Significance
Transformed Balb 3T3 cells consume glutamine at a much faster rate than nontransformed cells. The stepwise decrease in glutamine concentration is paralleled by the decreased growth rate of the transformed cells. At the lowest concentration of glutamine used here, the transformed cells stop increasing, while the nontransformed cells continue to increase at a relatively rapid rate without visible damage to the cells. Related results were obtained in NIH 3T3 cells during the process of transformed focus formation. Widely varying the background of amino acids and serum concentrations did not alter the basic results. The findings call for similar experiments with a wider range of cell culture types, extension to experimental animals, and clinical trials in human cancers.
Keywords: transformed cells, nontransformed cells, glutamine regulation
Abstract
The growth-stimulating capacity of calf serum (CS) in cell culture reaches a maximum of 10% with Balb 3T3 cells, remains at a plateau to 40% CS, and declines steeply to 100% CS. Growth capacity can be largely restored to the latter by a combination of cystine and glutamine. Glutamine is a conditionally essential amino acid that continues to function at very low concentrations to support the growth of nontransformed cells, but transformed cells require much larger concentrations to survive. These different requirements hold true over a 10-fold variation in background concentrations of CS and amino acids. The high requirement of glutamine for transformed cells applies to the development of neoplastically transformed foci. These observations have given rise to a novel protocol for cancer therapy based on the large difference in the need for glutamine between nontransformed and transformed cells. This protocol would stop the cumulative growth and survival of the transformed cells without reducing the growth rate of the nontransformed cells. The results call for studies of glutamine deprivation as a treatment for experimental cancer in rodents and clinical trials in humans.
This investigation began as an attempt to understand the observation that spontaneously transformed Balb 3T3 cells have a much higher capacity to multiply in cell culture than in mice (1). The procedure used was to compare the growth-promoting activity of calf lymph (CL), which closely resembles the interstitial fluid of calves, versus the growth-promoting activity of calf serum (CS). CL and CS were used in culture with increases from 10% to 100%, supplemented by the low molecular weight components of the synthetic medium in various combinations. The cell concentrations after 4 d of growth reached a plateau between 10% and 40% serum and a maximum number at 20% CL (1) (Fig. 1), then began to descend sharply after 40% serum and more moderately at 20–80% lymph. The maximum number of cells in CS was approximately threefold higher than that in CL, which approximates the difference in protein concentration between serum of 9–3% of lymph. There was a cross-over between the two at 60%, with a descent to no growth between 80% and 100%.
Fig. 1.
Multiplication of transformed cells in varying high concentrations of CS and CL. The transformed cells (104) were seeded in multiwells in a medium containing the concentration of CS or CL shown on the abscissa made up in MCDB 402. Cells were incubated for 4 d and counted. Reprinted with permission from ref. 1.
These results suggested that the decreased growth in high concentrations of CS and CL resulted from the decrease in low molecular weight components as the concentration of the synthetic medium components approached zero with 100% concentrations of the serum and lymph. This raised the question of what low molecular weight constituents when added to the 100% serum or lymph would most effectively raise the growth rate of the cells. We tested the combination of all 18 amino acids of MCDB 402, a combination of the 13 essential amino acids, and a combination of cystine and glutamine.
Interestingly, while omission of glutamine from the supplement containing all the other amino acids prevents the growth of cells, simply adding glutamine back did not permit growth. This only occurred with the coaddition of cystine, an amino acid that was not detectable in whole serum unless it was released from protein immediately after bleeding (2). It was concluded that half-cystine residues are bound through disulfide linkage to serum proteins. Cystine drives glutamine catabolism and sensitivity to glutaminase inhibitors (3). The high concentration of glutamine in most growth media, combined with its unique effects on cell growth, provide evidence of its central regulatory role in the growth of cells (4).
