μg/mL) and kanamycin (10 μg/mL) were added to broth medium and agar plates.
Construction of the F. tularensis LVS Δwzz1 Deletion Mutant and Plasmids.
The pMP812 and pMP633 plasmids were kind gifts of Martin Pavelka, University of Rochester, Rochester, NY (Table 3). A wzz homolog (FTL_0589) was identified in the F. tularensis LVS genome. A nonfunctional wzz homolog was identified in F. tularensis with a nonsense mutation (FTL_1037 and FTL_1038). The Δwzz1 (FTL_0589 deletion) mutant was constructed by allelic exchange using a procedure described previously (62). To achieve a clean wzz1 deletion, 0.5-kb upstream and downstream DNA regions were PCR-amplified by Q5 polymerase (New England Biolabs) using primers (Table 3) and cloned into pMP812 suicide plasmid using a Gibson Assembly Kit (New England Biolabs) following the manufacturer’s recommendations.
Table 3.
Primers and plasmids used in study
| Primer/plasmid | Sequence/description | Reference |
| Primers | ||
| NHL207 | 5′-gctgcaggaattcgatATCTCCAGTAGAAGCTACG | Upstream region forward wzz1 deletion |
| NHL208 | 5′-gcacaatattTAGCTTGGTTTAAATAACCAAG | Upstream region reverse, wzz1 deletion |
| NHL209 | 5′-ccaagctaAATATTGTGCAAAGACAGATTAAATTATG | Downstream region forward, wzz1 deletion |
| NHL210 | 5′-ggtatcgataagcttgatGATGATCTATGGTTGCTGAAG | Downstream region reverse, wzz1 deletion |
| NHL86 | 5′-atcaagcttatcgataccg | pMP812 forward primer |
| NHL87 | 5′-atcgaattcctgcagccc | pMP812 reverse primer |
| NHL66 | 5′-ctaagaaattctaataatgatTATACCCTTCAAGCTTTGAAAAATAAAC | groEL promoter forward, wzz1 expression |
| NHL203 | 5′-tcagccatAACAATCTTACTCCTTTGTTAAATTATTTTTG | groEL promoter reverse, wzz1 expression |
| NHL204 | 5′-taagattgttATGGCTGAAATTAAAAATGATGAATATATAG | FTL_0589 forward, wzz1 expression |
| NHL208 | 5′gcattatccaatgcagatttagtggtgatggtgatgatgTTGTTTAAGATTTCTATATTCAGTAATC | FTL_0589 reverse, wzz1 expression |
| NHL69 | 5′-atctgcattggataatgcattg | pMP633 forward primer |
| NHL70 | 5′-atcattattagaatttcttagaaatatgaa | pMP633 reverse primer |
| Plasmids | ||
| pMP812 | KmR, sacB suicide vector | (62) |
| pMP633 | HygR, E. coli-F. tularensis shuttle vector | (63) |
| pNA45 | pMP812-derived F. tularensis ∆wzz1 (FTL_0589) deletion plasmid | This work |
| pNA49 | pMP633-derived F. tularensis wzz1 (FTL_0589) expression under the control of groEL promoter | This work |
To generate the VHMW LPS-producing strain, two wzz homologs were identified in F. novicida U112 (FTN_1433 and FTN_0925), and each was separately cloned into pMP633 plasmid under groEL promoter using the Gibson Assembly Kit (Table 3). Plasmids were introduced into F. tularensis LVS by electroporation as described elsewhere (26). LPS analysis showed that the FTN_0925 (wzz2) gene is responsible for the production of VHMW LPS.
OAg Purification.
For the production of OAg, F. tularensis LVS from a fresh cysteine heart agar plate was inoculated into five 25-mL, vented-cap Erlenmeyer flasks, each containing 100 mL of tryptic soy broth with 0.1% cysteine, 0.025% ferric pyrophosphate, and 0.1% antifoam 204 (T-soy with additives). After incubation overnight at 37 °C, the cultures were used to inoculate five 3-L, vented-cap Erlenmeyer flasks containing 2 L of T-soy with additives, which were then incubated as described above for 72 h. Cells were harvested by centrifugation and frozen at −80 °C until used for LPS purification.
