Significance
We do not know how and why belowground biodiversity may change as soils develop over centuries to millennia, hampering our ability to predict the myriad of ecosystem processes regulated by belowground organisms under changing environments. We conducted a global survey of 16 soil chronosequences spanning a wide range of ecosystem types and found that in less productive ecosystems, increases in belowground biodiversity followed increases in plant cover, but in more productive ecosystems, acidification during soil development was often associated with declines in belowground biodiversity. The biodiversity of multiple soil organisms exhibited similar patterns over time, but in contrast to expectations, changes in plant diversity were not associated with corresponding changes in belowground biodiversity.
Keywords: soil biodiversity, ecosystem development, global scale, acidification, soil chronosequences
Abstract
Belowground organisms play critical roles in maintaining multiple ecosystem processes, including plant productivity, decomposition, and nutrient cycling. Despite their importance, however, we have a limited understanding of how and why belowground biodiversity (bacteria, fungi, protists, and invertebrates) may change as soils develop over centuries to millennia (pedogenesis). Moreover, it is unclear whether belowground biodiversity changes during pedogenesis are similar to the patterns observed for aboveground plant diversity. Here we evaluated the roles of resource availability, nutrient stoichiometry, and soil abiotic factors in driving belowground biodiversity across 16 soil chronosequences (from centuries to millennia) spanning a wide range of globally distributed ecosystem types. Changes in belowground biodiversity during pedogenesis followed two main patterns. In lower-productivity ecosystems (i.e., drier and colder), increases in belowground biodiversity tracked increases in plant cover. In more productive ecosystems (i.e., wetter and warmer), increased acidification during pedogenesis was associated with declines in belowground biodiversity. Changes in the diversity of bacteria, fungi, protists, and invertebrates with pedogenesis were strongly and positively correlated worldwide, highlighting that belowground biodiversity shares similar ecological drivers as soils and ecosystems develop. In general, temporal changes in aboveground plant diversity and belowground biodiversity were not correlated, challenging the common perception that belowground biodiversity should follow similar patterns to those of plant diversity during ecosystem development. Taken together, our findings provide evidence that ecological patterns in belowground biodiversity are predictable across major globally distributed ecosystem types and suggest that shifts in plant cover and soil acidification during ecosystem development are associated with changes in belowground biodiversity over centuries to millennia.
Belowground organisms play critical roles in maintaining the rates and stability of multiple ecosystem processes, including plant productivity, decomposition, and nutrient cycling (1–3). Complementary ecological theories have been proposed to explain belowground biodiversity patterns, including theories related to aboveground and belowground resource availability, nutrient stoichiometry, and abiotic environmental factors (1–12) (SI Appendix, Table S1). However, and despite a longstanding interest in the topic (4–8), the patterns in belowground biodiversity as soils develop over centuries to millennia (pedogenesis), as well as the environmental factors responsible for those patterns, remain largely unresolved. It is also unclear whether belowground biodiversity follows a similar trend to that of plant diversity during pedogenesis (4–6), which often exhibits a positive or hump-shaped relationship attributed to changes in abiotic environmental factors (e.g., acidification) and soil resource availability (e.g., soil phosphorus) as soils develop (4–6). Improving our knowledge of the mechanisms driving changes in belowground biodiversity during pedogenesis is critical for predicting both global ecological patterns and the many ecosystem processes regulated by belowground organisms (1–3).