Glutamine is considered a conditionally essential amino acid, meaning that it is necessary under particular conditions (5). For example, glutamine is nonessential for normal subjects where it is readily formed from common metabolite intermediates. Under certain stressful conditions, such as cancer, the need for glutamine may be greater than the endogenous rate of synthesis; thus, it is referred to as “conditionally essential.” However, it is generally considered an essential amino acid in cell culture research. Supplementation of 100% serum or lymph by cystine and glutamine by themselves has provided mixed results (1). For optimum growth, the concentration of cystine in the medium must be adjusted precisely. Deviation by a factor of 3 in either direction from the optimum concentration eliminates essentially all clonal growth (6).
A high concentration of glutamine is also needed for optimum clonal growth. Glutamine is the most abundant free amino acid in the circulation and intracellular pools, a precursor for the synthesis of amino acids, proteins, nucleotides and many other biologically important molecules (7). None of the combinations equaled the addition of all the nutrients of MCDB 402, indicating that such components as vitamins, glucose, and other organic compounds contribute to the growth capacity of cells.
Quantitative Differences in Growth Rates of Transformed and Nontransformed Cells in Response to a Wide Range of Glutamine Concentrations
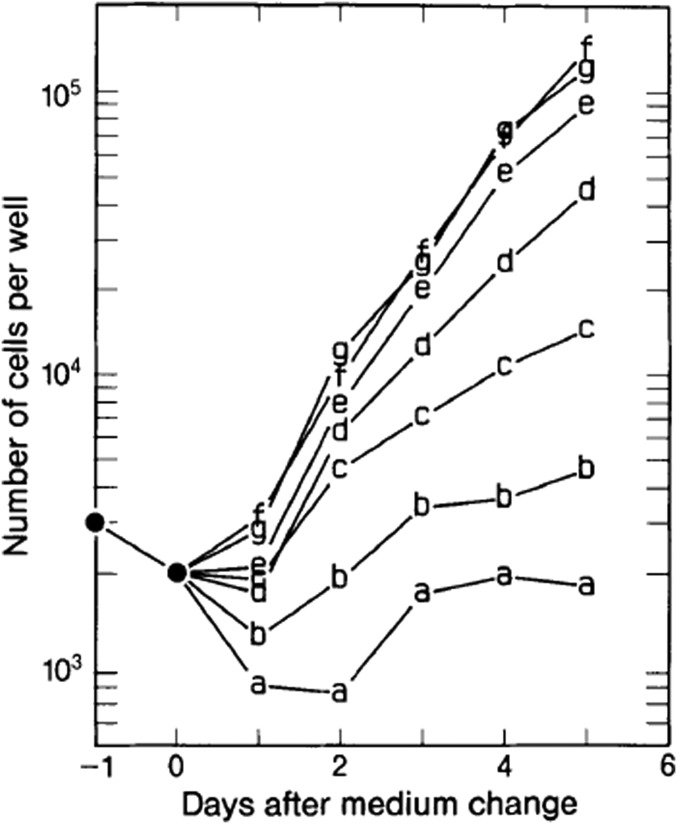
Experiments were conducted to quantify the dynamics of growth using a range of glutamine concentrations (8). The cystine concentration was 20 µM, while the other amino acids except glutamine were reduced to 1/10 the concentrations found in MCDB 402 to approximate physiological concentrations. The results are shown for transformed cells (Fig. 2) and nontransformed cells (Fig. 3) growing in 10% CS. The transformed cells grew at the same high rate descending from 1,627 µM to 427 µM glutamine with slight reductions between 427 µM and 127 µM, a greater decrease to 77 µM, and a much greater decrease to 27 µM (Fig. 2). The growth curves from 1,627 µM to 127 µM glutamine were exponential. The curve for 77 µM glutamine was initially parallel to those for the higher glutamine concentrations but then dropped to that occurring at 27 µM glutamine. The latter maintained a single, reduced exponential curve.
Fig. 2.
Multiplication of transformed cells with reduced concentrations of amino acids and different glutamine concentrations at 1 d after seeding 3 × 103 cells per well in MCDB 402 with 1/10 the standard concentration of amino acids of MCDB 402 plus 10% CS and varying amounts of glutamine as follows: 27 µM (A), 77 µM (B); 127 µM (C); 227 µM (D); 427 µM (E); 827 µM (F); and 1,627 µM (G). Cells were counted every day for 5 d. From ref. 8, copyright © 1988 by the Society for In Vitro Biology, formerly the Tissue Culture Association. Reproduced with permission of the copyright owner.