LPS was purified by the addition of a hot solution of fresh 50% phenol to thawed F. tularensis LVS cells (at a ratio of 1 g of cells to 10 mL of phenol solution), with subsequent mixing for 2 h at 68 °C with use of sterile glass beads and an overhead mixer. After further mixing overnight at 4 °C, cell debris and phenol were removed by centrifugation (6,000 × g for 20 min at 4 °C) in Teflon FEP centrifuge bottles (Nalgene). After the top aqueous phase was removed, additional phenol was removed by dilution with 1 vol of water and 2 vol of ether. The solution was mixed vigorously for 10 min in a separatory funnel and allowed to separate overnight at room temperature. The bottom aqueous phase was decanted, and residual ether was removed with a rotary evaporator. The sample was lyophilized to reduce its volume before dialysis against water and enzyme treatment. Nucleic acid and protein were degraded by sequential treatment with DNase, RNase, and pronase (Seward Ltd.). The solution was then clarified by low-speed centrifugation (5,000 × g overnight at 4 °C). LPS was sedimented and washed three times with water by ultracentrifugation (60,000 × g overnight at 4 °C).
To obtain different sizes of OAg, LPS was hydrolyzed by treatment with acetic acid [2% (vol/vol)] for 1 h at 90 °C (HMW and VHMW OAgs) or with acetic acid [6% (vol/vol)] for 3 h at 90 °C (LMW OAg). The solution was cooled to room temperature before centrifugation (15,000 × g for 10 min at 4 °C) to sediment the cleared lipid. O polysaccharide was further purified by gel filtration. NMR and wavelength scanning at 206 nm, 260 nm, and 280 nm were performed to establish purity, and a Micro BCA Protein Assay (Thermo Fisher Scientific) was conducted according to the manufacturer’s instructions to confirm the absence of protein/peptide contamination, with BSA as the standard.
Synthesis of OAg-TT Glycoconjugates.
LMW, HMW, and VHMW OAgs were conjugated to TT by a slightly modified, previously described protocol (13, 19). In brief, OAg was dissolved in water (10 mg/mL) at ice-bath temperature, and the pH was adjusted to 10.5 with 0.1 M NaOH. Cyanogen bromide [1:1 (wt/wt) with OAg] in CH3CN (100 mg/mL) was then added. The solution was kept at 0 °C for 2 min before being treated with a solution of adipic acid dihydrazide [1:1 (wt/wt) with OAg] in 0.5 M NaHCO3 (70 mg/mL), and the pH was adjusted to 8.5. The mixture was maintained overnight at 4 °C; following dialysis against distilled water for 3 d with use of a 10-kDa molecular-weight cut-off membrane, the retentate was lyophilized to yield activated OAg.
For conjugation, TT and activated OAg were dissolved (5 mg/mL) in 100 mM 2-(N-morpholino)-ethane sulfonic acid sodium salt buffer (pH 5.8), and the solution was placed on ice for 2 min. Thereafter, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride was added (1 mg/mL), with continuous stirring on ice for 12 h. The mixture was purified by SEC on a HiPrep 16/60 Sephacryl S-300 HR column, with PBS as a running buffer. UV detection at 280 nm was used for conjugate and free protein, and a differential refractometer (dRI) was used for OAg peak detection.
The total saccharide content of the glycoconjugates was quantified by ELISA inhibition (discussed below). Protein content was measured by Micro BCA Protein Assay, and the ratio of saccharide to protein was calculated.
Liquid SEC.
SEC profiles of hydrolyzed OAgs extracted under different hydrolytic conditions from F. tularensis LPS were run on a Sephacryl S-300 HR column (GE Healthcare Life Sciences) at 0.5 mL⋅min−1 in PBS (pH 7.4). The average molecular weight was calculated with a dextran calibration curve (Sigma–Aldrich).
LMW, HMW, and VHMW OAgs extracted from F. tularensis LVS were run on a Superose 6 10/300 GL column (GE Healthcare Life Sciences) at 0.5 mL⋅min−1 in PBS (pH 7.4). The average molecular weight was calculated with a dextran calibration curve (Sigma–Aldrich).
SEC analysis was also used to characterize conjugates, comparing them with free OAg and free TT. All samples were eluted on two Superose 6 10/300 GL columns connected in series for a better separation of conjugate from free saccharide and protein. The mobile phase consisted of PBS (pH 7.4) at 0.5 mL⋅min−1. Void- and bed-volume calibrations were performed with λ-DNA (λ-DNA Molecular Weight Marker III, 0.12–21.2 Kbp; Roche) and sodium azide (NaN3; Merck), respectively. OAg peaks were detected by a dRI, while UV detection at 214 nm and 280 nm was used for free protein and conjugate. For Kd determination, the following equation was used: Kd = (Te − T0)/(Tt − T0), where Te is the elution time of the analyte, T0 is the elution time of the biggest fragment of λ-DNA, and Tt is the elution time of NaN3.