There are two main reasons why we lack a mechanistic understanding of how belowground biodiversity changes during pedogenesis. First, studies of belowground biodiversity patterns with pedogenesis have mostly been conducted on a few individual soil chronosequences (13–17), with such work focusing mainly on a single group of belowground organisms, such as bacteria (16), fungi (18) or protists (19), or on changes in microbial biomass and community structure (17). Although such studies provide valuable information, pedogenesis often follows different trajectories depending on such factors as soil parent material and climate (7, 8, 19–21). Moreover, multiple taxa should be considered in concert to achieve a holistic understanding of how belowground biodiversity changes during pedogenesis. Second, most studies reported to date have focused on changes in belowground biodiversity during initial stages of primary succession (i.e., years to centuries) (13, 22), with few studies evaluating effects over much longer time scales (i.e., from centuries to thousands or millions of years) (13, 15, 16). The fate of belowground biodiversity is expected to differ between early and late stages of pedogenesis, because older ecosystems may enter a retrogressive phase (19, 23–25). This stage of ecosystem development is typically characterized by reduced resource availability [e.g., soil phosphorus (P), carbon (C), plant biomass], altered soil nutrient stoichiometry [e.g., increased nitrogen (N):P ratios], and soil acidification (19, 23–26), which could change the long-term development of belowground biodiversity.
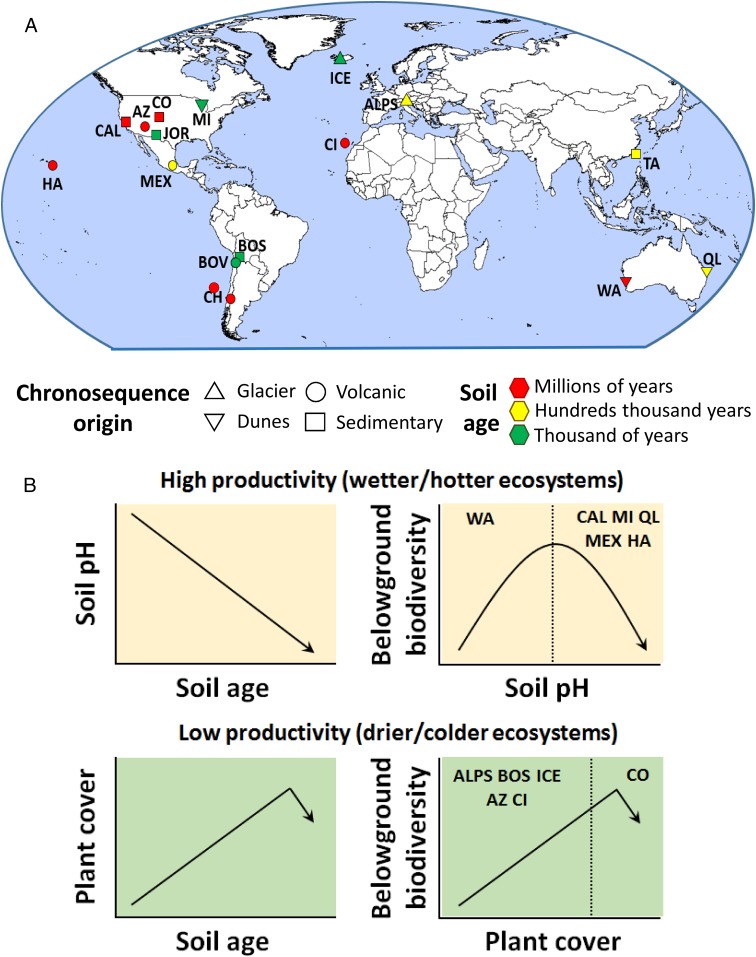
Here we considered multiple complementary ecological theories, based on aboveground and belowground resource availability, nutrient stoichiometry, and abiotic environmental factors (SI Appendix, Table S1), to identify the predominant mechanisms driving the changes in belowground biodiversity during pedogenesis across ecosystem types (SI Appendix, Materials and Methods). Toward this aim, we conducted soil and vegetation surveys across 16 globally distributed chronosequences ranging in age from hundreds to millions of years and encompassing a wide range of climatic conditions (tropical, temperate, continental, polar, and arid), vegetation types (grasslands, shrublands, forests, and croplands), and chronosequence origins (volcanic, sedimentary, dunes, and glaciers) (Fig. 1A and SI Appendix, Tables S2 and S3). The diversity of soil organisms (bacteria, fungi, protists, and invertebrates) was measured via marker gene amplicon sequencing. Data on the dominant bacterial, fungal, protist, and invertebrate taxa detected are provided in SI Appendix, Table S4.