Fig. 3.
Multiplication of nontransformed cells at varying concentrations of glutamine in a background of 1/10 the standard amino acid concentrations of MCDB 402: 27 µM (A), 77 µM (B); 127 µM (C); 227 µM (D); 427 µM (E); 827 µM (F); and 1,627 µM (G). Cells were counted every day for 5 d in 10% CS. From ref. 8, copyright © 1988 by the Society for In Vitro Biology, formerly the Tissue Culture Association. Reproduced with permission of the copyright owner.
The nontransformed cells grew more slowly than the transformed cells under the same glutamine conditions as in Fig. 3 but with no reduction in growth rate between 1,627 µM and 127 µM glutamine and slight reductions to 77 µM and 27 µM (Fig. 3). These results indicate that the transformed cells required much higher concentrations of glutamine for growth than the nontransformed cells and were able to use high concentrations of glutamine to grow to higher densities.
A second series of growth curves of transformed cells in glutamine was performed but with the same high background of individual amino acids as characteristic of MCDB 402 (8) (Fig. 4). The same general pattern was observed as seen in 1/10 the amino acid concentrations (Fig. 2) but with several specific differences. There were the same growth rates in 1,627 µM and 827 µM glutamine, with a slight reduction in 427 µM glutamine (Fig. 4). The size of the gaps down to 227 µM widened, with even larger downward gaps between 127 µM, 77 µM, and 27 µM glutamine. Of note, although progressive decreases in cell numbers on the first day at 77 µM and 27 µM glutamine are shown in both Figs. 2 and 4, the subsequent increase on days 3–5 is much less in Fig. 4.
Fig. 4.
Multiplication of nontransformed cells at varying concentrations of glutamine in a background of the full standard amino acid concentrations of MCDB 402: 27 µM (A), 77 µM (B); 127 µM (C); 227 µM (D); 427 µM (E); 827 µM (F); and 1,627 µM (G). Cells were counted every day for 5 d. From ref. 8, copyright © 1988 by the Society for In Vitro Biology, formerly the Tissue Culture Association. Reproduced with permission of the copyright owner.
The foregoing experiments indicate that transformed cells need more glutamine to survive compared with nontransformed cells. The question arose as to whether this difference was related to the faster growth of the transformed cells. To address this question, the growth rate of the transformed cells was reduced by lowering the concentration of CS to 1% while the nontransformed cells were maintained in 10% CS (8). The final glutamine concentrations were 1,002.7 µM and 2.7 µM for the transformed cells and 1,027 µM and 27 µM for the nontransformed cells. The nontransformed cells grew at virtually the same rate in 1,027 µM and 27 µM glutamine for 3 d (Fig. 5). The transformed cells in 1,002.7 µM glutamine and 1% CS grew at a slower rate than the nontransformed cells in 10% CS for 2 d, but slowly increased to 8 d. The transformed cells in 2.7 µM glutamine showed little or no further growth in the lowest glutamine concentration.
Fig. 5.
Comprehensive comparison of glutamine concentrations for nontransformed cells in 10% CS and transformed cells in 1% CS. Cells were counted every day for 8 d. ∆–Δ, nontransformed cells, 1,027 µM glutamine, 10% CS; ○–○, nontransformed cells, 27 µM glutamine, 10% CS; ▲–▲, transformed cells, 1,002.7 µM glutamine, 1% CS; ●–●, transformed cells, 2.7 µM glutamine, 1% CS. Media were changed every day. From ref. 8, copyright © 1988 by the Society for In Vitro Biology, formerly the Tissue Culture Association. Reproduced with permission of the copyright owner.
This experiment demonstrates the generality of the principle of the high resistance of nontransformed cells compared with transformed cells to the low glutamine concentrations shown in Figs. 2 and 3. The glutamine reduction obtained by application of a glutaminase-asparaginase enzyme mixture has been identified as a biochemical rationale for the treatment of patients with acute leukemia or intra-abdominal metastatic cancers (9, 10).