Immunization and Challenge Studies.
Male BALB/cByJ mice (6–8 wk old; The Jackson Laboratory) were caged in a microisolator in a pathogen-free environment in the animal facility at Harvard Medical School; groups of six to eight mice were used in each experiment. Mice received three doses (2 wk apart) of positive-control F. tularensis mutant ∆kdhAB (∼107 cfu, 50 μL per mouse, given intranasally), OAg-TT glycoconjugates bearing a different OAg size (10 μg of OAg, 200 μL per mouse, given i.p. with a 25-gauge needle), or adjuvant alone (negative control). Each dose of glycoconjugate was emulsified with PBS and 0.5 mg of Alhydrogel. On day 56 (4 wk after the third immunization), all mice were challenged intranasally with F. tularensis LVS organisms at different bacterial loads, depending on the experiment. Survival was monitored for 25 d after challenge, at which point the survivors were humanely killed.
Serological Studies.
Serum levels of IgG and IgM to OAg were measured by ELISA with slight modification of a previously described method (13). Microtiter plates (Nunc MaxiSorp flat-bottom) were coated with antigens by overnight incubation at 4 °C. LPS from F. tularensis LVS and from the F. tularensis ∆wzz1/wzz2Fn mutant strain was used at a concentration of 5 μg/mL (100 μL per well) in carbonate buffer, while UV-killed F. tularensis LVS was coated at 5 × 108 cfu/mL (100 μL per well) in carbonate buffer. One day later, excess antigen was removed by washing the wells three times using an automated plate washer with PBS containing 0.05% (vol/vol) Tween 20. Mouse sera were diluted at a starting dilution of 1:100 through a twofold dilution series in incubation buffer composed of PBS containing 0.05% Tween 20 and 0.1% BSA (100 μL per well). Plates were then incubated for 2 h at room temperature and washed three more times as described above. Next, goat anti-mouse IgG or goat anti-mouse IgM conjugated to alkaline phosphatase (Cedarlane Laboratories) at a dilution of 1:3,000 in incubation buffer was added at 100 μL per well and incubated for 2 h at room temperature. Plates were rewashed, and a 100-μL volume of p-nitrophenylphosphate substrate in diethanolamine buffer (phosphatase substrate kit; Kirkegaard and Perry Laboratories) was added to each well. The yellow color that developed was read at 405 nm with a microplate reader. Titers were determined from plots of absorbance at 405 nm versus dilution and were defined as the reciprocal of the dilution giving an A405 equivalent to 0.5.
Data are presented as dots for individual mouse IgG ELISA units. ELISA units were expressed relative to the mouse anti-OAg standard serum curves. One ELISA unit was defined as the reciprocal of the standard serum dilution that gives an A405 value equal to 0.5 in this assay. Horizontal bars represent means ± SD values. Sera were run in triplicate, and results are reported as absorbance values (arithmetic means of the three replicates).
F. tularensis OAg ELISA Inhibition Assay.
An F. tularensis OAg competitive ELISA was performed with slight modification of a previously described protocol (32, 33). In this assay, we measured the recognition of α-F. tularensis LVS rabbit polyclonal serum (Lampire Biological Laboratories) or OAg-specific mAb 2034 by either LPS-coated or UV-killed F. tularensis-coated ELISA plates in the presence of soluble competitors. In particular, the differently sized OAgs (LMW, HMW, and VHMW) or the corresponding molecular-weight glycoconjugates (LMW-TT, HMW-TT, and VHMW-TT) were used as competitors. The α-F. tularensis LVS rabbit polyclonal serum was preabsorbed overnight at 4 °C with an F. tularensis LVS wbtA mutant (7), which expressed no OAg, before the start of the assay to increase the sensitivity of detection. The ELISA protocol is identical to that described above for IgG detection. The differently sized OAgs and glycoconjugate competitors were added at different concentrations at the beginning of the incubation step.