Fig. 1.
Geographical distribution and major patterns showing the fate of belowground biodiversity during pedogenesis. Belowground biodiversity is defined as the standardized average of the diversity of soil bacteria, fungi, protists, and invertebrates. (A) Locations of the 16 soil chronosequences (87 plots) included in this study. (B) A conceptual figure summarizing the major ecological patterns observed (data provided in Figs. 2 and 3). Acronyms for each chronosequence are listed in SI Appendix, Table S2. Correlations between pH and plant cover with belowground diversity across sites are shown in SI Appendix, Fig. S26.
Results and Discussion
Species richness (i.e., number of phylotypes) and Shannon diversity of soil bacteria, fungi, protists, and invertebrates were highly correlated (SI Appendix, Figs. S1–S4). Consequently, we used richness as our metric of diversity in further analyses. Importantly, we found that the richness (“diversity” hereinafter) of soil bacteria, fungi, protists, and invertebrates across each chronosequence was generally well correlated over time (SI Appendix, Tables S5 and S6), and so we used an integrated index of belowground biodiversity to evaluate changes in diversity with pedogenesis (SI Appendix, Material and Methods). This index was positively and significantly correlated with the biodiversity of the major groups of organisms in >92% of the cases (59 out of 64 cases; SI Appendix, Table S6). The strong positive correlations among soil bacteria, fungi, protists, and invertebrates suggest that the changes in the biodiversity of multiple soil organisms during pedogenesis are driven by similar ecological factors.
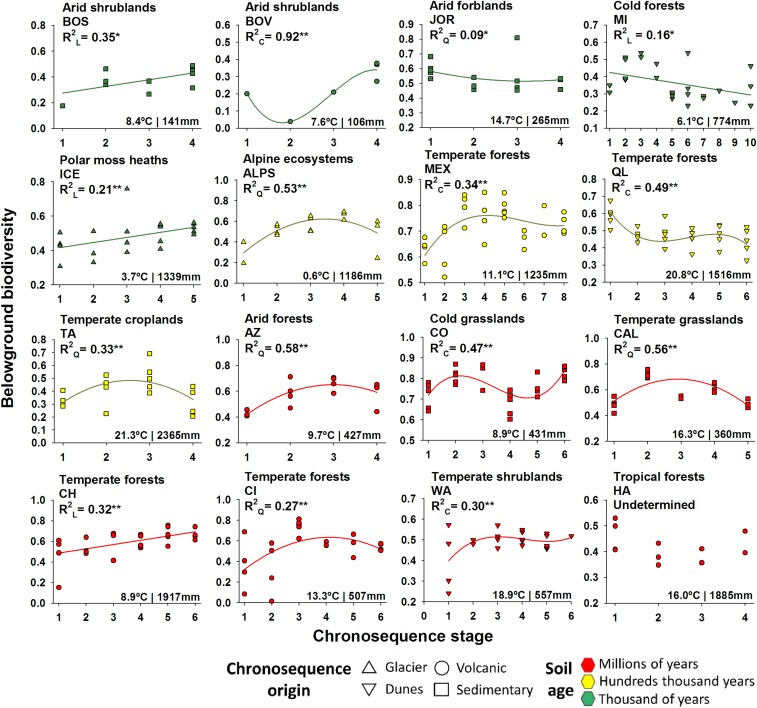
We then identified the form of the relationship between chronosequence stage and belowground biodiversity within each chronosequence. For this, we considered the three most common regression models used to evaluate changes in soil attributes during pedogenesis: linear, quadratic, and cubic (4–6, 17, 27) (SI Appendix, Material and Methods and Table S7). We found a high degree of variation in the observed patterns across the 16 soil chronosequences (Fig. 2). In most cases, belowground biodiversity took thousands to millions of years to reach its maximum as it followed either a positive (linear or cubic: seven cases) or hump-shaped (quadratic; five cases) relationship with chronosequence stage (Fig. 2 and SI Appendix, Figs. S5–S8 and Table S7). Changes in belowground biodiversity during pedogenesis were not influenced by including chronosequences of very different age ranges (from thousands to millions of years), as several patterns were found within each soil age range (Fig. 2). We found similar results when evaluating the relationships between chronosequence stage and the diversity of soil bacteria, fungi, protists, and invertebrates individually (SI Appendix, Figs. S5–S8 and Table S7). In support of this, the dissimilarity in belowground community composition consistently increased with chronosequence stage (SI Appendix, Figs. S9–S13 and Table S8), which suggests that belowground communities become more dissimilar as pedogenesis proceeds. Further discussions about the changes in belowground community composition during ecosystem development, based on the results reported below, are available in SI Appendix, Extended Discussion.