Effects of Glutamine on the Process of Transformation in NIH 3T3 Cells
All the foregoing experiments in cell culture were executed with Balb 3T3 cells, using fully transformed and nontransformed cells. An experiment was conducted with nontransformed NIH 3T3 cells to explore the effects of glutamine concentration on the process of transformation. The time of appearance and number of transformed foci were followed over a 30-d period at glutamine concentrations between 0.16 and 5.0 mM (the latter the concentration in MCDB 402) (11) (Fig. 6). The plateau levels of focus formation increased with increasing glutamine concentration, starting from zero foci in 0.16 mM glutamine. No level overlapped with another level at the multiple points over 28 d of incubation. Thus, the process of transformation bears a similar type of requirement for glutamine as the growth of fully transformed cells. It is significant to note that the transformed NIH 3T3 cells observed here are capable of producing malignant fibrosarcomas in athymic mice (12, 13). Such is also the case with the transformed Balb 3T3 cells (14).
Fig. 6.
Dependence of focus formation in NIH 3T3 cells on time and on glutamine concentration in the medium. At 1 d after seeding of 104 nontransformed cells per 60 mm dish, the 10% FBS-containing MCDB 402 medium was replaced with similar medium but containing the following glutamine concentrations: ○, 5.0 mM; ●, 1.0 mM; ■, 0.6 mM; ▲, 0.4 mM; □, 0.2 mM; and ∆, 0.16 mM. 105 cells were seeded per 60-mm Petri dish and fixed and stained for transformed foci every 3–4 d. Reprinted from ref. 11 with permission from AACR.
Extensive transformation studies have shown that both Balb 3T3, and NIH 3T3 cells undergo conversion to the transformed state by a process of phenotypic selection (13). The transformed cells undergo reversion to the nontransformed state by passaging them frequently at low density in high serum concentrations. It is largely on this basis that transformation has been recognized as an epigenetic process. That makes it possible to study the response to glutamine of all phases of transformation, making it possible to optimize the minimal dosage of glutamine treatment to select against cancer cell proliferation while sparing damage to the surrounding normal cells.
Discussion
As shown in Fig. 5, the nearly constant number of transformed cells in 2.7 µM glutamine over an 8-d period raised the question of whether that flat level is due to cessation of growth or to an equilibrium between cell growth and death. This question could be answered by labeling with 3H-thymidine and autoradiography to determine the proportion of actively replicating cells. The answer has significance for the potential of using low glutamine concentrations for long-term treatment of cancer.
This points to a novel approach to the problem. The proposed protocol was developed in the treatment of phenylketonuria, a metabolic disorder secondary to an inherited deficiency of phenylalanine hydroxylase (15) that results in the accumulation of phenylalanine in plasma and cells. High levels of phenylalanine in body fluids inhibit the transport of amino acids into cells. The biochemical abnormalities can be corrected by preventing the accumulation of phenylalanine. This is done by using a special diet in which protein is replaced by a free amino acid mixture low in phenylalanine. Supplementary foods are given to supply only the amount of l-phenylalanine needed for body growth.
In the case of cancer therapy as proposed in this paper, the level of glutamine would be reduced by eliminating protein from the diet of experimental animals to be replaced by an amino acid mixture low in glutamine. Supplementary foods would be given to supply only the amount of glutamine needed for body growth. Our experiments show a marked difference in the glutamine requirement of neoplastically transformed cells and nontransformed cells. The transformed cells fail to multiply and some degenerate in 20–30 µM of glutamine, whereas nontransformed cells in the same glutamine concentrations remain healthy and continue to multiply.
The switch of the reduced glutamine concentration experiment from cell culture to animals must take into consideration the conditionally essential nature of glutamine in animals (16, 17). Under these conditions, glutamine is secreted and taken up by a diversity of organs. This contributes to the complexity of determining the dose required to produce the desired biological effect of stopping or reversing tumor growth.