ELISA inhibition was also used to determine the sugar content of the glycoconjugates. In this assay, we measured the recognition of F. tularensis OAg-specific mAb 2034 by LPS-coated ELISA plates in the presence of either the glycoconjugate or the corresponding molecular-weight OAg (as competitors) in relation to the ELISA signal (A405) with no competition. A standard curve generated by competing with OAg at 500, 100, 20, 4, 0.8, and 0.16 μg/mL was used to measure sugar content.
In all experiments, the inhibition percentage was calculated in relation to the ELISA signal (A405) with no competition. Data points represent competition percentage values at indicated inhibitor concentrations. Data are presented as mean ± SD values for triplicate determinations.
Immunoblotting.
Samples for tricine-SDS/PAGE were solubilized by boiling for 5 min in sample buffer [62.5 mM Tris⋅HCl (pH 6.8) containing 5% SDS, 10% glycerol, 10% β-mercaptoethanol, and 0.02% bromophenol] and separated on precast gels containing 10% acrylamide (Invitrogen). For western blot analysis, bands separated on acrylamide gels were transferred onto nitrocellulose membranes using Towbin buffer [25 mM Tris⋅HCl, 192 mM glycine, and 20% methanol (pH 8.3)] and probed with mouse sera diluted 1:200. Membranes were incubated with alkaline phosphatase-conjugated goat-anti-mouse polyclonal antibody (no. M30808; Caltag) and detected using BioRad’s color development kit (no. 170-6432).
Statistical Analysis.
Statistical significance was determined with ordinary one-way ANOVA; GraphPad Prism 7.0c was used. P values of ≤0.05 were considered statistically significant (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001).
Supplementary Material
Acknowledgments
This work was supported by Grants 5R01AI089915 and 5U19AI109764 from the National Institute of Allergy and Infectious Diseases; and by funding from the European Union’s Horizon 2020 Research and Innovation Programme under Marie Skłodowska Curie Grant Agreement 661138.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1900144116/-/DCSupplemental.
References
- 1.Tärnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11:440–451. [PubMed] [Google Scholar]
- 2.McLendon MK, Apicella MA, Allen L-AH. Francisella tularensis: Taxonomy, genetics, and immunopathogenesis of a potential agent of biowarfare. Annu Rev Microbiol. 2006;60:167–185. doi: 10.1146/annurev.micro.60.080805.142126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tärnvik A, Berglund L. Tularaemia. Eur Respir J. 2003;21:361–373. doi: 10.1183/09031936.03.00088903. [DOI] [PubMed] [Google Scholar]
- 4.Eigelsbach HT, Tulis JJ, Overholt EL, Griffith WR. Aerogenic immunization of the monkey and guinea pig with live tularemia vaccine. Proc Soc Exp Biol Med. 1961;108:732–734. doi: 10.3181/00379727-108-27049. [DOI] [PubMed] [Google Scholar]
- 5.Oyston PCF, Quarry JE. Tularemia vaccine: Past, present and future. Antonie van Leeuwenhoek. 2005;87:277–281. doi: 10.1007/s10482-004-6251-7. [DOI] [PubMed] [Google Scholar]
- 6.Conlan JW. Vaccines against Francisella tularensis–Past, present and future. Expert Rev Vaccines. 2004;3:307–314. doi: 10.1586/14760584.3.3.307. [DOI] [PubMed] [Google Scholar]
- 7.Sebastian S, et al. A defined O-antigen polysaccharide mutant of Francisella tularensis live vaccine strain has attenuated virulence while retaining its protective capacity. Infect Immun. 2007;75:2591–2602. doi: 10.1128/IAI.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, et al. Attenuation and protective efficacy of an O-antigen-deficient mutant of Francisella tularensis LVS. Microbiology. 2007;153:3141–3153. doi: 10.1099/mic.0.2007/006460-0. [DOI] [PubMed] [Google Scholar]
- 9.Twine S, et al. A mutant of Francisella tularensis strain SCHU S4 lacking the ability to express a 58-kilodalton protein is attenuated for virulence and is an effective live vaccine. Infect Immun. 2005;73:8345–8352. doi: 10.1128/IAI.73.12.8345-8352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjöstedt A. Virulence determinants and protective antigens of Francisella tularensis. Curr Opin Microbiol. 2003;6:66–71. doi: 10.1016/s1369-5274(03)00002-x. [DOI] [PubMed] [Google Scholar]
- 11.Fulop M, Manchee R, Titball R. Role of lipopolysaccharide and a major outer membrane protein from Francisella tularensis in the induction of immunity against tularemia. Vaccine. 1995;13:1220–1225. doi: 10.1016/0264-410x(95)00062-6. [DOI] [PubMed] [Google Scholar]