Fig. 2.
Changes in belowground biodiversity during pedogenesis. Shown are the relationships between chronosequence stage and belowground biodiversity across 16 globally distributed soil chronosequences. *P ≤ 0.05; **P < 0.01.
Perennial plant diversity (“plant diversity” hereinafter) was not correlated with belowground biodiversity in 75% of the studied soil chronosequences (12 out of 16 cases; SI Appendix, Fig. S14). Furthermore, and unlike previously reported positive relationships between chronosequence stage and plant diversity (4–6), we detected a high degree of variation in the responses of plant diversity to pedogenesis (SI Appendix, Fig. S15; SI Appendix, Table S9 presents the most important environmental factors associated with perennial plant diversity). In particular, we found positive (25% of cases), negative (18% of cases), and neutral (57% of cases) relationships between the diversity of plants and belowground communities. Matching patterns of plant and soil biodiversity were not associated with any particular type of ecosystem (SI Appendix, Table S9). In contrast to expectations (4–6), which have developed largely from work on individual soil chronosequences typically located in temperate environments, the observed changes in plant diversity during pedogenesis were highly variable. We acknowledge that directly comparing patterns in the diversity of plants and soil organisms is not straightforward, due to differences in spatial scales, organism sizes, and taxonomic resolution; however, despite this important caveat, we still compared soil and plant diversity patterns during pedogenesis (SI Appendix, Figs. S14 and S15; compare with Fig. 2). Our findings challenge the common expectation that belowground biodiversity mirrors aboveground diversity during pedogenesis (4, 9, 16).
We then sought to identify the most important environmental factors associated with belowground biodiversity across the 16 chronosequences studied (Materials and Methods). We first used random forest modeling to identify those environmental factors that change during pedogenesis related to each chronosequence (SI Appendix, Fig. S16). Environmental factors included aboveground (plant cover) and belowground (soil total organic C and available P) resource availability, nutrient stoichiometry (soil C:N and N:P ratios, calculated from soil total organic C, total N, and total P) and other soil abiotic factors (soil salinity, pH, and texture: % clay + silt). These factors were then selected as potential predictors of changes in belowground biodiversity and the diversity of individual taxonomic groups during pedogenesis. Statistical modeling was conducted independently for each of the 16 soil chronosequences. The rationale for including soil available P and plant cover in our models is provided in SI Appendix, Environmental Predictors of Belowground Biodiversity During Pedogenesis.