Analogs of glutamine have been used as possible chemotherapeutic agents of cancers in animals and patients (16–18). They are antagonists of l-glutamine, and act by interrupting cellular nucleotide synthesis and thereby stopping the synthesis of DNA and/or RNA in the tumor cells. They also inhibit a number of other biochemical reactions that require glutamine. All the anticancer agents are limited by side effects, including nausea, mucositis, and pancytopenia. In one case, a reduction in tumor growth was demonstrated with acivicin, which lowered glutamine when combined with insulin (19). Unfortunately, all of the clinical trials were associated with toxic side effects, especially involving the rate-limiting enzymes of purine and pyrimidine biosynthesis (16).
These results are in contrast to the experiments in cell culture described here of simply lowering the concentration of glutamine to 27 µM in 10% CS or 2.7 µM in 1% CS, in which the number of transformed cells remained constant for many passages but have very little or no reduction in growth rate of nontransformed cells (Figs. 3 and 5). Allowing nontransformed cells to remain confluent over a wide range of glutamine concentrations from 5,000 µM to 160 µM exhibited a parallel reduction in transformed foci, reaching zero with 160 µM glutamine and remaining there for the full 27 d of the experiment (Fig. 6). The concentration of glutamine in MCDB 402 is 5,000 µM, far greater than the 270 µM concentration in CS (8). The implication is that 27 µM may be low enough to prevent further growth of tumor cells and possibility reverse tumor size, while having virtually no effect on nontransformed cells. However, that depends on the contribution of the conditionally essential behavior of glutamine. There is no evidence in the cell culture reduction of glutamine equivalent to the damaging side effects produced by the glutamine analogs in vivo (16–19).
Dysfunction in glucose metabolism has received most attention as a hallmark of cancer cells (20), yet limitations in glucose in the extracellular environment do not clearly limit the growth and malignancy of transformed cancer cells relative to normal cell counterparts. Glucose deprivation revealed that glutamine, the most abundant amino acid in serum, provides sufficient metabolic energy and blocks in the absence of glucose. In fact, even when glucose is readily available, glutamine provides up to 40% of tumor cell metabolism. In normal resting cell metabolism, pathways of glucose and glutamine metabolism are tightly regulated and result in complete oxidation with maximal efficiency. However, transiently in rapidly growing cells or constitutively in transformed cancer cells, both pathways are uncoupled from complete oxidation of pyruvate and each other, resulting in predominantly glycolysis and glutaminolysis to pyruvate and lactate. These results and glutaminolysis have received far less attention than the Warburg phenomena and dysfunctional glucose metabolism in tumors. There have been few studies exploiting glutaminolysis in chemotherapy (or diagnosis) relative to glutaminolysis.
It has been shown that glutamine deprivation causes differentiation accompanied by decreased proliferation of leukemia cells (21). The present study would be enhanced by showing that glutamine deprivation reverses the phenotype of transformed cells, as has been shown by manipulation of the cell culture conditions (13) and amplified by the unique morphology of descendants of selection (14). This would be a strong indication that glutamine deprivation would be of utility in the induction of differentiation mechanisms rather than limiting cell growth mechanisms to limit the size of tumors. Such a result would be of great interest in the specific model since it was contended in previous studies that transformation and reversion have an underlying epigenetic mechanism (22).
Materials and Methods
The spontaneously transformed clone of BALB 3T3 cells used here had been passaged twice by tumor formation in nude mice, and the nontransformed Balb 3T3 clone produced no tumors in mice. Cells were maintained in MCDB 402 (23) with 10% CS in 60-mm plastic culture dishes. The transformed cells were transferred twice a week, and the nontransformed cells were transferred once weekly, with incubation in 5% CO2 and 95% air in 37 °C. Cells were removed from the dish with 0.01% trypsin and 0.5 mM EDTA in Tris-buffered saline. The suspended cells were diluted into medium and seeded at 1–10 × 102 per 60-mm dish for transformed cells and 8–20 × 104 per 60-mm dish for nontransformed cells.