- 12.Fulop M, Manchee R, Titball R. Role of two outer membrane antigens in the induction of protective immunity against Francisella tularensis strains of different virulence. FEMS Immunol Med Microbiol. 1996;13:245–247. doi: 10.1111/j.1574-695X.1996.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 13.Conlan JW, Shen H, Webb A, Perry MB. Mice vaccinated with the O-antigen of Francisella tularensis LVS lipopolysaccharide conjugated to bovine serum albumin develop varying degrees of protective immunity against systemic or aerosol challenge with virulent type A and type B strains of the pathogen. Vaccine. 2002;20:3465–3471. doi: 10.1016/s0264-410x(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 14.Conlan JW, Sjöstedt A, North RJ. CD4+ and CD8+ T-cell-dependent and -independent host defense mechanisms can operate to control and resolve primary and secondary Francisella tularensis LVS infection in mice. Infect Immun. 1994;62:5603–5607. doi: 10.1128/iai.62.12.5603-5607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conlan JW, Vinogradov E, Monteiro MA, Perry MB. Mice intradermally-inoculated with the intact lipopolysaccharide, but not the lipid A or O-chain, from Francisella tularensis LVS rapidly acquire varying degrees of enhanced resistance against systemic or aerogenic challenge with virulent strains of the pathogen. Microb Pathog. 2003;34:39–45. doi: 10.1016/s0882-4010(02)00194-8. [DOI] [PubMed] [Google Scholar]
- 16.Elkins KL, Rhinehart-Jones T, Nacy CA, Winegar RK, Fortier AH. T-cell-independent resistance to infection and generation of immunity to Francisella tularensis. Infect Immun. 1993;61:823–829. doi: 10.1128/iai.61.3.823-829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreisbach VC, Cowley S, Elkins KL. Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon. Infect Immun. 2000;68:1988–1996. doi: 10.1128/iai.68.4.1988-1996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fulop M, Mastroeni P, Green M, Titball RW. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine. 2001;19:4465–4472. doi: 10.1016/s0264-410x(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 19.Sebastian S, et al. Cellular and humoral immunity are synergistic in protection against types A and B Francisella tularensis. Vaccine. 2009;27:597–605. doi: 10.1016/j.vaccine.2008.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westphal O, Westphal O, Jann K. 1965 Bacterial lipopolysaccharide-extraction with phenol water and further application of procedure. Available at https://www.scienceopen.com/document?vid=6865c59b-264b-4993-ae31-5cdfbc50571a. Accessed August 9, 2018.
- 21.Vinogradov EV, et al. Structure of the O-antigen of Francisella tularensis strain 15. Carbohydr Res. 1991;214:289–297. doi: 10.1016/0008-6215(91)80036-m. [DOI] [PubMed] [Google Scholar]
- 22.Vinogradov E, Perry MB, Conlan JW. Structural analysis of Francisella tularensis lipopolysaccharide. Eur J Biochem. 2002;269:6112–6118. doi: 10.1046/j.1432-1033.2002.03321.x. [DOI] [PubMed] [Google Scholar]
- 23.Szu SC, et al. Comparative immunogenicities of Vi polysaccharide-protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high- or lower-molecular-weight Vi. Infect Immun. 1989;57:3823–3827. doi: 10.1128/iai.57.12.3823-3827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peeters CC, et al. A comparative study of the immunogenicity of pneumococcal type 4 polysaccharide and oligosaccharide tetanus toxoid conjugates in adult mice. J Immunol. 1991;146:4308–4314. [PubMed] [Google Scholar]
- 25.Wessels MR, et al. Structural properties of group B streptococcal type III polysaccharide conjugate vaccines that influence immunogenicity and efficacy. Infect Immun. 1998;66:2186–2192. doi: 10.1128/iai.66.5.2186-2192.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okan NA, et al. Kdo hydrolase is required for Francisella tularensis virulence and evasion of TLR2-mediated innate immunity. MBio. 2013;4:e00638-12. doi: 10.1128/mBio.00638-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reeves PR, et al. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 1996;4:495–503. doi: 10.1016/s0966-842x(97)82912-5. [DOI] [PubMed] [Google Scholar]