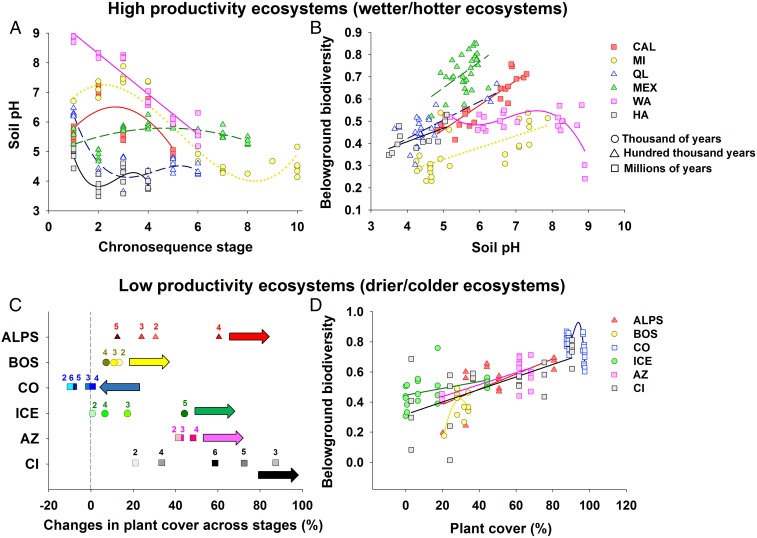
Our random forest analyses provided evidence that plant cover and soil pH are the most important statistical predictors of changes in belowground biodiversity during pedogenesis (Fig. 3 and SI Appendix, Figs. S17–S23). We then used hierarchical clustering to test the importance of environmental factors in predicting belowground biodiversity (from random forest modeling) and to classify our 16 soil chronosequences by the major ecological patterns associated with the observed changes in belowground biodiversity during pedogenesis. Most chronosequences were clustered by either soil pH or plant cover (6 out of 16 in both cases) as the major factors associated with the changes in belowground biodiversity during pedogenesis (Fig. 3 and SI Appendix, Fig. S24). Interestingly, on average, locations for chronosequences in which belowground biodiversity was associated with plant cover also had significantly lower ecosystem productivity and harsher climatic conditions (i.e., lower temperature and precipitation) compared with those in which belowground biodiversity was associated with soil pH (SI Appendix, Fig. S25). In other words, soil chronosequences in which belowground biodiversity was positively correlated with plant cover had lower ecosystem productivity and corresponded with colder and drier ecosystems (SI Appendix, Figs. S24 and S25). In these ecosystems, increases in plant cover during pedogenesis were typically associated with increases in belowground biodiversity (Figs. 1B and 3 and SI Appendix, Tables S10 and S11). The sole exception to this pattern was a very old (millions of years) chronosequence located in semiarid grasslands in Colorado, in which a reduction in plant cover late in pedogenesis was associated with reductions in belowground biodiversity (Figs. 1B and 3).
Fig. 3.
Major ecological drivers of the fate of belowground biodiversity during pedogenesis. (A and B) High-productivity ecosystems. (C and D) Low-productivity ecosystems. Statistical support for these patterns is provided in SI Appendix, Tables S10 and S11. In C, the numbers indicate the chronosequence stage, and the arrows indicate the overall directions for the changes in plant cover across stages. Changes in plant cover across chronosequence stages are calculated from stage 1 in each chronosequence.