In these growth experiments, the stock cells suspended in MCDB 402 plus 2% serum were seeded in 24-well plates (Falcon) at 1.84 cm2/well. The next day, the medium was removed, the cells were carefully washed once with 1.5 mL of 0.082% BSA (fraction V) in Tris-saline per well, and experimental medium was added. Cells were counted in a Coulter electronic counter. CL was obtained from the thoracic duct of 400-lb calves and processed as described previously (1). It contained 15 U of heparin/mL. Heparin in >10-fold higher concentration had no measurable effect on multiplication of the Balb 3T3 cells. CS was obtained from HyClone Laboratories, and CL was obtained from Bio-Response Solutions. A batch of CS was carefully chosen from several lots and was not changed throughout the course of the experiments. Amino acid stock solutions were prepared at 40–1,000× concentrations and stored at −20 °C.
The concentrations of amino acids in the CS, CL, mouse plasma, and culture medium were determined in a Beckman Coulter 6300 amino acid analyzer. The mouse plasma was treated with 10% trichloroacetic acid immediately after bleeding to prevent binding of half-cystine residues.
The cells were cryopreserved after a single passage in MCDB 402 medium supplemented with 10% CS or 10% FBS (HyClone Laboratories). The cells used to initiate experiments were maintained in a constant state of exponential growth by passaging them three times weekly in 10% CS or 10% FBS at densities between 2 × 104 and 105 cells per 60-mm plastic culture dish. This treatment prevented the cultures from undergoing progressive transformation (24). During the long-term experiments described here, cells were passaged once weekly at a density of 104 per dish in the appropriate medium containing 10% FBS. Transformed foci, visible in dishes that had been fixed in methanol and stained in a 4% aqueous solution of Giemsa, were distinct from the nontransformed surrounding areas by virtue of their higher cell density. The number of focus-forming units at a given time was determined by dividing the number of transformed foci by the plating efficiency of the experimental cells on plastic. At the outset of this work, transformation assays were performed by plating 104 cells in medium containing 5 mM glutamine supplemented by 10% FBS, but in the quantitative determination of focus-forming units, 102–104 test cells were inoculated in the presence of 105 nontransformed background cells in 2% CS-containing medium.
Cell numbers were determined electronically after trypsinization. MCDB 402 medium (23) with or without glutamine was prepared in the laboratory. The glutamine concentration in the FBS supplement was determined on a Beckman Coulter amino acid analyzer. For the two lots of FBS used in these experiments, the glutamine concentrations were 1.00 and 1.15 mM. l-glutamine was added directly to the serum-supplemented media from 200 mM stock solutions to obtain the appropriate concentrations.
Acknowledgments
I thank Dorothy M. Rubin for assistance in preparing the manuscript and Drs. Allen Mayer and Andrew L. Rubin for helpful comments.
Footnotes
The author declares no conflict of interest.
References
- 1.Rubin H, Nomura T. Use of lymph in cell culture to model hormonal and nutritional constraints on tumor growth in vivo. Cancer Res. 1987;47:4924–4931. [PubMed] [Google Scholar]
- 2.Eagle H, Oyama VI, Piez KA. The reversible binding of half-cystine residues to serum protein, and its bearing on the cystine requirement of cultured mammalian cells. J Biol Chem. 1960;235:1719–1726. [PubMed] [Google Scholar]
- 3.Muir A, Vander Heiden MG. The nutrient environment affects therapy. Science. 2018;360:962–963. doi: 10.1126/science.aar5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zetterberg A, Engström W. Glutamine and the regulation of DNA replication and cell multiplication in fibroblasts. J Cell Physiol. 1981;108:365–373. doi: 10.1002/jcp.1041080310. [DOI] [PubMed] [Google Scholar]