- 28.Islam ST, Lam JS. Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway. Can J Microbiol. 2014;60:697–716. doi: 10.1139/cjm-2014-0595. [DOI] [PubMed] [Google Scholar]
- 29.Kim T-H, et al. Characterization of the O-antigen polymerase (Wzy) of Francisella tularensis. J Biol Chem. 2010;285:27839–27849. doi: 10.1074/jbc.M110.143859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinogradov E, Conlan WJ, Gunn JS, Perry MB. Characterization of the lipopolysaccharide O-antigen of Francisella novicida (U112) Carbohydr Res. 2004;339:649–654. doi: 10.1016/j.carres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 31.Thomas RM, et al. The immunologically distinct O antigens from Francisella tularensis subspecies tularensis and Francisella novicida are both virulence determinants and protective antigens. Infect Immun. 2007;75:371–378. doi: 10.1128/IAI.01241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wessels MR, Muñoz A, Kasper DL. A model of high-affinity antibody binding to type III group B Streptococcus capsular polysaccharide. Proc Natl Acad Sci USA. 1987;84:9170–9174. doi: 10.1073/pnas.84.24.9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wessels MR, Kasper DL. Antibody recognition of the type 14 pneumococcal capsule. Evidence for a conformational epitope in a neutral polysaccharide. J Exp Med. 1989;169:2121–2131. doi: 10.1084/jem.169.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulu-Kilic A, Doganay M. An overview: Tularemia and travel medicine. Travel Med Infect Dis. 2014;12:609–616. doi: 10.1016/j.tmaid.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Oyston PCF, Sjostedt A, Titball RW. Tularaemia: Bioterrorism defence renews interest in Francisella tularensis. Nat Rev Microbiol. 2004;2:967–978. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- 36.Sunagar R, Kumar S, Franz BJ, Gosselin EJ. Tularemia vaccine development: Paralysis or progress? Vaccine (Auckl) 2016;6:9–23. doi: 10.2147/VDT.S85545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elkins KL, Rhinehart-Jones TR, Culkin SJ, Yee D, Winegar RK. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect Immun. 1996;64:3288–3293. doi: 10.1128/iai.64.8.3288-3293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yee D, Rhinehart-Jones TR, Elkins KL. Loss of either CD4+ or CD8+ T cells does not affect the magnitude of protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J Immunol. 1996;157:5042–5048. [PubMed] [Google Scholar]
- 39.Ericsson M, Kroca M, Johansson T, Sjöstedt A, Tärnvik A. Long-lasting recall response of CD4+ and CD8+ alphabeta T cells, but not gammadelta T cells, to heat shock proteins of francisella tularensis. Scand J Infect Dis. 2001;33:145–152. doi: 10.1080/003655401750065562. [DOI] [PubMed] [Google Scholar]
- 40.Ericsson M, Sandström G, Sjöstedt A, Tärnvik A. Persistence of cell-mediated immunity and decline of humoral immunity to the intracellular bacterium Francisella tularensis 25 years after natural infection. J Infect Dis. 1994;170:110–114. doi: 10.1093/infdis/170.1.110. [DOI] [PubMed] [Google Scholar]
- 41.Poquet Y, et al. Expansion of Vgamma9 Vdelta2 T cells is triggered by Francisella tularensis-derived phosphoantigens in tularemia but not after tularemia vaccination. Infect Immun. 1998;66:2107–2114. doi: 10.1128/iai.66.5.2107-2114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowley SC, et al. CD4-CD8- T cells control intracellular bacterial infections both in vitro and in vivo. J Exp Med. 2005;202:309–319. doi: 10.1084/jem.20050569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barry EM, Cole LE, Santiago AE. Vaccines against tularemia. Hum Vaccin. 2009;5:832–838. doi: 10.4161/hv.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drabick JJ, Narayanan RB, Williams JC, Leduc JW, Nacy CA. Passive protection of mice against lethal Francisella tularensis (live tularemia vaccine strain) infection by the sera of human recipients of the live tularemia vaccine. Am J Med Sci. 1994;308:83–87. doi: 10.1097/00000441-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Kirimanjeswara GS, Golden JM, Bakshi CS, Metzger DW. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J Immunol. 2007;179:532–539. doi: 10.4049/jimmunol.179.1.532. [DOI] [PubMed] [Google Scholar]