Conversely, our findings indicate that on average, chronosequences in which soil pH was strongly correlated with changes in belowground biodiversity during pedogenesis had higher ecosystem productivity, corresponding with warmer and wetter ecosystems (SI Appendix, Figs. S24 and S25). In these ecosystems, a drop in soil pH during pedogenesis was associated (Fig. 3A) with a reduced number of soil taxa in most cases (Figs. 1B and 3 and SI Appendix, Tables S10 and S11). This pattern likely can be attributed to environmental filtering linked to soil acidification, which is a result of intense weathering (Figs. 1B and 3 and SI Appendix, Figs. S25 and S26). Such a pattern has been reported for another highly productive and wet chronosequence from New Zealand not included in our study (16). The sole exception to this pattern was observed in an ecosystem with very high initial soil pH located in warm Mediterranean shrublands from Western Australia. Alkaline soils in young sand dunes (pH ∼9) from this chronosequence support low belowground biodiversity, explaining the increase in belowground biodiversity as pH declines during pedogenesis (SI Appendix, Fig. S26). Thus, our results suggest that pH deviations away from neutral are associated with decreased belowground biodiversity during ecosystem development, supporting an overall hump-shaped relationship between soil pH and belowground diversity (Fig. 1B and SI Appendix, Fig. S26). Taken together, these findings reveal the prevalent patterns associated with the changes in belowground biodiversity during pedogenesis and across resource gradients worldwide, and suggest that changes in belowground biodiversity during pedogenesis are predictable across major ecosystem types. We note that the observed soil biodiversity patterns associated with changes in plant cover and pH can be found in soil chronosequences with very different age ranges (Figs. 2 and 3), suggesting that the extent of changes in these key factors, rather than soil age per se, drives soil biodiversity during pedogenesis.
Our findings indicate that the fate of belowground biodiversity during pedogenesis is associated with two major ecological factors across a wide range of globally distributed ecosystem types and environmental conditions: plant cover in less productive systems and acidification in more productive systems. These results are valid for soil chronosequences with very different age ranges (thousands to millions of years). Our results suggest that more productive, wetter, and hotter ecosystems can potentially limit the development of belowground biodiversity as a consequence of the soil acidification associated with pedogenesis. Conversely, in low-productivity, colder, and drier ecosystems, plant cover is positively correlated with the changes in belowground biodiversity during ecosystem development across multiple chronosequences with very different age ranges. Of course, plants not only are a source of C for soil organisms (via litter and root exudates), but also improve microclimatic conditions, especially in the low productivity ecosystems often found in low-temperature and/or arid climates (SI Appendix, Fig. S25). This could explain, for instance, the reduction in belowground biodiversity at the Colorado chronosequence as plant cover declined with soil age in this relatively dry and cold region. In more productive ecosystems (SI Appendix, Fig. S25), acidification can potentially constrain the diversity of soil organisms (SI Appendix, Fig. S26) via multiple interactive mechanisms, including metal toxicity, solubility of essential nutrients, enzyme stability, and internal cell pH regulation.
We also found two other, less common patterns of the changes in belowground biodiversity during pedogenesis. For instance, soil salinity was identified as the most important environmental factor associated with the changes in belowground diversity during pedogenesis in a nonsaline (0.01–0.29 dS m−1) temperate forest from Chile (SI Appendix, Table S2). In addition, soil texture was identified as the most important environmental factor associated with the changes in belowground diversity during ecosystem development in a temperate cropland ecosystem with very high levels of silt and clay (79.5–86.4%) and very high potential weathering rates (i.e., high levels of precipitation and temperature) (SI Appendix, Fig. S25).
The observed correlation between soil pH and belowground biodiversity could be an indirect consequence of reductions in soil P availability as soil develops (23, 24), but our results suggest otherwise. In fact, we expected to identify soil C, N, and P concentrations (or their stoichiometric ratios) as important factors associated with belowground biodiversity during pedogenesis, because soil C is a major energy source for heterotrophic microbes and because resource quality (i.e., C:N and N:P) and soil P concentrations are commonly considered limiting factors for belowground biodiversity during pedogenesis (16, 23, 24). However, in our models, soil N:P ratio, soil total organic C concentration, and soil P availability were never identified as the most important factors associated with observed changes in belowground diversity (SI Appendix, Figs. S17 and S18), and soil C:N ratio was identified as the most important environmental factor only in a volcanic arid chronosequence (SI Appendix, Fig. S27 and Table S2). More importantly, a survey conducted in a 27-y N and P fertilization experiment (24) showed that nutrient additions did not increase belowground biodiversity in very young (0.3 ky; stage 1 in our study) and very old (4,100 ky; stage 4 in our study) soils from Hawaii (SI Appendix, Fig. S28).