- 5.Lacey JM, Wilmore DW. Is glutamine a conditionally essential amino acid? Nutr Rev. 1990;48:297–309. doi: 10.1111/j.1753-4887.1990.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 6.Ham RG, Hammond SL, Miller LL. Critical adjustment of cysteine and glutamine concentrations for improved clonal growth of WI-38 cells. In Vitro. 1977;13:1–10. doi: 10.1007/BF02615497. [DOI] [PubMed] [Google Scholar]
- 7.Smith RJ. Glutamine metabolism and its physiologic importance. JPEN J Parenter Enteral Nutr. 1990;14(4 Suppl):40S–44S. doi: 10.1177/014860719001400402. [DOI] [PubMed] [Google Scholar]
- 8.Nomura T, Rubin H. Quantitative studies of amino acid and growth factor requirements of transformed and nontransformed cells in high concentrations of serum or lymph. In Vitro Cell Dev Biol. 1988;24:878–884. doi: 10.1007/BF02623897. [DOI] [PubMed] [Google Scholar]
- 9.Spiers ASD, Wade HE. Bacterial glutaminase in treatment of acute leukaemia. BMJ. 1976;1:1317–1319. doi: 10.1136/bmj.1.6021.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holcenberg J, et al. Intraperitoneal chemotherapy with melphalan plus glutaminase. Cancer Res. 1983;43:1381–1388. [PubMed] [Google Scholar]
- 11.Rubin AL. Suppression of transformation by and growth adaptation to low concentrations of glutamine in NIH-3T3 cells. Cancer Res. 1990;50:2832–2839. [PubMed] [Google Scholar]
- 12.Rubin AL, Arnstein P, Rubin H. Physiological induction and reversal of focus formation and tumorigenicity in NIH 3T3 cells. Proc Natl Acad Sci USA. 1990;87:10005–10009. doi: 10.1073/pnas.87.24.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin H, Rubin AL. Phenotypic selection as the biological mode of epigenetic conversion and reversion in cell transformation. Proc Natl Acad Sci USA. 2018;115:E725–E732. doi: 10.1073/pnas.1717299115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin H. Uniqueness of each spontaneous transformant from a clone of BALB/c 3T3 cells. Cancer Res. 1988;48:2512–2518. [PubMed] [Google Scholar]
- 15.Smith LH. Harrison’s Principles of Internal Medicine. 7th Ed. McGraw-Hill; New York: 1974. pp. 592–596. [Google Scholar]
- 16.Medina MA, Sánchez-Jiménez F, Márquez J, Rodríguez Quesada A, Núñez de Castro I. Relevance of glutamine metabolism to tumor cell growth. Mol Cell Biochem. 1992;113:1–15. doi: 10.1007/BF00230880. [DOI] [PubMed] [Google Scholar]
- 17.Souba WW. Glutamine and cancer. Ann Surg. 1993;218:715–728. doi: 10.1097/00000658-199312000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahluwalia GS, Grem JL, Hao Z, Cooney DA. Metabolism and action of amino acid analog anti-cancer agents. Pharmacol Ther. 1990;46:243–271. doi: 10.1016/0163-7258(90)90094-i. [DOI] [PubMed] [Google Scholar]
- 19.Chance WT, Cao L, Fischer JE. Insulin and acivicin improve host nutrition and prevent tumor growth during total parenteral nutrition. Ann Surg. 1988;208:524–531. doi: 10.1097/00000658-198810000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apple S. 2016 An old idea, revived: Starve cancer to death. Available at https://www.nytimes.com/2016/05/15/magazine/warburg-effect-an-old-idea-revived-starve-cancer-to-death.html. Accessed February 20, 2019.
- 21.Spittler A, et al. Low glutamine concentrations induce phenotypical and functional differentiation of U937 myelomonocytic cells. J Nutr. 1997;127:2151–2157. doi: 10.1093/jn/127.11.2151. [DOI] [PubMed] [Google Scholar]
- 22.McKeehan WL. Glycolysis, glutaminolysis and cell proliferation. Cell Biol Int Rep. 1982;6:635–650. doi: 10.1016/0309-1651(82)90125-4. [DOI] [PubMed] [Google Scholar]
- 23.Shipley GD, Ham RG. Improved medium and culture conditions for clonal growth with minimal serum protein and for enhanced serum-free survival of Swiss 3T3 cells. In Vitro. 1981;17:656–670. doi: 10.1007/BF02628401. [DOI] [PubMed] [Google Scholar]
- 24.Rubin H, Xu K. Evidence for the progressive and adaptive nature of spontaneous transformation in the NIH 3T3 cell line. Proc Natl Acad Sci USA. 1989;86:1860–1864. doi: 10.1073/pnas.86.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]