- 46.Lavine CL, et al. Immunization with heat-killed Francisella tularensis LVS elicits protective antibody-mediated immunity. Eur J Immunol. 2007;37:3007–3020. doi: 10.1002/eji.200737620. [DOI] [PubMed] [Google Scholar]
- 47.Lu Z, et al. Protective B-cell epitopes of Francisella tularensis O-polysaccharide in a mouse model of respiratory tularaemia. Immunology. 2012;136:352–360. doi: 10.1111/j.1365-2567.2012.03589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forestal CA, et al. Francisella tularensis has a significant extracellular phase in infected mice. J Infect Dis. 2007;196:134–137. doi: 10.1086/518611. [DOI] [PubMed] [Google Scholar]
- 49.Casadevall A, Pirofski LA. A reappraisal of humoral immunity based on mechanisms of antibody-mediated protection against intracellular pathogens. Adv Immunol. 2006;91:1–44. doi: 10.1016/S0065-2776(06)91001-3. [DOI] [PubMed] [Google Scholar]
- 50.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166:7381–7388. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 51.Joller N, et al. Antibodies protect against intracellular bacteria by Fc receptor-mediated lysosomal targeting. Proc Natl Acad Sci USA. 2010;107:20441–20446. doi: 10.1073/pnas.1013827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Avery OT, Goebel WF. Chemo-immunological studies on conjugated carbohydrate-proteins: II. Immunological specificity of synthetic sugar-protein antigens. J Exp Med. 1929;50:533–550. doi: 10.1084/jem.50.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avery OT, Goebel WF. Chemo-immunological studies on conjugated carbohydrate-proteins: V. The immunological specifity of an antigen prepared by combining the capsular polysaccharide of type III pneumococcus with foreign protein. J Exp Med. 1931;54:437–447. doi: 10.1084/jem.54.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuccui J, et al. Exploitation of bacterial N-linked glycosylation to develop a novel recombinant glycoconjugate vaccine against Francisella tularensis. Open Biol. 2013;3:130002. doi: 10.1098/rsob.130002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen L, et al. Outer membrane vesicles displaying engineered glycotopes elicit protective antibodies. Proc Natl Acad Sci USA. 2016;113:E3609–E3618. doi: 10.1073/pnas.1518311113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boltje TJ, et al. Chemical synthesis and immunological evaluation of the inner core oligosaccharide of Francisella tularensis. J Am Chem Soc. 2012;134:14255–14262. doi: 10.1021/ja306274v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim T-H, Pinkham JT, Heninger SJ, Chalabaev S, Kasper DL. Genetic modification of the O-polysaccharide of Francisella tularensis results in an avirulent live attenuated vaccine. J Infect Dis. 2012;205:1056–1065. doi: 10.1093/infdis/jir620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daniels C, Griffiths C, Cowles B, Lam JS. Pseudomonas aeruginosa O-antigen chain length is determined before ligation to lipid A core. Environ Microbiol. 2002;4:883–897. doi: 10.1046/j.1462-2920.2002.00288.x. [DOI] [PubMed] [Google Scholar]
- 59.Murray GL, Attridge SR, Morona R. Regulation of Salmonella typhimurium lipopolysaccharide O antigen chain length is required for virulence; identification of FepE as a second Wzz. Mol Microbiol. 2003;47:1395–1406. doi: 10.1046/j.1365-2958.2003.03383.x. [DOI] [PubMed] [Google Scholar]
- 60.Hegerle N, et al. Overexpression of O-polysaccharide chain length regulators in Gram-negative bacteria using the Wzx-/Wzy-dependent pathway enhances production of defined modal length O-polysaccharide polymers for use as haptens in glycoconjugate vaccines. J Appl Microbiol. 2018;125:575–585. doi: 10.1111/jam.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marston HD, Paules CI, Fauci AS. Monoclonal antibodies for emerging infectious diseases–Borrowing from history. N Engl J Med. 2018;378:1469–1472. doi: 10.1056/NEJMp1802256. [DOI] [PubMed] [Google Scholar]
- 62.LoVullo ED, Molins-Schneekloth CR, Schweizer HP, Pavelka MS., Jr Single-copy chromosomal integration systems for Francisella tularensis. Microbiology. 2009;155:1152–1163. doi: 10.1099/mic.0.022491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.LoVullo ED, Sherrill LA, Pavelka MS., Jr Improved shuttle vectors for Francisella tularensis genetics. FEMS Microbiol Lett. 2009;291:95–102. doi: 10.1111/j.1574-6968.2008.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.