In summary, we found that plant cover and soil pH were the most important environmental factors associated with changes in belowground biodiversity during pedogenesis across a wide range of globally distributed ecosystem types. In less productive, drier, and colder ecosystems increases in plant cover during pedogenesis were related to increases in belowground biodiversity, whereas in more productive ecosystems, which are also warmer and wetter, declines in soil pH during pedogenesis were associated with declines in belowground diversity. Moreover, our results suggest that the temporal changes in aboveground plant diversity and belowground biodiversity are not correlated, challenging the common perception that belowground biodiversity should follow similar patterns to those of plant diversity during ecosystem development. Our results also indicate that we need to consider multiple soil chronosequences simultaneously to identify consistent ecological patterns. Taken together, our findings provide insight into the fate of belowground biodiversity during pedogenesis, and ultimately suggest that plant cover and soil acidification drive belowground biodiversity over centuries to millennia on a global scale.
Materials and Methods
Complete documentation of the study sites, field survey, sample collection, and laboratory procedures, as well as additional details on the statistical analyses, are provided in SI Appendix, Materials and Methods. Field data were collected between 2016 and 2017 from 16 soil age chronosequences located in nine countries from six continents (Fig. 1A). Each of the 16 chronosequences studied included between 4 and 10 chronosequence age-based stages (SI Appendix, Tables S2 and S3). At each stage, we conducted a vegetation survey and collected five composite samples of mineral soil (five soil cores 0–10 cm deep, a total of 435 soil samples) and obtained information on aboveground and belowground resource availability, nutrient stoichiometry, and other abiotic factors. The diversity of soil organisms was measured via marker gene amplicon sequencing. Belowground biodiversity was calculated as the standardized average of the diversity (i.e., richness; number of phylotypes) of soil bacteria, fungi, protists, and invertebrates. Detailed information on our regression, random forest, and hierarchical clustering analyses is provided in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
This project received funding from the European Union’s Horizon 2020 research and innovation program under Marie Sklodowska-Curie Grant Agreement 702057. N.F. was supported through grants from the US National Science Foundation (EAR1331828, DEB 1556090). Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the US Government. An extended version of the acknowledgments is provided in SI Appendix.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The primary data used in this paper have been deposited in Figshare: https://figshare.com/s/0dc27c87f09d0a1c6ca3 (doi: 10.6084/m9.figshare.7556675).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1818400116/-/DCSupplemental.
References
- 1.Bardgett RD, van der Putten WH. Belowground biodiversity and ecosystem functioning. Nature. 2014;515:505–511. doi: 10.1038/nature13855. [DOI] [PubMed] [Google Scholar]
- 2.Wagg C, Bender SF, Widmer F, van der Heijden MG. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc Natl Acad Sci USA. 2014;111:5266–5270. doi: 10.1073/pnas.1320054111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delgado-Baquerizo M, et al. Soil microbial communities drive the resistance of ecosystem multifunctionality to global change in drylands across the globe. Ecol Lett. 2017;20:1295–1305. doi: 10.1111/ele.12826. [DOI] [PubMed] [Google Scholar]
- 4.Wardle DA, et al. The response of plant diversity to ecosystem retrogression: Evidence from contrasting long-term chronosequences. Oikos. 2008;117:93–103. [Google Scholar]
- 5.Laliberté E, et al. How does pedogenesis drive plant diversity? Trends Ecol Evol. 2013;28:331–340. doi: 10.1016/j.tree.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Laliberté E, Zemunik G, Turner BL. Environmental filtering explains variation in plant diversity along resource gradients. Science. 2014;345:1602–1605. doi: 10.1126/science.1256330. [DOI] [PubMed] [Google Scholar]
- 7.Jenny H. Factors of Soil Formation: A System of Quantitative Pedology. Dover; New York: 1941. [Google Scholar]
- 8.Crews TE, et al. Changes in soil phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology. 1995;76:1407–1424. [Google Scholar]
- 9.De Deyn GB, Van der Putten WH. Linking aboveground and belowground diversity. Trends Ecol Evol. 2005;20:625–633. doi: 10.1016/j.tree.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Wu T, Ayres E, Bardgett RD, Wall DH, Garey JR. Molecular study of worldwide distribution and diversity of soil animals. Proc Natl Acad Sci USA. 2011;108:17720–17725. doi: 10.1073/pnas.1103824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tedersoo L, et al. Fungal biogeography: Global diversity and geography of soil fungi. Science. 2014;346:1256688. doi: 10.1126/science.1256688. [DOI] [PubMed] [Google Scholar]
- 12.Fierer N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat Rev Microbiol. 2017;15:579–590. doi: 10.1038/nrmicro.2017.87. [DOI] [PubMed] [Google Scholar]
- 13.Tripathi BM, et al. Soil pH mediates the balance between stochastic and deterministic assembly of bacteria. ISME J. 2018;12:1072–1083. doi: 10.1038/s41396-018-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rillig MC, et al. Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant Soil. 2001;233:167–177. [Google Scholar]
- 15.Tarlera S, Jangid K, Ivester AH, Whitman WB, Williams MA. Microbial community succession and bacterial diversity in soils during 77,000 years of ecosystem development. FEMS Microbiol Ecol. 2008;64:129–140. doi: 10.1111/j.1574-6941.2008.00444.x. [DOI] [PubMed] [Google Scholar]
- 16.Jangid K, et al. Progressive and retrogressive ecosystem development coincide with soil bacterial community change in a dune system under lowland temperate rainforest in New Zealand. Plant Soil. 2013;367:235–247. [Google Scholar]
- 17.Wardle DA, Walker LR, Bardgett RD. Ecosystem properties and forest decline in contrasting long-term chronosequences. Science. 2004;305:509–513. doi: 10.1126/science.1098778. [DOI] [PubMed] [Google Scholar]
- 18.Roy-Bolduc A, Laliberté E, Hijri M. High richness of ectomycorrhizal fungi and low host specificity in a coastal sand dune ecosystem revealed by network analysis. Ecol Evol. 2015;6:349–362. doi: 10.1002/ece3.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlson ML, et al. Community development along a proglacial chronosequence: Are above-ground and below-ground community structure controlled more by biotic than abiotic factors? J Ecol. 2010;98:1084–1095. [Google Scholar]
- 20.Walker LR, et al. The use of chronosequences in studies of ecological succession and soil development. J Ecol. 2010;98:725–736. [Google Scholar]
- 21.Alfaro FD, et al. Microbial communities in soil chronosequences with distinct parent material: The effect of soil pH and litter quality. J Ecol. 2017;105:1709–1722. [Google Scholar]
- 22.Ortiz-Álvarez R, Fierer N, de Los Ríos A, Casamayor EO, Barberán A. Consistent changes in the taxonomic structure and functional attributes of bacterial communities during primary succession. ISME J. 2018;12:1658–1667. doi: 10.1038/s41396-018-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker TW, Syers JK. The fate of phosphorus during pedogenesis. Geoderma. 1976;15:1–19. [Google Scholar]
- 24.Vitousek PM. Nutrient Cycling and Limitation: Hawai’i as a Model System. Princeton Univ Press; Princeton, NJ: 2004. [Google Scholar]
- 25.Peltzet DA, et al. Understanding ecosystem retrogression. Ecol Monogr. 2010;80:509–529. [Google Scholar]
- 26.McGill WB, Cole CV. Comparative aspects of cycling organic C, N, S, and P through soil organic matter. Geoderma. 1981;26:267–286. [Google Scholar]
- 27.Wardle DA, et al. Among- and within-species variation in plant litter decomposition in contrasting long-term chronosequences. Funct Ecol. 2009;23:442–453. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.